In this issue of Blood, Kuter et al report the results of a phase 3 randomized and placebo-controlled trial showing the efficacy and safety of rilzabrutinib, a Bruton tyrosine kinase (BTK) inhibitor, for treating persistent and chronic adult immune thrombocytopenia (ITP) in heavily pretreated patients.1
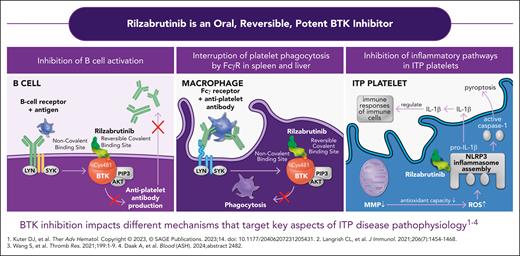
ITP is a rare autoimmune disease leading to accelerated platelet destruction and impaired platelet production, which is associated with an increased risk of bleeding when the platelet count decreases below 30 × 109/L.2 In the last 20 years, the number of therapeutic options for managing adult persistent or chronic ITP has sequentially increased with the off-label use of rituximab,3 then the approval of different thrombopoietin-receptor agonists (Tpo-RAs),4 and more recently the Syk inhibitor fostamatinib.5 Despite this progress, some adults with ITP may fail to respond to or not tolerate several treatment lines, with only a minority of patients with chronic ITP achieving a sustained response without therapy.6 BTK is a key actor in the signal transduction of the B-cell antigen receptor that plays an important role in the development and maturation of the B-cell lineage. The development of BTK inhibitors has revolutionized the treatment of chronic lymphocytic leukemia (CLL) and other B-cell malignancies. Ibrutinib was the first covalent, irreversible BTK inhibitor approved in 2013 as a breakthrough therapy for CLL. Early studies of ibrutinib in patients with B-cell malignancy complicated by ITP demonstrated high rates of ITP remission after initiation of ibrutinib treatment for the malignancy.7 Rilzabrutinib is an oral covalent, reversible, highly selective, and potent BTK inhibitor, which, unlike ibrutinib, does not interact with platelet functions, does not alter platelet aggregation, and has less off-target side effects.8 The rationale for using rilzabrutinib in ITP is based not only on its ability to inhibit the maturation of autoreactive B and autoantibody production, but also to impair macrophage Fcγ receptor-mediated signaling and to inhibit inflammatory pathways in ITP platelets8,9 (see figure). In a previous phase 1/2 open trial followed by a long-term extension study, rilzabrutinib at a daily dose of 400 mg twice daily showed promising efficacy and a good safety profile in adults with ITP.10 The phase 3 randomized placebo-controlled study (LUNA3) further assessed efficacy and safety of rilzabrutinib for treating ITP. Eligible adults with persistent or chronic primary ITP had to have a previous transient response to corticosteroids or intravenous immunoglobulin or anti-D and a platelet count below 30 × 109/L; concomitant treatment with corticosteroids or a Tpo-RA at a stable dose was allowed.
Different mechanisms of action of rilzabrutinib targeting some key aspects of ITP disease. IL-1β, interleukin 1β; MMP, matrix metalloproteinase; ROS, reactive oxydative species. Reproduced with permission from Sanofi. © 2025 Sanofi. All rights reserved.
Different mechanisms of action of rilzabrutinib targeting some key aspects of ITP disease. IL-1β, interleukin 1β; MMP, matrix metalloproteinase; ROS, reactive oxydative species. Reproduced with permission from Sanofi. © 2025 Sanofi. All rights reserved.
Patients were randomized in a 2:1 ratio to rilzabrutinib at 400 mg twice daily or placebo for 24 weeks of the blinded treatment period. At the end of week 12, patients were evaluated for initial “platelet response,” and responders could continue double-blinded treatment through week 24. The primary end point was durable response, which was defined, according to the requirements of medical agencies, as a platelet count ≥50 × 109/L either on at least 8 platelet weekly measurements (European Union and United Kingdom) in the absence of rescue therapy, or, in patients from other countries, two-thirds of at least 8 measurements scheduled over the last 12 weeks of the blind treatment period, with at least 2 counts beyond this threshold in 2 out of the 6 last weeks. These criteria, with questionable clinical relevance, raise the issue of the prominent role taken by the major regulatory agencies for defining platelet response in ITP.
In total, 202 patients with mostly chronic ITP (median ITP duration of 7 years) were included in the LUNA3 trial. Of note, these patients were previously heavily treated, with approximately half of the patients having previously received at least 5 different treatment lines for ITP including 28% who had undergone splenectomy, a percentage that reflects a patient population with mostly chronic refractory ITP. Among them, 85 (64%) patients taking rilzabrutinib and 22 (32%) patients taking placebo achieved platelet response during the first 12 weeks and were eligible to complete the double-blind period. Overall, a durable response was achieved in 31 of 133 (23%) patients on rilzabrutinib vs 0% in the placebo arm (P < .001). Interestingly, and as previously shown in the phase 2 trial, the median time to first response to rilzabrutinib was relatively short (ie, 15 days). Sixty percent of the patients from the rilzabrutinib arm were receiving concomitant ITP therapy at time of inclusion. Although subgroup analysis did not show any significant difference in terms of durable response rates between patients receiving or not receiving concomitant treatment, a synergistic effect of the combination cannot be fully ruled out, and whether some patients in the rilzabrutinib arm were able to decrease or stop that concomitant therapy has not been assessed. Rilzabrutinib was associated with a significant reduction of physical fatigue, and other indicators of health-related quality of life were improved. In terms of safety, most of adverse events (mostly diarrhea) were of grade 1 or 2, and only 3 patients had to discontinue rilzabrutinib because of gastrointestinal adverse events. Very few cases of infections graded ≥2 were observed in the rilzabrutinib arm, and 1 patient died from pneumonia, which was not considered as being related to rilzabrutinib.
In conclusion, the lessons that can be drawn from the LUNA3 trial are the following:
The major progress made in the treatment of B-cell lymphoid malignancies often paves the way for subsequent therapeutic progress in ITP as in other autoantibody-mediated autoimmune diseases.3,7,9
Rilzabrutinib, given alone or in combination with corticosteroids or Tpo-RAs, is the first in class of BTK inhibitors to show convincing efficacy and safety data for treating adults with persistent or chronic ITP.
The initial response to rilzabrutinib may be relatively rapid, a factor that could be helpful for its future use.
The place of rilzabrutinib in the actual ITP therapeutic landscape will depend on the data from ongoing long-term extension studies and also from real-world evidence data throughout large-scale prospective registries. The cost-effectiveness ratio of rilzabrutinib and its potential ability to induce durable responses will be among the factors that could influence its future positioning.
Conflict-of-interest-disclosure: M.M. received honoraria for consultancy and/or as a speaker from Alexion, Amgen, Grifols, Novartis, Sanofi, and Sobi.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal