In this issue of Blood, Kulasekararaj et al show that danicopan (an oral complement factor D inhibitor) as add-on therapy to a C5 inhibitor can improve hemoglobin ≥ 2 g/dL in patients with paroxysmal nocturnal hemoglobinuria (PNH) experiencing clinically significant extravascular hemolysis.1
PNH is a clonal hematopoietic disease caused by somatic mutation of the X-linked gene PIGA. The PIGA gene product is required for making glycosylphosphatidylinositol (GPI), an anchoring moiety that attaches dozens of proteins to the cell membrane.2 Two GPI-anchored proteins, CD55 and CD59, are complement regulatory proteins missing on PNH red cells. CD59 inhibits downstream of C5 by regulating terminal complement, and CD55 works upstream of C5 to regulate both the C3 and C5 convertases. Deficient complement regulation on PNH blood cells leads to intravascular hemolysis, fatigue, and thrombosis. Thrombosis is the leading cause of death from PNH. Terminal complement inhibitors (eculizumab and ravulizumab) target C5, reducing the risk for thrombosis, stabilizing hemoglobin, and improving quality of life as well as overall survival. Eculizumab was approved for the treatment of PNH in 20073 and ravulizumab in 2019.4 Most patients with PNH do well on intravenous ravulizumab administered every 8 weeks, but up to 30% develop clinically significant extravascular hemolysis, defined in this study as a hemoglobin of 9.5 g/dL or less with an absolute reticulocyte count over 120 × 109/L. Since CD55 is upstream of C5, surviving PNH red cells are opsonized with C3 fragments and prematurely phagocytosed in the liver and spleen.
In an attempt to improve upon ravulizumab, pharmaceutical companies have developed so-called “upstream” complement inhibitors that inhibit complement upstream to CD55 and CD59 (see figure). Targets include C3 (pegcetacoplan),5 factor B (iptacopan),6 and factor D (danicopan).7 Indeed, these targets are upstream of C5 in the alternative pathway, but none of these new agents inhibit the classical pathway of complement; furthermore, they all have very short half-lives (hours to days). A more accurate description of these drugs would be alternative pathway inhibitors. All 3 control intravascular and extravascular hemolysis at steady state in patients with PNH as single agents, often resulting in hemoglobin values >2 g/dL higher than ravulizumab or eculizumab. However, a strong classical pathway stimulus (infections, trauma, surgery, etc) can lead to massive intravascular hemolysis (breakthrough hemolysis) in patients on alternative pathway inhibitors.8 Even patients on C5 inhibitors may experience breakthrough hemolysis with strong complement-amplifying conditions; however, breakthrough events on C5 inhibitors are less severe because only 1 membrane attack complex is formed per molecule of C5 that escapes ravulizumab.9 In contrast, incomplete C3 inhibition with alternative pathway inhibitors catalyzes the cleavage of multiple C5 molecules, thus amplifying the breakthrough. Furthermore, the PNH erythrocyte clone size increases on upstream complement inhibitor, approximating the size of the granulocyte clone, due to increased survival of the GPI-deficient red cells. Therefore, a larger clone is vulnerable to breakthrough events.
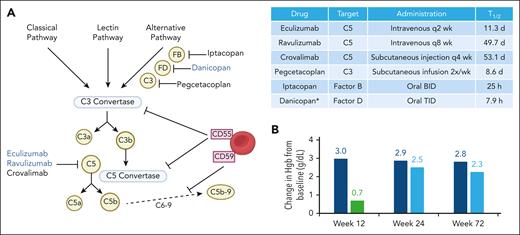
(A) Schematic of complement pathway and targets of currently approved complement inhibitors for PNH. PNH blood cells are deficient in CD55 and CD59, leading to complement dysregulation. Table of approved complement inhibitors in PNH. Drug half-life as listed in Food and Drug Administration labeling. (B) Change in hemoglobin from baseline with add-on danicopan to a C5 inhibitor in participants with clinically significant extravascular hemolysis in the phase 3 ALPHA trial. Dark blue bar indicates danicopan-danicopan group, green is placebo, and light blue is placebo-danicopan crossover arm. ∗All drugs are approved as single agents except danicopan, which is add-on therapy to eculizumab or ravulizumab. BID, twice a day; Hgb, hemoglobin; q, every; TID, 3 times a day.
(A) Schematic of complement pathway and targets of currently approved complement inhibitors for PNH. PNH blood cells are deficient in CD55 and CD59, leading to complement dysregulation. Table of approved complement inhibitors in PNH. Drug half-life as listed in Food and Drug Administration labeling. (B) Change in hemoglobin from baseline with add-on danicopan to a C5 inhibitor in participants with clinically significant extravascular hemolysis in the phase 3 ALPHA trial. Dark blue bar indicates danicopan-danicopan group, green is placebo, and light blue is placebo-danicopan crossover arm. ∗All drugs are approved as single agents except danicopan, which is add-on therapy to eculizumab or ravulizumab. BID, twice a day; Hgb, hemoglobin; q, every; TID, 3 times a day.
The phase 3 ALPHA trial, a study sponsored by Alexion, Astra Zeneca Rare Disease, took a “belt-and-suspenders” approach to managing PNH by adding danicopan to a C5 inhibitor in patients with clinically significant extravascular hemolysis on a C5 inhibitor. The initial 12-week treatment period, including 63 participants randomized to danicopan or placebo plus a C5 inhibitor, was previously published.7 In this issue of Blood, Kulasekararaj et al report the long-term efficacy and safety data up to 72 weeks including the double-blind randomized control treatment period (weeks 0-12), open-label crossover period (weeks 12-24), and 2-year long-term extension study in all 86 randomized participants. Efficacy and safety were overall unchanged in the long-term study. Approximately half of participants on danicopan maintained a ≥2 g/dL increase in hemoglobin in the absence of transfusions at 72 weeks, and ∼80% achieved transfusion avoidance between weeks 48 to 72 with 65% transfusion independence throughout the entire study. Mean lactate dehydrogenase remained <1.5 × the upper limit of normal (ULN) through week 72, and participant-reported improvements in fatigue were maintained. A total of 16 participants discontinued study, 6 in the treatment periods and 10 in the long-term extension study. The main dose-limiting toxicity was liver function abnormalities, leading to study discontinuation in 5 participants. Breakthrough hemolysis of mild to moderate severity (lactate dehydrogenase ≤ 2.2 × ULN) was reported in 5 participants (7 events), none leading to trial discontinuation, dose adjustments, or need for transfusions. One death occurred due to pneumonia in a participant with aplastic anemia receiving immunosuppression. No meningococcal infections were reported.
The recent approval of multiple complement inhibitors with different targets and modes of administration represents a significant advance in the treatment of PNH. Differing entry criteria and definitions of breakthrough hemolysis limit exact comparison between the different upstream inhibitors. Danicopan as an add-on therapy to C5 inhibitors may offer more complete control of complement dysregulation, especially in the setting of a complement-amplifying condition, and the long half-life C5 inhibitor serves as a backstop against recurrent intravascular hemolysis in cases of missed medication doses. Lost pills, food poisoning, and severe viral gastroenteritis, among others, are real-world events that are underrepresented in clinical trials with stand-alone alternative pathway inhibitors. What is the contingency plan for managing these events, especially surrounding travel? Will these severe breakthrough events lead to thrombosis? How will clinicians distinguish between breakthrough vs missed doses? The long half-life of ravulizumab has stood the test of time so the addition of danicopan for the 25% to 30% of patients with a suboptimal response to monotherapy may be the safest approach. Danicopan’s 3 times daily administration represents a downside. The potential advantage of dual therapy (ravulizumab + danicopan) compared with alternative pathway monotherapy remains to be determined in a real-world setting and hopefully head-to-head trials.
Conflict-of-interest disclosures: G.F.G. reports advisory board participation for Alexion Pharmaceuticals and Apellis Pharmaceuticals. R.A.B. has received research funding and provided consultancy for Alexion Pharmaceuticals.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal