We report our single-center experience demonstrating that HLA class I alloimmunization predicts longer time to platelet engraftment, increased bleeding complications, and higher transfusion requirements in patients undergoing gene-modified hematopoietic stem cell transplant for transfusion-dependent β thalassemia.
TO THE EDITOR:
Gene-modified hematopoietic stem cell transplant (GM-HCT) represents a revolutionary curative therapy for transfusion-dependent β thalassemia (TDT), inducing lasting transfusion independence with few complications.1-3 However, significantly longer platelet engraftment times have been observed compared with patients undergoing allogeneic stem cell transplant (alloSCT).1,2 Although complications, including hypersplenism and veno-occlusive disease (VOD), may delay platelet engraftment in both curative modalities, the reasons for longer platelet engraftment time in GM-HCT remain uncertain. Possible additional mechanisms include components of stem cell health and GM-HCT manufacturing.
Patients with TDT receive lifelong packed red blood cell (pRBC) transfusions before transplant, which can lead to transfusion-associated HLA class I alloimmunization, introducing an additional risk factor for delayed platelet engraftment. Although pRBC leukoreduction diminishes the risk of HLA alloimmunization, phagocytosis of donor platelets allows interaction between donor HLA class I complexes and recipient lymphocytes, forming HLA class I antibodies.4 A total of 29% to 85% of patients with hemoglobinopathies receiving transfusions have evidence of HLA alloimmunization.5,6 HLA class I antibodies opsonize transfused platelets, sequestering them within the spleen.7 During SCT, numerous platelet transfusions are required before engraftment. HLA-alloimmunized patients may exhibit platelet refractoriness, increasing the risk of bleeding complications. Higher transfusion requirements have been observed among patients with HLA alloimmunization undergoing matched related donor (MRD) alloSCTs for hemoglobinopathies.8,9
We conducted a retrospective single-center cohort study of patients undergoing GM-HCT and MRD alloSCT for TDT to evaluate HLA class I alloimmunization as an independent risk factor for delayed platelet engraftment and increased platelet and pRBC transfusions.
Patients with TDT receiving gene therapy participated in clinical trials or received postapproval betibeglogene autotemcel or exagamglogene autotemcel following conditioning with pharmacokinetic-adjusted busulfan over 4 days. Busulfan area under the curve (AUC) targeted trial-specific requirements using either every 6 hours or every 24 hours dosing for patients treated on clinical trials. For postapproval GM-HCT, busulfan AUC at 24 hours was initially targeted to 3600 to 6000 μmol/L∗min for daily dosing per institutional standards for busulfan transplant indications. This target was later narrowed to busulfan AUC at 24 hours 3800 to 5000 μmol/L∗min (international harmonized units for total 4-day AUC: 62.4-82.1 mg∗h/L). Patients receiving MRD alloSCT received distal alemtuzumab (48 mg total from day −22 to day −19), fludarabine (30 mg/m2 per day, daily, over 5 days), and melphalan (140 mg/m2 once). Then, 28-day hydroxyurea prophase (30 mg/kg per day) and thiotepa (8 mg/kg once) were added in patients treated after 2016.
HLA class I antibodies were assessed using the Luminex Single Antigen Bead Assay, which provides a reactivity percentage against potential platelet donors, termed the panel-reactive antibody percentage (PRA). Patients were considered PRAhigh if testing indicated reactivity to ≥80% of platelet donors. For early patients participating in clinical trials, PRAs were assessed during their transplant course only with signs of platelet refractoriness or delayed engraftment. On the basis of clinical experience, we changed our institutional policy to assess PRAs before transplant in all patients with preceding blood transfusion exposure, which included all patients with TDT receiving GM-HCT.
Platelet transfusions were given for platelets <20 × 103/μL in both GM-HCT and alloSCT per institutional standards while inpatient. Thresholds were increased for clinical bleeding episodes. pRBCs were given for hemoglobin <7 g/dL in both GM-HCT and alloSCT, for patients treated in 2017 or later. Patients 3 and 8 were treated before 2017, and underwent transfusion for hemoglobin <8 g/dL. Platelet and pRBC doses were 10 to 15 mL/kg for children <20 kg, and 1 to 2 units for children >20 kg. HLA-matched platelets were given in patients with clinical concern for platelet refractoriness, symptomatic bleeding, and evidence of HLA class I alloimmunization. Platelet engraftment was defined per Center for International Blood and Marrow Transplant Research consensus criteria.10 Statistical analyses used the Wilcoxon rank-sum test using Stata BE v17.0.
This study has been submitted to the institutional review board at Children's Hospital of Philadelphia and was deemed exempt from institutional review board review.
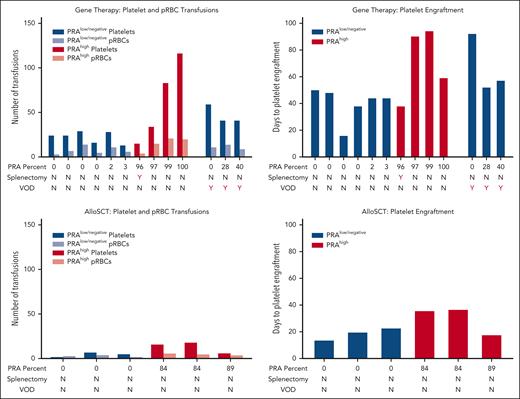
Eighteen patients underwent GM-HCT for TDT from 2015 to 2024 (supplemental Figure 1). Thirteen had PRA testing before or during hematopoietic stem cell transplant admission. Of these, 9 were categorized as PRAlow. Three were diagnosed with VOD, a known risk factor for delayed platelet engraftment and bleeding complications, and were analyzed separately (Figure 1). The 6 remaining patients with PRAlow achieved platelet engraftment at a median of 44 days (range, 16-50 days). They received a median of 24 platelet transfusions (range, 13-29 platelet transfusions) and 7 pRBC transfusions (range, 3-14 pRBC transfusions).
Platelet engraftment and transfusion requirements in gene therapy and MRD alloSCT. N, no; Y, yes.
Platelet engraftment and transfusion requirements in gene therapy and MRD alloSCT. N, no; Y, yes.
Four of 13 patients were classified as PRAhigh. They required a median of 75 days (range, 38-94 days) to achieve platelet engraftment, 30 days longer than patients with PRAlow. Patients with PRAhigh received a median of 59 platelet transfusions (range, 15-116 platelet transfusions), twice the number required for patients with PRAlow. They received 18 pRBC transfusions (range, 4-21 pRBC transfusions), a threefold increase compared with patients with PRAlow.
Bleeding complications are listed in Table 1. Patient 10 had 100% PRAs and experienced subarachnoid hemorrhages and severe mucocutaneous bleeding, leading to respiratory failure and a 15-day intubation. He required support with continuous transfusions of HLA-matched platelets, romiplostim, rituximab, IVIG, and plasmapheresis. In the setting of elevated terminal complement and persistent platelet refractoriness, he received eculizumab, despite not meeting other diagnostic criteria for thrombotic microangiopathy. He eventually achieved platelet engraftment after 59 days and received 116 units of platelets.
Patient demographics, outcomes, and complications
| Patient no. . | Age at transplant, y . | Sex . | Transplant type . | PRA, % . | Vector copy no., vc/dg . | CD34+ cells/kg . | Platelet engraftment, d . | Platelet transfusions . | pRBC transfusions . | VOD . | Bleeding complications . | Status, follow-up, mo . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 7.7 | F | GM-HCT∗,§ | 0 | 6.3 | 9.6 × 106 | 50 | 24¶ | 3 | N | Epistaxis | A/W/TI, 14.7 |
| 2 | 14.7 | F | GM-HCT∗,§ | 0 | 3.8 | 9.0 × 106 | 48 | 24 | 7 | N | Epistaxis | A/W/TI, 3.1 |
| 3 | 13.2 | M | GM-HCT∗,‡ | 0 | Not reported | 18.2 × 106 | 16 | 29¶ | 14 | N | Severe epistaxis | A/W/TI, 95.7 |
| 4 | 13.1 | M | GM-HCT∗,§ | 0 | 4.4 | 10.4 × 106 | 38 | 16 | 5 | N | None | A/W/TI, 10.8 |
| 5 | 20.0 | F | GM-HCT∗,§ | 2 | 5.2 | 11.1 × 106 | 44 | 28 | 11 | N | None | A/W/TI, 8.9 |
| 6 | 10.1 | M | GM-HCT∗,§ | 3 | 2.9 | 6.0 × 106 | 44 | 13 | 6 | N | Epistaxis | A/W/TI, 16.8 |
| 7 | 12.5 | F | GM-HCT∗,‡ | 96‖ | 1.9 | 6.8 × 106 | 38 | 15¶ | 4 | N | None | A/W/TI, 60 |
| 8 | 31.7 | F | GM-HCT∗,‡ | 97 | Not reported | 5.8 × 106 | 90 | 34 | 15 | N | Gum bleeding, bruising | A/W/TI, 103.9 |
| 9 | 17.8 | M | GM-HCT†,‡ | 99 | N/A | 10.2 × 106 | 94 | 83 | 21 | N | Severe epistaxis, gum bleeding, facial bruising | A/W/TI, 20.1 |
| 10 | 20.1 | M | GM-HCT∗,§ | 100 | 3.3 | 7.5 × 106 | 59 | 116¶ | 20 | N | Subarachnoid hemorrhage, hematuria, epistaxis, mucocutaneous bleeding leading to intubation | A/W/TI, 7.1 |
| 11 | 9.4 | M | GM-HCT∗,§ | 0 | 6.1 | 7.8 × 106 | 92 | 59¶ | 11 | Y | Severe epistaxis | A/W/TI, 10.3 |
| 12 | 19.8 | M | GM-HCT∗,§ | 28 | 5.2 | 5.9 × 106 | 57 | 41 | 14 | Y | Epistaxis, hematemesis, gum bleeding, subconjunctival hemorrhage | A/W/TI, 3.1 |
| 13 | 9.1 | M | GM-HCT∗,§ | 40 | 5.0 | 6.5 × 106 | 52 | 41 | 9 | Y | Epistaxis, facial bruising | A/W/TI, 4.9 |
| 14 | 4.4 | F | AlloSCT | 0 | N/A | 16.0 × 106 | 18 | 6 | 4 | N | None | A/W/TI, 11.7 |
| 15 | 1.6 | M | AlloSCT | 0 | N/A | 13.8 ×106 | 20 | 7 | 4 | N | None | A/W/TI, 54.8 |
| 16 | 8.7 | M | AlloSCT | 0 | N/A | 3.4 × 106 | 23 | 5 | 2 | N | None | A/W/TI, 6.1 |
| 17 | 20.1 | F | AlloSCT | 84 | N/A | 4.7 × 106 | 36 | 16 | 6 | N | Heavy menses, gum bleeding | A/W/TI, 33.9 |
| 18 | 8.1 | F | AlloSCT | 84 | N/A | 8.7 × 106 | 37 | 18 | 4 | N | None | A/W/TI, 61.6 |
| 19 | 3.1 | M | AlloSCT | 89 | N/A | 6.7 × 106 | 18 | 6 | 4 | N | None | A/W/TI, 37.8 |
| Patient no. . | Age at transplant, y . | Sex . | Transplant type . | PRA, % . | Vector copy no., vc/dg . | CD34+ cells/kg . | Platelet engraftment, d . | Platelet transfusions . | pRBC transfusions . | VOD . | Bleeding complications . | Status, follow-up, mo . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 7.7 | F | GM-HCT∗,§ | 0 | 6.3 | 9.6 × 106 | 50 | 24¶ | 3 | N | Epistaxis | A/W/TI, 14.7 |
| 2 | 14.7 | F | GM-HCT∗,§ | 0 | 3.8 | 9.0 × 106 | 48 | 24 | 7 | N | Epistaxis | A/W/TI, 3.1 |
| 3 | 13.2 | M | GM-HCT∗,‡ | 0 | Not reported | 18.2 × 106 | 16 | 29¶ | 14 | N | Severe epistaxis | A/W/TI, 95.7 |
| 4 | 13.1 | M | GM-HCT∗,§ | 0 | 4.4 | 10.4 × 106 | 38 | 16 | 5 | N | None | A/W/TI, 10.8 |
| 5 | 20.0 | F | GM-HCT∗,§ | 2 | 5.2 | 11.1 × 106 | 44 | 28 | 11 | N | None | A/W/TI, 8.9 |
| 6 | 10.1 | M | GM-HCT∗,§ | 3 | 2.9 | 6.0 × 106 | 44 | 13 | 6 | N | Epistaxis | A/W/TI, 16.8 |
| 7 | 12.5 | F | GM-HCT∗,‡ | 96‖ | 1.9 | 6.8 × 106 | 38 | 15¶ | 4 | N | None | A/W/TI, 60 |
| 8 | 31.7 | F | GM-HCT∗,‡ | 97 | Not reported | 5.8 × 106 | 90 | 34 | 15 | N | Gum bleeding, bruising | A/W/TI, 103.9 |
| 9 | 17.8 | M | GM-HCT†,‡ | 99 | N/A | 10.2 × 106 | 94 | 83 | 21 | N | Severe epistaxis, gum bleeding, facial bruising | A/W/TI, 20.1 |
| 10 | 20.1 | M | GM-HCT∗,§ | 100 | 3.3 | 7.5 × 106 | 59 | 116¶ | 20 | N | Subarachnoid hemorrhage, hematuria, epistaxis, mucocutaneous bleeding leading to intubation | A/W/TI, 7.1 |
| 11 | 9.4 | M | GM-HCT∗,§ | 0 | 6.1 | 7.8 × 106 | 92 | 59¶ | 11 | Y | Severe epistaxis | A/W/TI, 10.3 |
| 12 | 19.8 | M | GM-HCT∗,§ | 28 | 5.2 | 5.9 × 106 | 57 | 41 | 14 | Y | Epistaxis, hematemesis, gum bleeding, subconjunctival hemorrhage | A/W/TI, 3.1 |
| 13 | 9.1 | M | GM-HCT∗,§ | 40 | 5.0 | 6.5 × 106 | 52 | 41 | 9 | Y | Epistaxis, facial bruising | A/W/TI, 4.9 |
| 14 | 4.4 | F | AlloSCT | 0 | N/A | 16.0 × 106 | 18 | 6 | 4 | N | None | A/W/TI, 11.7 |
| 15 | 1.6 | M | AlloSCT | 0 | N/A | 13.8 ×106 | 20 | 7 | 4 | N | None | A/W/TI, 54.8 |
| 16 | 8.7 | M | AlloSCT | 0 | N/A | 3.4 × 106 | 23 | 5 | 2 | N | None | A/W/TI, 6.1 |
| 17 | 20.1 | F | AlloSCT | 84 | N/A | 4.7 × 106 | 36 | 16 | 6 | N | Heavy menses, gum bleeding | A/W/TI, 33.9 |
| 18 | 8.1 | F | AlloSCT | 84 | N/A | 8.7 × 106 | 37 | 18 | 4 | N | None | A/W/TI, 61.6 |
| 19 | 3.1 | M | AlloSCT | 89 | N/A | 6.7 × 106 | 18 | 6 | 4 | N | None | A/W/TI, 37.8 |
A/W/TI, alive, well, transfusion independent; F, female; M, male; N/A, not applicable (patient received exagamglogene autotemcel, which does not have a vector); N, no; vc/dg; vector copy number per decagram; Y, yes.
Betibeglogene autotemcel.
Exagamglogene autotemcel.
Clinical trial.
Commercial product.
Had prior splenectomy.
Platelet threshold increased to 30 during periods of active bleeding.
In contrast, patient 7 had 96% PRAs and experienced no transplant complications. Notably, she was the only patient in this cohort with a prior splenectomy. She only required 15 platelet transfusions and achieved platelet engraftment in 38 days. Splenectomy likely prevented clearance of opsonized platelets, leading to engraftment time and transfusion requirements comparable to patients with PRAlow.
Excluding patient 7, differences between patients with high and low PRAs in platelet engraftment time and platelet and pRBC transfusion requirements reached statistical significance, P = .02 for all outcomes.
Thirteen patients underwent MRD alloSCT from 2012 to 2024, and 6 had PRAs tested before or during SCT. None experienced VOD. Three patients had PRAlow and engrafted platelets in a median of 20 days (range, 14-23 days), requiring a median of 5 platelet transfusions (range, 2-7 platelet transfusions) and 3 pRBC transfusions (range, 2-4 pRBC transfusions). Three patients with PRAhigh (PRA level range, 84-89) were engrafted at a median of 36 days (range, 18-37 days), and required a median of 16 platelet transfusions (range, 6-18 platelet transfusions) and 5 pRBC transfusions (range, 4-6 pRBC transfusions). These differences did not reach statistical significance.
We observed a 2-fold increase in platelet engraftment time in patients receiving GM-HCT compared with alloSCT, a 4.5-fold increase in platelet transfusions, and a threefold increase in pRBC transfusions. Differences between groups were statistically significant, P < .01 for all outcomes.
Severe HLA alloimmunization was associated with dramatically longer platelet engraftment times and higher platelet and pRBC transfusion needs in patients undergoing GM-HCT. A similar trend, although smaller in amplitude and not statistically significant, was observed in MRD alloSCT. Although the underlying mechanisms remain unknown, we theorize that this effect may be due to increased subclinical bleeding in PRA-positive, platelet-refractory patients. Once the bone marrow begins to produce platelets, increased bleeding could result in platelet consumption, further prolonging engraftment time. In GM-HCT, the additional time required for CD34-selected, gene-modified and cryopreserved stem cell grafts to produce platelets compared with alloSCT grafts (which are usually given as fresh products and contain more hematopoietic progenitors) may exacerbate this effect. We observed substantially more bleeding complications in patients with PRAhigh receiving GM-HCT compared with alloSCT.
This study is limited by small sample size, as few patients have received GM-HCT for TDT, and even fewer underwent HLA antibody testing. Additional studies evaluating larger sample sizes are necessary to draw firm conclusions. Our population may be skewed toward patients with more bleeding complications, as in 4 cases PRAs were not tested before patients showed signs of platelet refractoriness.
Immunomodulation with IVIG, plasmapheresis, and rituximab has been used to reduce donor-specific HLA antibodies in patients receiving alloSCTs to prevent graft rejection.11,12 Eculizumab and splenectomy have also been used successfully to prevent graft rejection in patients with donor-specific HLA antibodies receiving solid organ transplants.13,14 Although not a standard approach in GM-HCT, pretransplant immunomodulation could similarly be used in patients with high PRAs to promote platelet engraftment and decrease risks of bleeding. We suggest all patients undergoing GM-HCT should have PRA testing to provide a complete bleeding risk assessment. In patients with high PRAs, prophylactic immunomodulation or even splenectomy for those with hypersplenism are potential interventions requiring future study with the goal of improving platelet engraftment and decreasing bleeding in GM-HCT.
Authorship
Contribution: N.M.G. analyzed data and wrote the manuscript; E.K. performed research and analyzed data; C.W.E. analyzed data and edited the manuscript; E.W. performed research; D.S.M. contributed analytical tools and edited the manuscript; A.A.T. performed research and edited the manuscript; J.L.K. performed and designed research and edited the manuscript; and T.S.O. performed and designed research and wrote and edited the manuscript.
Conflict-of-interest disclosure: E.K., C.W.E., A.A.T., J.L.K., and T.S.O. conducted research with bluebird bio and Vertex Pharmaceuticals. A.A.T., J.L.K., and T.S.O. consulted for bluebird bio and Vertex Pharmaceuticals. J.L.K. consulted for Editas. D.S.M. is chair of the scientific advisory board of Omixon, and receives royalties and owns options in Omixon. The remaining authors declare no competing financial interests.
Correspondence: Timothy S. Olson, Colket Translational Research Building, Room 3010, 3501 Civic Center Blvd, Philadelphia, PA 19104; email: olsont@chop.edu.
References
Author notes
For original data, please contact the corresponding author, Timothy S. Olson (olsont@chop.edu).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal