In this issue of Blood, Abagnale et al1 reveal the effect of the BRAFV600E mutation on induced pluripotent stem cell (iPSC)–derived hematopoietic progenitor cells and microglial lineages. This study reveals that the BRAFV600E mutation in CD34+ hematopoietic progenitor cells induces the differentiation of these cells into Langerhans cell histiocytosis cells.
Langerhans cell histiocytosis (LCH) is a rare inflammatory myeloid neoplasm affecting both children and adults.2 It is characterized by the accumulation of CD1a+/CD207+ histiocytes in various tissues.3 Typical clinical features include bone pain, skin lesions, lung cysts, diabetes insipidus, and central nervous system involvement. In 2010, the discovery of recurrent BRAF mutations underlying the V600E variant of LCH revealed the neoplastic nature of this disease.4 Recurrent somatic BRAFV600E mutations were subsequently found in 50% to 60% of patients with LCH.2,4 Other mutations in MAP kinase (MAPK) pathway genes have since been described, including downstream (MEK1 encoded by MAP2K1) and upstream (KRAS, NRAS, and the tyrosine kinase receptors ALK, CSF1R, NTRK1, and RET) components.5 MAPK pathway mutations are almost universal in LCH. These findings paved the way for the use of targeted therapies, which have revolutionized the management of patients with histiocytosis.6
Despite these advances, the mechanisms by which these mutations drive the disease remain unclear. The origin of LCH histiocytes had puzzled the scientific community for many years. The unique phenotype of CD1a+/CD207+ histiocytes suggested that epidermal Langerhans cells were a likely culprit. However, genomic studies identified a signature in LCH cells resembling that of immature myeloid precursors.3 The discovery of the BRAFV600E mutation made it possible to test the hypothesis that LCH arises from hematopoietic precursors as it provided a molecular tag for lineage tracing. In 2014, Berres et al7 revealed that the BRAFV600E mutation was present in circulating monocytes and CD34+ bone marrow progenitor cells from children with high-risk multifocal LCH. These data strongly suggested that mutated bone marrow progenitor cells were the precursors ultimately differentiating into tissue-infiltrating histiocytes. However, this result was not duplicated in patients with low-risk unifocal LCH disease.7 It has been suggested that these conflicting results are due to an association between the extent of the disease and the differentiation stage of the cell in which the activating somatic MAPK gene mutation arises. This LCH ontogeny model is known as the “misguided myeloid differentiation model.”2,7 However, many questions remain: is this model valid? Why do some patients develop neurodegeneration whereas others with similar mutations do not? How should LCH be treated?
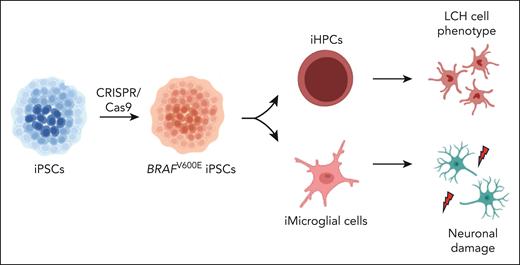
In this issue of Blood, Abagnale et al address some of these questions (see figure). They used 2 Nobel Prize–winning techniques: iPSC reprogramming and CRISPR/Cas9 genome editing. The authors induced PSCs from the peripheral blood mononuclear cells of 2 children (1 patient with LCH, 1 healthy control) and performed genome editing by CRISPR/Cas9 methods to obtain cells with the BRAFV600E mutation. These cells were then differentiated into various lineages including iPSC-derived hematopoietic progenitor cells (iHPCs) and induced microglia-like cells (iMGLs) bearing the BRAFV600E mutation. They found the following 3 principal results: First, iHPCs differentiated into monocytic cells that had key features of LCH. Second, MEK inhibitor resistance was observed in immature progenitors whereas mature LCH-like cells were sensitive to these inhibitors. Third, iMGL induced neurodegeneration in iPSC-derived neurons (iNeurons).
BRAFV600E-mutated PSCs. Abagnale et al induced PSCs from the peripheral blood mononuclear cells of 2 subjects (1 with childhood-onset LCH, 1 healthy control). They, then, performed genome editing with CRISPR/Cas9 to obtain iPSCs with the BRAFV600E mutation. The authors differentiated these cells into various cell lineages, including CD34+ iHPCs and iMGLs. They found that (1) the iHPCs spontaneously acquired key features of LCH histiocytes and (2) the iMGLs induced neurodegeneration.
BRAFV600E-mutated PSCs. Abagnale et al induced PSCs from the peripheral blood mononuclear cells of 2 subjects (1 with childhood-onset LCH, 1 healthy control). They, then, performed genome editing with CRISPR/Cas9 to obtain iPSCs with the BRAFV600E mutation. The authors differentiated these cells into various cell lineages, including CD34+ iHPCs and iMGLs. They found that (1) the iHPCs spontaneously acquired key features of LCH histiocytes and (2) the iMGLs induced neurodegeneration.
These findings may have a direct clinical impact. For example, stopping targeted therapy has been reported to frequently result in disease relapse.8 Abagnale et al provide an explanation for this phenomenon. They reveal that MAPK inhibitors target exclusively the mature CD14+ monocytic population, with no effect on myeloid progenitors. Thus, the study results support the use of conventional chemotherapy to target immature clones. But do all patients have immature clones? Published data suggest that they do not.7 So, how can we identify patients with immature mutated clones? Should a baseline bone marrow assessment be performed in all patients with LCH? Could the decision to use chemotherapy or targeted agents be guided by the presence of a detectable bone marrow clone? The data presented by Abagnale et al provide a valuable basis for upcoming prospective trials addressing these questions.
Abagnale et al also shed light on the pathophysiology of neurodegeneration in LCH. The origin of BRAFV600E microglial clones remains unclear. Neurodegenerative LCH may be caused by mutated cells migrating to the brain from the periphery or by resident microglia acquiring the BRAF mutation early in fetal development, as previously suggested.9 Abagnale et al found that iMGL induced neurodegeneration in vitro. These data confirm that bone marrow precursors can differentiate into pathogenic microglial cells. They indirectly support the hypothesis that microglial clones originate from circulating peripheral precursors. Vicario et al10 recently reported data from the postmortem examinations of 8 patients with L-histiocytosis. There were 5 (2 cases of pediatric-onset LCH and 3 of adult-onset LCH/Erdheim-Chester disease [ECD]) who had no detectable BRAFV600E variant in the bone marrow, suggesting that the clones with BRAF mutations in the brain might have originated from the resident macrophage lineage. Conversely, 3 other adult patients (2 ECD and 1 mixed LCH/ECD form) had bone marrow clones similar to the clones found in the brain. The authors concluded that the origin of the mutated microglial cells was patient dependent.
The main limitation of this study is that the model the authors developed does not encompass the spatial dimension of this disease. LCH is a tumoral process affecting tissues, with various inflammatory cells frequently organized into granulomas. Studies of the interactions of cells with their microenvironment will be important to providing clear understanding of the pathophysiology of LCH.
Despite this limitation, this study opens up new avenues of research for histiocytosis and beyond. Other studies harnessing the same framework but for other mutations (of the NRAS, KRAS, and MAP2K1 genes, for example), other cell lineages, and other candidate therapies may provide significant insights in the future. This study by Abagnale et al is a breakthrough in our understanding of histiocytic disorders because it provides mechanistic insights into a rare disease, a platform for future research, and a rationale for upcoming clinical trials.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal