In this issue of Blood, Prasad et al1 analyzed the impact of antibiotics on survival outcomes in a large international cohort of patients with lymphoma who received anti-CD19 chimeric antigen receptor T-cell (CAR-T) therapy. They found that broad-spectrum antibiotics deplete metabolically active commensals, the metabolites of which are essential for enhancing CAR-T efficacy (see figure).
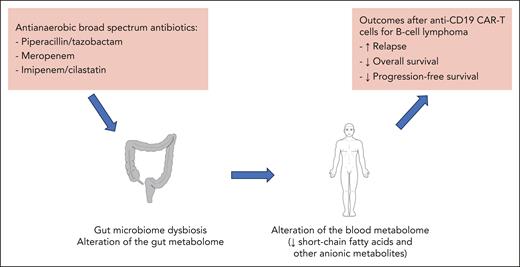
Detrimental effect of antibiotics with a broad antianaerobic spectrum on patients with B-cell lymphoma after anti-CD19 CAR-T therapy.
Detrimental effect of antibiotics with a broad antianaerobic spectrum on patients with B-cell lymphoma after anti-CD19 CAR-T therapy.
The standard of care treatment strategy for patients with relapsed or refractory B-cell lymphoma now includes new immunotherapeutic approaches, in particular anti-CD19 CAR-Ts, including usage as second-line therapy.2 Despite CAR-Ts significantly improving patient outcomes compared with conventional approaches with chemotherapy, including high-dose chemotherapy followed by autologous hematopoietic cell transplantation, a significant number of patients still do not achieve a complete response after receiving CAR-T therapy.2 Possible determinants of CAR-T failure are numerous3 but of particular interest was the finding that exposure to antibiotics with a broad antianaerobic spectrum (piperacillin/tazobactam, meropenem or imipenem/cilastatin) in the 4 weeks before therapy was associated with worse overall survival.4 These findings were confirmed in a second study,5 but the authors also found that patients with high tumor burden and systemic inflammation were more likely to receive antibiotics prior to CAR-T therapy, both of which possibly contributed to the association with worse outcomes.5 Therefore, to address these confounding factors, the gut microbiome was analyzed in the subgroup of patients not exposed to broad-spectrum antibiotics, and significant correlation was found between gut microbiome composition and patients’ survival and lymphoma progression. In particular, Bacteroides, Ruminococcus, Eubacterium, and Akkermansia were found to be the most important in determining CAR-T responsiveness.5 However, the functional consequences of microbial effectors such as metabolites during antibiotic-induced microbiome dysbiosis has not been explored in this context.
With this background, the same group investigated the gut microbiome along with the gut and blood metabolome in a large international cohort of patients with B-cell lymphoma that had received CAR-Ts. First, they confirmed that prior exposure to antibiotics with a broad antianaerobic spectrum induced gut microbiome dysbiosis and was associated with worse overall survival after CAR-T therapy. Interestingly, they found that gut microbiome dysbiosis resulted in significant alteration not only of the gut but also of the blood metabolome. Thus, use of piperacillin/tazobactam, meropenem or imipenem/cilastatin led to decreased levels of microbiome effectors such as short-chain fatty acids (SCFAs) and anionic metabolites. Overall, these findings suggest that the association between use of antibiotics with a broad antianaerobic spectrum and poor outcomes after CAR-T therapy may be related to a systemic effect of broad-spectrum antibiotics impacting the CAR-T function. But given the confounding factors associated with the use of antibiotics, particularly of high disease burden, the authors explored their findings in an immune-competent CAR-T mouse model. They confirmed that in this model meropenem induced gut microbiota dysbiosis and a systemic dysbiotic metabolome, associated with significantly increased A20 lymphoma tumor growth and poorer survival, compared with mice treated with CAR-Ts but not exposed to meropenem.
Therefore, based on these data, modulation of the gut microbiota to restore its composition before CAR-T infusion seems a promising approach to improve patient outcomes after CAR-T therapy. One of the most promising approaches would be the use of fecal microbiota transfer that consists of the transfer of a whole ecosystem from a single donor or a pool of donors to restore the patient’s microbiome.6 This approach was proven to be safe and effective in highly immunosuppressed patients with graft-versus-host disease after allogeneic hematopoietic cell transplantation, with a good restoration of gut microbiome diversity, particularly of the bacteria that produce SCFAs.7 The authors are currently conducting a randomized clinical trial (NCT06218602) to evaluate this approach to correct antibiotic-induced microbiota dysbiosis to improve CAR-T therapy. However, SCFAs have a pleiotropic effect on the immune system and contribute to the stimulation of regulatory immune cell subsets, which may be detrimental to CAR-T efficacy. Therefore, the authors evaluated an alternative approach, where they exposed CAR-Ts to SCFAs ex vivo, during their manufacturing, leading to an increased cytotoxicity of those CAR-Ts against tumors cells. This approach may also warrant further investigation as a way to enhance cytotoxicity of next-generation CAR-Ts.
Of note, although some mechanistic insights are still lacking, this study does provide important evidence confirming the detrimental impact of exposure to broad-spectrum antibiotics before CAR-T therapy on patients’ outcomes. Although gut microbiota modulation through fecal microbiota transfer is already under investigation, it would seem important to change our practice regarding use of antibiotics for patients eligible for CAR-Ts, in particular by avoiding the use of piperacillin/tazobactam, meropenem or imipenem/cilastatin when feasible in those patients. Finally, given the impact of diet on gut microbiota composition,8 and the fact that patients with lymphoma frequently have a poor diet due to decreased appetite, these factors should be more closely analyzed in future studies to evaluate the respective effects of diet and antibiotics on gut microbiota dysbiosis in this setting. Indeed, improvement of nutritional status with well-designed dietary approaches and use of prebiotics may help to further enhance the potential efficacy of fecal microbiota transfer in these patients.
Conflict-of-interest disclosure: M.M. reports grants and lecture honoraria from Janssen, Sanofi, Maat Pharma, and Jazz Pharmaceuticals; lecture honoraria from Celgene, Amgen, Bristol Myers Squibb (BMS), Takeda, and Pfizer; and grants from Roche, all outside the submitted work. F.M. reports lecture honoraria from Therakos/Mallinckrodt, BMS, MSD, Sanofi, Novartis, AstraZeneca, and Jazz Pharmaceuticals, all outside the submitted work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal