Key Points
BRAFV600E/WT iPSCs show monocytic skewing and develop into CD1a+/CD207+ LCH-like cells and microglia-like cells, which cause neurodegeneration.
MAPKis target mature monocytic cells and revert transcriptomic changes induced by BRAFV600E but do not eradicate progenitors.
Visual Abstract
Langerhans cell histiocytosis (LCH) is a clonal hematopoietic disorder defined by tumorous lesions containing CD1a+/CD207+ cells. Two severe complications of LCH are systemic hyperinflammation and progressive neurodegeneration. The scarcity of primary samples and lack of appropriate models limit our mechanistic understanding of LCH pathogenesis and affect patient care. We generated a human in vitro model for LCH using induced pluripotent stem cells (iPSCs) harboring the BRAFV600E mutation, the most common genetic driver of LCH. We show that BRAFV600E/WT iPSCs display myelomonocytic skewing during hematopoiesis and spontaneously differentiate into CD1a+/CD207+ cells that are similar to lesional LCH cells and are derived from a CD14+ progenitor. We show that BRAFV600E modulates the expression of key transcription factors regulating monocytic differentiation and leads to an upregulation of proinflammatory molecules and LCH marker genes early during myeloid differentiation. In vitro drug testing revealed that BRAFV600E-induced transcriptomic changes are reverted upon treatment with mitogen-activated protein kinase (MAPK) pathway inhibitors (MAPKis). Importantly, MAPKis do not affect myeloid progenitors but reduce only the mature CD14+ cell population. Furthermore, iPSC-derived neurons (iNeurons) cocultured with BRAFV600E/WT iPSC-derived microglia-like cells, differentiated from iPSC-derived CD34+ progenitors, exhibit signs of neurodegeneration with neuronal damage and release of neurofilament light chain. In summary, the iPSC-based model described here provides a platform to investigate the effects of BRAFV600E in different hematopoietic cell types and provides a tool to compare and identify novel approaches for the treatment of BRAFV600E-driven diseases.
Introduction
Langerhans cell histiocytosis (LCH) is a rare disease characterized by lesions infiltrated by CD1a+/CD207+ cells.1,2 The discovery of mitogen-activated protein kinase (MAPK)-activating mutations, predominantly BRAFV600E,3 a common pleiotropic oncogene,4 and the clonal expansion of CD1a+/CD207+ cells,5,6 led to its classification as an inflammatory myeloid neoplasm. Although patients with single-system involvement have favorable prognoses, with lesions often resolving spontaneously, those with multisystem involvement, particularly affecting the risk-organs hematopoietic system, spleen, and liver, face poorer prognoses. These patients may also develop a life-threatening form of LCH characterized by hyperinflammation with high fever, pancytopenia, and a cytokine storm.7,8 Moreover, up to 30% of patients develop devastating and irreversible neurodegeneration years after remission.9,10 MAPK-pathway inhibitors (MAPKis) are currently used in patients with LCH,11 but monotherapy does not seem to eradicate the malignant clone.12,13 Important pathophysiological aspects of LCH remain elusive: although BRAFV600E is present in different hematopoietic cells,14 including CD34+ progenitor cells,15,16 the immediate precursor of the pathognomonic LCH cells is uncertain because both monocytes and dendritic cells (DCs) can acquire CD1a/CD207 expression.16-21 Additionally, it is unclear which cells cause systemic inflammation, because these symptoms can persist even after lesions disappear,22 nor which downstream targets are affected by MAPKis in LCH. Furthermore, it is unresolved whether neurodegenerative central nervous system (CNS) LCH (ND-CNS-LCH) is caused by mutated cells migrating to the brain from the periphery or by resident microglia acquiring the BRAFV600E mutation early during fetal development.23-25 A lack of mechanistic models contributes to this knowledge gap: LCH is rare and predominantly affects children, thus primary samples are scarce and small in size. Moreover, no distinct marker distinguishes mutated cells in the periphery or bone marrow (BM). Furthermore, no LCH cell lines exist and current murine models, in which BRAFV600E is often expressed at nonphysiological levels, do not fully recapitulate the disease, showing an extremely aggressive phenotype with massive liver and spleen infiltration, not seen in humans.26-28 Few human LCH in vitro models exist but they either exploit JAG2 stimulation to induce a LCH phenotype in healthy donor monocytes or require introducing BRAFV600E into CD34+ hematopoietic progenitor cells (HPCs) with limited proliferation and differentiation potential.18,29
Induced pluripotent stem cells (iPSCs) can self-renew indefinitely and differentiate into all human cell types.30 Over the past decade, they emerged as a powerful disease-modeling31,32 tool for different cell types, including mononuclear phagocytes.33,34 Here, we develop an iPSC-based LCH model in which BRAFV600E/WT iPSCs exhibit myelomonocytic skewing during hematopoiesis and differentiate into LCH-like CD1a+/CD207+ cells deriving from CD14+ progenitors. We demonstrate that BRAFV600E triggers an overexpression of inflammatory cytokines and deregulation of transcription factors (TFs), promoting monocytic differentiation, and cell type–specific transcriptomic changes. MAPKis revert the transcriptomic signature induced by BRAFV600E and progressively reduce cell numbers as they differentiate into CD14+ monocytes but have little impact on immature progenitors. Finally, we show that BRAFV600E/WT iPSC-derived HPCs (iHPCs) can differentiate into iPSC-derived microglia-like cells (iMGLs) that induce neurodegenerative features, including high neurofilament light protein (NFL) release35,36 and neuronal damage, when cocultured with iPSC-derived neurons (iNeurons).
Methods
Patient samples
Samples were collected and analyzed after written informed consent with approval of the ethics committee of the Medical University of Vienna and according to the Declaration of Helsinki.
Flow cytometry and immunofluorescence staining
Supplemental Tables 1 and 2, available on the Blood website, list antibodies used in this study. For immunofluorescence, cells were fixed with 4% paraformaldehyde, permeabilized with 0.5% Triton X, and analyzed on a Leica SP8 or Operetta CLS. For flow cytometry and fluorescence-activated cell sorting, a BD LSR Fortessa or BD fluorescence-activated cell sorter Aria II were used. Cell cycle analysis was performed with propidium iodide and RNase A (Sigma).
scRNA-seq and bioinformatics analysis
Living single cells were sorted in bovine serum albumin 0.1%, and single-cell RNA sequencing (scRNA-seq) libraries were generated using the Chromium iX Controller and Chromium Next GEM single cell 3′ kit version 3.1 (Dual Index; 10X Genomics), according to the manufacturer’s instructions. After quality control, libraries were sequenced on the Illumina NovaSeq 6000. Raw data were preprocessed and demultiplexed with Cell Ranger version 7.1.0 (10X Genomics). scRNA-seq data were processed using Seurat (version 5.0.3) in R, with initial quality control steps to remove low-quality cells, followed by data normalization and integration to account for batch effects. Integrated data were clustered to identify distinct cellular populations and annotated based on gene expression profiles. Differential expression analysis was conducted using DESeq2.37,38 Cellular differentiation pathways and gene regulatory networks were further analyzed using Monocle339 and SCENIC.40 Details are included in supplemental Methods.
iPSCs reprogramming and genomic editing
To reprogram patient cells into iPSCs, peripheral blood mononuclear cells (PBMCs) of a 2-year-old male patient with risk-organ involving, multisystem LCH treated with vemurafenib at sampling (13.4% BRAFV600E alleles) were thawed and transfected using a Sendai Virus 2.0 kit (Thermo Fisher). Single colonies were picked and expanded on hESC Qualified Matrigel (Corning). For CRISPR-associated protein 9 editing, single cell suspensions of patient-derived and ShiPS-miFF3 iPSCs41 (derived from a healthy male infant) were treated with Accutase plus 10 μM Y-27632 (STEMCELL) and electroporated with guide RNA, ssODN, HiFi CRISPR-associated protein 9 (supplemental Table 3) using an Amaxa NF-2b and Lonza electroporation kit. Cells were seeded on vitronectin (STEMCELL) with CloneR (STEMCELL) and HDR enhancer V2 (IDT) for the first 24 hours. DNA fragments containing the BRAFV600E mutation was isolated from single colony lysates and sequenced. BRAFV600E/WT and BRAFWT/WT iPSCs with normal karyotype and similar expression of pluripotency markers and genetic background (supplemental Figure 1; supplemental Tables 4 and 5) were generated. One patient-derived BRAFV600E/WT iPSC clone used in 3 replicates of Figure 1C and supplemental Figure 4C, and in 1 scRNA-seq replicate of BRAFV600E/WT iPSCs at day 14 had a XYY karyotype. Although it did not generate outliers, it was excluded from further experiments. iPSCs were kept in mTesR Plus (STEMCELL) with 1% penicillin/streptomycin (Invitrogen) on vitronectin.
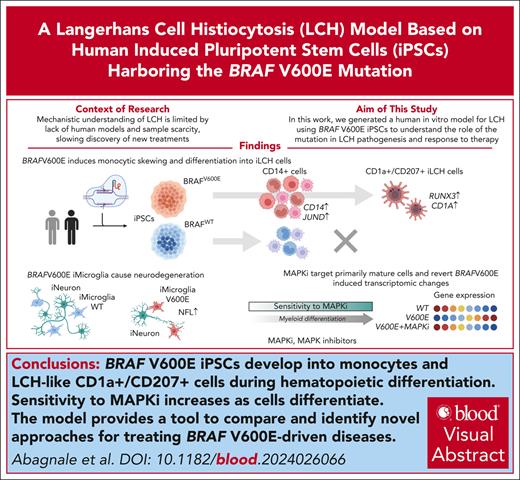
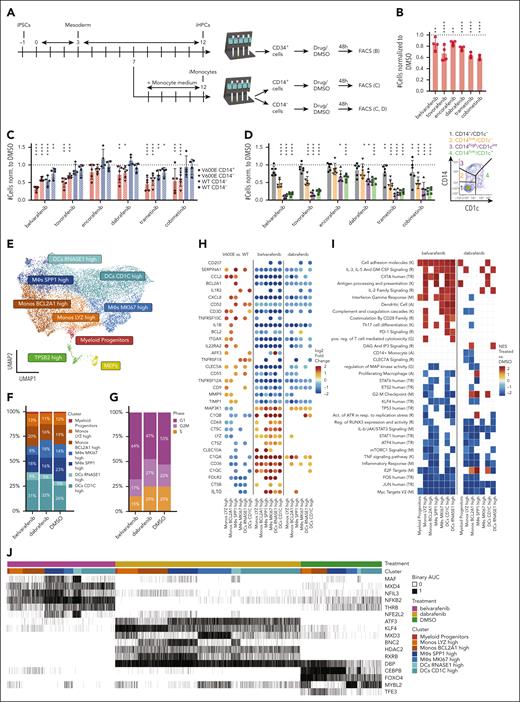
Generation and phenotypic characterization of iPSC-derived BRAFV600E/WT cells differentiating toward the monocytic lineage. (A) PBMCs of a patient with Langerhans cell histiocytosis (LCH) were reprogrammed into induced pluripotent stem cells (iPSCs) and then edited via CRISPR-associated protein 9 (Cas9) to introduce the V600E mutation in 1 endogenous BRAF allele as shown by Sanger sequencing. A synonymous mutation was introduced as part of the editing to prevent recutting by Cas9. A second iPSC line (ShiPS-miFF3) derived from a healthy infant was edited in similar way with Cas9 to introduce the V600E mutation in 1 endogenous BRAF allele. (B) Schematic of iPSCs differentiation toward CD14+ iMonocytes using STEMdiff Monocyte kit: a monolayer of iPSCs colonies is first differentiated toward mesoderm, then toward iHPCs, and finally toward CD14+ iMonocytes. First microscopy image on the left was acquired with a 5× air objective at room temperature on a Zeiss Axio Vert.A1 microscope; the remaining microscopy images were acquired with 20× objective with the same setup; scale bar, 100 μm. Images were processed with ImageJ. (C) Density plots of iPSCs at day 13 to 14 of monocytic differentiation showing smaller CD33−/CD34+ and CD33−/CD34− fractions for mutated than WT cells (n = 9). (D) BRAFV600E/WT iPSCs express CD14 at day 10 of differentiation, as shown by flow cytometry analysis (n = 5). (E) More BRAFV600E/WT iPSCs-derived CD14+ cells coexpressed CD11c than their WT counterpart as shown by flow cytometry analysis at day 13 of iMonocyte differentiation (n = 6). (F-G) BRAFV600E/WT develop unique CD14low/CD1c+ (n = 7) and CD1a+ (n = 8) populations that are missing in the WT isogenic control while they differentiate into iMonocytes as shown by flow cytometry density plots. (H) Schematic of developmental trajectory experiment. BRAFV600E/WT iPSCs differentiating toward iMonocytes were sorted in 4 fractions via fluorescence-activated cell sorting (FACS): (1) CD14−/CD1c−, (2) CD14low/CD1c−, (3) CD14high/CD1cint, and (4) CD14low/CD1c+. Each fraction was then cultured separately from the others for 4 days after which cells were restained and analyzed as shown in panel I and J. (I) Density plot showing the 4 fractions sorted in panel H analyzed after 4 days of culture: CD14−/CD1c− can reconstitute all other fractions but developmental potential decreases as cells differentiate toward CD14low/CD1c+ cells. (J) Same cells shown in panel I were also stained and gated for CD1a and CD207 expression to demonstrate that CD14low/CD1c+ fraction is enriched of CD1a+/CD207+ iLCH cells that derive from a CD14+ precursor and have a similar surface marker expression to lesional LCH cells. (K) Representative FACS plot of BRAFV600E/WT CD14low/CD1c+, sorted cells as in panel I and cultured for 7 days with granulocyte-macrophage colony-stimulating factor (GM-CSF), TNF-α, and transforming growth factor β1 (TGF-β1). (L) Quantification of CD1a+/CD207+ iLCH cells (n = 4) obtained from cells sorted as in panel I and cultured for 7 days with GM-CSF, TNF-α, and TGF-β1. For statistical significance of panels C-G unpaired parametric Student t test was used with Welch correction when variance was significant between groups, ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001. All histograms except in panel L include replicates from patient-derived iPSCs as well as ShiPS-MIFF3 iPSCs cell line. iPSC, induced pluripotent stem cells; LCH, Langerhans cell histiocytosis.
Generation and phenotypic characterization of iPSC-derived BRAFV600E/WT cells differentiating toward the monocytic lineage. (A) PBMCs of a patient with Langerhans cell histiocytosis (LCH) were reprogrammed into induced pluripotent stem cells (iPSCs) and then edited via CRISPR-associated protein 9 (Cas9) to introduce the V600E mutation in 1 endogenous BRAF allele as shown by Sanger sequencing. A synonymous mutation was introduced as part of the editing to prevent recutting by Cas9. A second iPSC line (ShiPS-miFF3) derived from a healthy infant was edited in similar way with Cas9 to introduce the V600E mutation in 1 endogenous BRAF allele. (B) Schematic of iPSCs differentiation toward CD14+ iMonocytes using STEMdiff Monocyte kit: a monolayer of iPSCs colonies is first differentiated toward mesoderm, then toward iHPCs, and finally toward CD14+ iMonocytes. First microscopy image on the left was acquired with a 5× air objective at room temperature on a Zeiss Axio Vert.A1 microscope; the remaining microscopy images were acquired with 20× objective with the same setup; scale bar, 100 μm. Images were processed with ImageJ. (C) Density plots of iPSCs at day 13 to 14 of monocytic differentiation showing smaller CD33−/CD34+ and CD33−/CD34− fractions for mutated than WT cells (n = 9). (D) BRAFV600E/WT iPSCs express CD14 at day 10 of differentiation, as shown by flow cytometry analysis (n = 5). (E) More BRAFV600E/WT iPSCs-derived CD14+ cells coexpressed CD11c than their WT counterpart as shown by flow cytometry analysis at day 13 of iMonocyte differentiation (n = 6). (F-G) BRAFV600E/WT develop unique CD14low/CD1c+ (n = 7) and CD1a+ (n = 8) populations that are missing in the WT isogenic control while they differentiate into iMonocytes as shown by flow cytometry density plots. (H) Schematic of developmental trajectory experiment. BRAFV600E/WT iPSCs differentiating toward iMonocytes were sorted in 4 fractions via fluorescence-activated cell sorting (FACS): (1) CD14−/CD1c−, (2) CD14low/CD1c−, (3) CD14high/CD1cint, and (4) CD14low/CD1c+. Each fraction was then cultured separately from the others for 4 days after which cells were restained and analyzed as shown in panel I and J. (I) Density plot showing the 4 fractions sorted in panel H analyzed after 4 days of culture: CD14−/CD1c− can reconstitute all other fractions but developmental potential decreases as cells differentiate toward CD14low/CD1c+ cells. (J) Same cells shown in panel I were also stained and gated for CD1a and CD207 expression to demonstrate that CD14low/CD1c+ fraction is enriched of CD1a+/CD207+ iLCH cells that derive from a CD14+ precursor and have a similar surface marker expression to lesional LCH cells. (K) Representative FACS plot of BRAFV600E/WT CD14low/CD1c+, sorted cells as in panel I and cultured for 7 days with granulocyte-macrophage colony-stimulating factor (GM-CSF), TNF-α, and transforming growth factor β1 (TGF-β1). (L) Quantification of CD1a+/CD207+ iLCH cells (n = 4) obtained from cells sorted as in panel I and cultured for 7 days with GM-CSF, TNF-α, and TGF-β1. For statistical significance of panels C-G unpaired parametric Student t test was used with Welch correction when variance was significant between groups, ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001. All histograms except in panel L include replicates from patient-derived iPSCs as well as ShiPS-MIFF3 iPSCs cell line. iPSC, induced pluripotent stem cells; LCH, Langerhans cell histiocytosis.
iPSCs differentiation
iPSCs differentiation into iHPCs, iPSC-derived monocytes (iMonocytes), iMGLs, and iNeurons was performed using a STEMdiff Hematopoietic,42 Monocyte, Microglia, Forebrain, and Midbrain Neuron kit, respectively (STEMCELL) according to the manufacturer’s instruction. For iLCH differentiation, cells were cultured in GMP-DC medium (Cellgenix) with granulocyte-macrophage colony-stimulating factor, transforming growth factor β1, tumor necrosis factor α (TNFα; Miltenyi), and 1% penicillin/streptomycin.
MAPKi screening
On day 12 of hematopoietic/monocytic differentiation, cells were harvested and seeded on Matrigel with HematoDiff B medium or monocytic differentiation medium with MAPKis or dimethyl sulfoxide (DMSO). For MACS separation, the CD34 MicroBeads kit and CD14 MicroBeads kit (Miltenyi) were used according to the manufacturer’s instructions. After 48 hours, plates were centrifuged, supernatants were removed for ToxiLight BioAssay (Lonza), and antibodies plus counting beads (Thermo Fisher) were added.
iNeuron–iMGL coculture
iNeurons were seeded into poly-l-ornithine/laminin-coated (Sigma) PhenoPlate 96-well TC (Revvity). For forebrain and midbrain iNeurons, iMGLs were added after 7 or 14 days, respectively, at 1:1 ratio in the corresponding neuron maturation medium with Supplement2 of the STEMdiff microglia medium (STEMCELL). Plates were imaged on Operetta CLS (Revvity) and analyzed using Harmony Software. Details of image analysis are provided in the supplemental Methods.
Enzyme-linked immunosorbent assay
Enzyme-linked immunosorbent assay (Cusabio Biotech) was used to determine NFL concentration according to the manufacturer’s instructions.
ddPCR
Droplet-digital polymerase chain reaction (ddPCR) for BRAFV600E on complementary DNA was performed using the QX200 ddPCR system (Bio-Rad) using probes and primers described previously.43
Results
BRAFV600E causes myeloid skewing and drives iPSCs differentiation toward CD14+ monocytes that acquire CD1a/CD207 expression
To model the effects of BRAFV600E on hematopoiesis, we stably introduced the mutation via genome editing in 2 iPSC lines; (1) 1 generated in our laboratory from a patient with LCH; and (2) a defined iPSC line from a healthy newborn (ShiPS-miFF3)41,44 (Figure 1A; supplemental Figure 1; supplemental Tables 4 and 5). We then differentiated BRAFV600E/WT and BRAFWT/WT isogenic iPSCs from both lines into iMonocytes (Figure 1B; supplemental Figure 1F-G). Mutated cells had smaller fractions of CD33−/CD34− cells and CD33−/CD34+ progenitors (Figure 1C), moreover, BRAFV600E/WT iPSCs expressed CD14 earlier than BRAFWT/WT cells, with little difference in CD14+ cell numbers at day 13 to 14 (Figure 1D; supplemental Figure 1H), and coexpressed CD11c (Figure 1E), a mature monocyte marker. Hence, BRAFV600E seems to induce myelomonocytic skewing in our iPSCs-LCH model, consistent with the current paradigm of LCH.
Notably, BRAFV600E/WT iPSCs, unlike the wild-type (WT) control, acquired a CD14low/CD1c+ population (Figure 1F) and expressed CD1a, a key LCH marker (Figure 1G). To determine the origin of this CD1a+ population we sorted 4 fractions of BRAFV600E/WT cells (CD14−/CD1c−, CD14low/CD1c−, CD14high/CD1cint, CD14low/CD1c+), which were then cultured separately (Figure 1H). After 4 days, CD14−/CD1c− cells reconstituted all existing fractions, but CD14low/CD1c− only gave rise to CD14high/CD1cint or CD14low/CD1c+ cells whereas CD14high/CD1cint reconstituted just CD14low/CD1c+ cells (Figure 1I). Our results suggest that BRAFV600E/WT iPSCs differentiate from CD14−/CD1c− cells toward CD14low/CD1c+ cells along a clockwise developmental trajectory shaped like a horseshoe. Importantly, CD1a+ cells are enriched within the CD14low/CD1c+ fraction with some coexpressing CD207 (Figure 1J; supplemental Figure 1I). Addition of granulocyte-macrophage colony-stimulating factor, transforming growth factor β1, and TNF-α improved the yield of CD1a+/CD207+ cells (Figure 1K-L). Thus, with our model we obtained iPSC-derived CD1a+/CD207+ BRAFV600E/WT cells (iLCH cells) originating from CD14+ progenitors (supplemental Figure 1J-K), suggesting that the cell of origin of lesional LCH cells belong to the monocytic lineage.
Transcriptomic regulation of inflammation and cell differentiation induced by BRAFV600E is cell type–specific and begins in myeloid progenitors
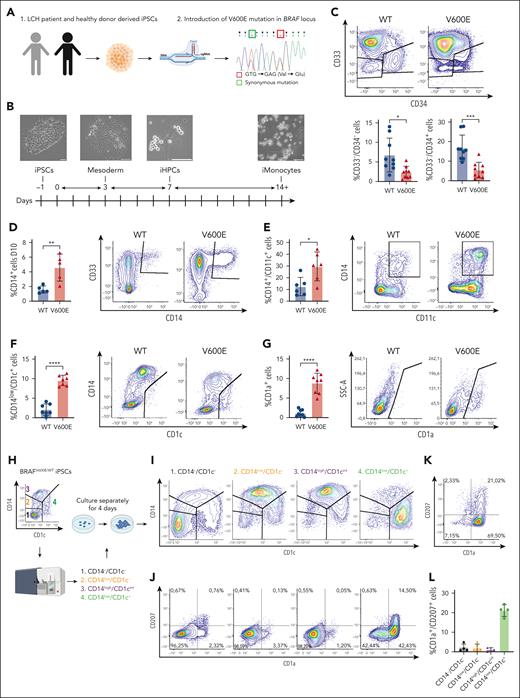
To identify BRAFV600E-induced transcriptomic changes during myelopoiesis, we performed scRNA-seq at day 11 and day 14 of iMonocyte differentiation. Our data set comprises various iPSC-derived cell types (Figure 2A; supplemental Figure 2A-B) and we focused on clusters annotated as Myeloid Progenitors, Monocytes, or Macrophages (MΦs)/DCs (supplemental Figure 2C). Myeloid Progenitors and Promonocytes were reduced in mutated samples, consistent with fewer CD34+ progenitors and earlier maturation into monocytes displayed by BRAFV600E/WT cells (Figure 2B) and further supported by pseudotime analysis (Figure 2C). Compared with BRAFWT/WT, mutated cells differentially expressed genes (DEGs) including inflammatory mediators and LCH-associated transcripts (Figure 2D-E): multiple clusters upregulated CCR6, whose expression by LCH cells has been linked to peripheral tissues accumulation.45,46 Furthermore, inflammatory cytokines (IL1B, CXCL8, CCL2, and TNF) were significantly upregulated in mutated Monocytes and Myeloid Progenitor clusters rather than MΦs/DCs, suggesting that in vivo BM progenitors and circulating monocytes might contribute to systemic inflammation in LCH, thus contributing to the clinical picture of LCH. Indeed, many DEGs (supplemental Figure 3A) and enriched pathways (Figure 2F,H; supplemental Figure 3B-C) were cell type specific. Most clusters showed positive enrichment for “inflammatory response” and “LCH” data sets, while only MΦs/DCs were negatively enriched for “MHC class 2”, consistent with reports describing LCH cells as poor antigen-presenting cells.47 Interestingly, mitosis-related genes (E2F2) were negatively enriched in most mutated clusters, with MΦ/DCs also downregulating apoptosis. This resembles oncogene-induced senescence in LCH24,26; however, only few senescence-associated genes were upregulated by BRAFV600E (supplemental Figure 4A), and senescence was negatively enriched among DEGs in 3 clusters of mutated cells (Figure 2H). Furthermore, cell cycle analysis and scoring with senescence signatures48,49 showed few differences between isogenic pairs (supplemental Figure 4B-D). Moreover, immunohistochemistry in biopsies showed LCH cells coexpressing KI67, arguing against senescence as a key driver in LCH lesions (supplemental Figure 4E). Rather, our data suggest that altered expression of key TFs might cause the myelomonocytic skewing of BRAFV600E/WT cells (Figure 2G,I): BRAFV600E/WT Myeloid Progenitors and Promonocytes upregulate inducers of monocytic fate JUND, CEBPB, and MAFB50-52 in addition to lineage markers CD33 and CD14. Simultaneously, they downregulate CEBPE, an essential TF for granulopoiesis53 and neutrophil markers (ELANE, MPO, and PRTN3). Notably, ERG, BCL11A, and RUNX1 regulons identified by SCENIC40 analysis are absent in BRAFV600E/WT clusters (Figure 2J-K; supplemental Figure 5A-B). Loss of ERG induces HSCs differentiation,54 consistent with the smaller CD33−/CD34+ BRAFV600E/WT population (Figure 1C), whereas BCL11A is essential for lymphopoiesis55,56 and RUNX1 promotes lymphoid vs myeloid differentiation.57 Conversely, FOS and IRF8 regulons, that are key to monocytic and DC differentiation,58-61 are only present in mutated cells. Interestingly, in cluster Monos LYZ high, BRAFV600E/WT cells upregulate RUNX3 and downregulate KLF4 and PPARG, whose respective expression and repression promote differentiation into CD1a+/CD207+ cells.62,63 Intriguingly, BRAFV600E/WT Monos LYZ high upregulate several LCH-associated transcripts, including CD1A (Figure 2D), suggesting this cluster might contain the early CD14+/CD1a+ cells (supplemental Figure 1I). Overall, our data show that BRAFV600E leads to differential regulation of key TFs skewing cells toward the monocytic lineage.
Transcriptomic regulation of inflammation and cell differentiation induced by BRAFV600E is cell type–specific and begins in myeloid progenitors. (A) Uniform manifold approximation and projection (UMAP) of 64 167 single living cells sequenced: 6 samples (3 × BRAFV600E/WT and 3 × BRAFWT/WT) were harvested at day 14, and 2 samples at day 11 (1 × BRAFV600E/WT and 1 × BRAFWT/WT) of differentiation toward iMonocytes. The main clusters discussed in this paper are highlighted as Myeloid Progenitors, Monocytes, and MΦs/DCs. (B) Frequencies of cell types between mutated and WT samples at day 14: BRAFV600E/WT samples show fewer Myeloid Progenitors and Promonocytes than BRAFWT/WT, consistent with a monocytic skewing induced by the mutation. (C) Pseudotime analysis using Monocle 339 of selected clusters showing that BRAFV600E/WT cells have higher density at later pseudotimes, supportive of their ability to differentiate faster than their WT counterpart. (D) Dot plots showing DEGs (adjusted P value <.05, positive fold change means higher in BRAFV600E/WT) associated with LCH and (E) inflammatory mediators. Dots for gene/cluster combinations, which are not significant, are not shown. (F) Gene set enrichment analysis (GSEA) shows that some pathways were uniquely enriched (adjusted P value <.05) for each cluster whereas others displayed similar normalized enrichment scores (NESs) among several clusters. A NES of >0 indicates positive enrichment in BRAFV600E/WT clusters. (G) Dot plot of differentially expressed TFs and modulators between clusters: most DEGs are in the Myeloid Progenitors and Monocyte clusters. Dots for gene/cluster combinations, which are not significant, are not shown. (H) Enrichr64 analysis of downregulated DEGs in mutated vs WT samples (adjusted P value <.05) with enrichment scores shown as negative log10 of their adjusted P value. The letter in brackets in panels F,H indicates the database of each ontology/pathway of the heat map: Reactome_2022 (R), Transcription_Factor_PPIs (T), GO_Biological_Process_2021 (G), Rare_Diseases_GeneRIF_ARCHS4_Predictions (D), MSigDB_Hallmark_2020 (M), and KEGG_2021_Human (K). (I) Dot plot of DEGs at day 11 of differentiation involved in myelomonocytic differentiation. BRAFV600E/WT induces downregulation of key genes associated with granulopoiesis (CEBPE, PRTN3, MPO, ELANE, and AZU1) and upregulation of monocyte-specific markers and TFs (MAFB, CD14, and S100A12). Dots for gene/cell type combinations, which are not significant, are not shown. (J) Regulon analysis showing the top 5 scoring regulons for Monos LYZ High, Myeloid Progenitors, and Promonocytes clusters in mutated and WT samples. (K) Heat map highlighting regulons that are uniquely present in either WT or mutated Monos LYZ High, Myeloid Progenitors, and Promonocytes clusters by showing their binary SCENIC area under the curve scores.
Transcriptomic regulation of inflammation and cell differentiation induced by BRAFV600E is cell type–specific and begins in myeloid progenitors. (A) Uniform manifold approximation and projection (UMAP) of 64 167 single living cells sequenced: 6 samples (3 × BRAFV600E/WT and 3 × BRAFWT/WT) were harvested at day 14, and 2 samples at day 11 (1 × BRAFV600E/WT and 1 × BRAFWT/WT) of differentiation toward iMonocytes. The main clusters discussed in this paper are highlighted as Myeloid Progenitors, Monocytes, and MΦs/DCs. (B) Frequencies of cell types between mutated and WT samples at day 14: BRAFV600E/WT samples show fewer Myeloid Progenitors and Promonocytes than BRAFWT/WT, consistent with a monocytic skewing induced by the mutation. (C) Pseudotime analysis using Monocle 339 of selected clusters showing that BRAFV600E/WT cells have higher density at later pseudotimes, supportive of their ability to differentiate faster than their WT counterpart. (D) Dot plots showing DEGs (adjusted P value <.05, positive fold change means higher in BRAFV600E/WT) associated with LCH and (E) inflammatory mediators. Dots for gene/cluster combinations, which are not significant, are not shown. (F) Gene set enrichment analysis (GSEA) shows that some pathways were uniquely enriched (adjusted P value <.05) for each cluster whereas others displayed similar normalized enrichment scores (NESs) among several clusters. A NES of >0 indicates positive enrichment in BRAFV600E/WT clusters. (G) Dot plot of differentially expressed TFs and modulators between clusters: most DEGs are in the Myeloid Progenitors and Monocyte clusters. Dots for gene/cluster combinations, which are not significant, are not shown. (H) Enrichr64 analysis of downregulated DEGs in mutated vs WT samples (adjusted P value <.05) with enrichment scores shown as negative log10 of their adjusted P value. The letter in brackets in panels F,H indicates the database of each ontology/pathway of the heat map: Reactome_2022 (R), Transcription_Factor_PPIs (T), GO_Biological_Process_2021 (G), Rare_Diseases_GeneRIF_ARCHS4_Predictions (D), MSigDB_Hallmark_2020 (M), and KEGG_2021_Human (K). (I) Dot plot of DEGs at day 11 of differentiation involved in myelomonocytic differentiation. BRAFV600E/WT induces downregulation of key genes associated with granulopoiesis (CEBPE, PRTN3, MPO, ELANE, and AZU1) and upregulation of monocyte-specific markers and TFs (MAFB, CD14, and S100A12). Dots for gene/cell type combinations, which are not significant, are not shown. (J) Regulon analysis showing the top 5 scoring regulons for Monos LYZ High, Myeloid Progenitors, and Promonocytes clusters in mutated and WT samples. (K) Heat map highlighting regulons that are uniquely present in either WT or mutated Monos LYZ High, Myeloid Progenitors, and Promonocytes clusters by showing their binary SCENIC area under the curve scores.
iPSC-derived CD1a+/CD207+ cells are transcriptionally close to LCH cells and recapitulate subsets found in lesions
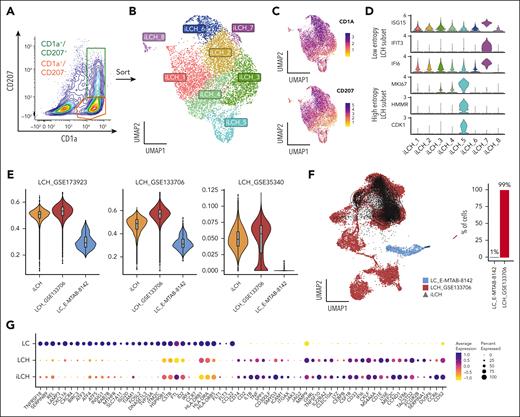
Next, we investigated whether iPSCs-derived BRAFV600E/WT iLCH were closer to epidermal Langerhans cells (LCs) or lesional LCH cells. We sequenced CD1a+/CD207+ and CD1a+/CD207− cells enriched from a CD14low/CD1c+ BRAFV600E/WT population (Figure 3A-C). Some iLCH clusters expressed markers similar to high- and low-entropy LCH subsets found in biopsy samples65 (Figure 3D). We then created 3 LCH cell signatures using published LCH data sets.19,20,65 The comparison of the iLCH transcriptome to lesional LCH cells (Figure 3E) cells44 and healthy LCs45 showed that iLCH resemble LCH cells rather than LCs. We confirmed this by projecting iLCH cells on a data set containing only LCH cells and LCs, in which iLCH aligned with lesional LCH cells (Figure 3F). Moreover, LC-specific genes were downregulated in LCH and iLCH cells and vice versa (Figure 3G). Altogether, our results show that iLCH are transcriptionally closer to LCH cells than LCs.
Transcriptomic characterization of iPSC-derived CD1a+/CD207+ iLCH cells using scRNA-seq reveals similarity to LCH subsets found in lesions. (A) BRAFV600E/WT CD14low/CD1c+ were cultured with GM-CSF, TNF-α, and TGF-β1 to generate iLCH cells. Living CD1a+/CD207− and CD1a+/CD207+ cells were sorted and used for scRNA-seq. (B) UMAP of 8483 sequenced cells from 2 independent experiments. (C) Feature plot showing the expression of CD1A and CD207 in the data set. (D) Violin plots showing that iLCH cells are heterogeneous and express markers defining different subsets of lesional cells in LCH biopsies. Cluster iLCH_5 expresses proliferative markers (HMMR, CDK1, and MKI67) similar to high-entropy LCH subsets, whereas cluster iLCH_7 expresses IFI6, IFIT3, and ISG15, similar to low-entropy LCH subsets.65 (E) UCell66 scoring was created using gene lists from 3 different data sets: (1) GSE173923, top100 makers (using FindMarkers function in Seurat) of LCH cell cluster, of Kvedaraite et al19; (2) GSE133706, 77 marker genes distinguishing LCH from non-LCH immune cells described in Table S2 of Halbritter et al65; and (3) GSE35340 884 genes that are differentially expressed in LCH vs LC described in Hutter et al20 (and listed as supplemental File 1 in Schwentner et al18). The results show that iLCH cells are much closer to lesional LCH cells than to physiological LCs (E-MTAB-8142). (F) Projection of iLCH on a scRNA-seq data set composed of healthy LCs (E-MTAB-8142) and tumoral LCH cells from biopsies.65 Overall, 99% of iLCH cells project on the LCH data set and only 1% on the subset of healthy epidermal LCs. (G) Dot plot of several transcripts expressed in LCH vs LC cells showing similar expression profiles between iLCH and LCH cells.
Transcriptomic characterization of iPSC-derived CD1a+/CD207+ iLCH cells using scRNA-seq reveals similarity to LCH subsets found in lesions. (A) BRAFV600E/WT CD14low/CD1c+ were cultured with GM-CSF, TNF-α, and TGF-β1 to generate iLCH cells. Living CD1a+/CD207− and CD1a+/CD207+ cells were sorted and used for scRNA-seq. (B) UMAP of 8483 sequenced cells from 2 independent experiments. (C) Feature plot showing the expression of CD1A and CD207 in the data set. (D) Violin plots showing that iLCH cells are heterogeneous and express markers defining different subsets of lesional cells in LCH biopsies. Cluster iLCH_5 expresses proliferative markers (HMMR, CDK1, and MKI67) similar to high-entropy LCH subsets, whereas cluster iLCH_7 expresses IFI6, IFIT3, and ISG15, similar to low-entropy LCH subsets.65 (E) UCell66 scoring was created using gene lists from 3 different data sets: (1) GSE173923, top100 makers (using FindMarkers function in Seurat) of LCH cell cluster, of Kvedaraite et al19; (2) GSE133706, 77 marker genes distinguishing LCH from non-LCH immune cells described in Table S2 of Halbritter et al65; and (3) GSE35340 884 genes that are differentially expressed in LCH vs LC described in Hutter et al20 (and listed as supplemental File 1 in Schwentner et al18). The results show that iLCH cells are much closer to lesional LCH cells than to physiological LCs (E-MTAB-8142). (F) Projection of iLCH on a scRNA-seq data set composed of healthy LCs (E-MTAB-8142) and tumoral LCH cells from biopsies.65 Overall, 99% of iLCH cells project on the LCH data set and only 1% on the subset of healthy epidermal LCs. (G) Dot plot of several transcripts expressed in LCH vs LC cells showing similar expression profiles between iLCH and LCH cells.
MAPKis target CD14+ cells and revert the BRAFV600E-induced phenotype but do not eliminate progenitors
Next, we tested 6 MAPKis in our model (Figure 4A; supplemental Figure 6A-B). MAPKis elicited only moderate decrease of CD34+ BRAFV600E/WT iHPCs (Figure 4B; supplemental Figure 6C), whereas stronger reduction was observed in BRAFV600E/WT and BRAFWT/WT cells cultured in monocyte differentiation medium that were separated into CD14+ and CD14− fractions and then treated for 48 hours (Figure 4C; supplemental Figure 6D). This suggests that MAPKis target cells committed to the monocytic lineage rather than immature progenitors. Tovorafenib, dabrafenib, and cobimetinib significantly reduced only BRAFV600E/WT cell numbers, whereas trametinib and belvarafenib (the drugs eliciting the strongest effect) targeted both WT and mutated cells. Drug effects were similar for populations arising from CD14+ (which expressed CD14 before treatment) or CD14− fractions (still undergoing differentiation into CD14+ monocytes at beginning of treatment). This indicates that inhibition of monopoiesis is not the primary mechanism causing cell number reduction and that CD14+ cells are directly affected. Indeed, treatment of the BRAFV600E/WT CD14− fraction revealed that MAPK-pathway inhibition targeted primarily mature cells of the monocytic lineage: the CD14−/CD1c− population was least affected by drugs, but cell numbers decreased as they progressed along the horseshoe (Figure 4D; supplemental Figure 7A-D). Hence, although MAPKis could not deplete progenitors, they gradually reduced cell numbers as cells differentiated, with higher selectivity for mutated cells. Significant cytotoxicity was detected only in BRAFV600E/WT cells treated with pan-RAFi (supplemental Figure 7E), suggesting that drugs eliciting similar cell number reductions can nonetheless act through distinct mechanisms.
Effect of MAPKis on cell numbers and single-cell transcriptome of different populations of iPSC-derived myelomonocytic cells. (A) Schematic of drug treatments on BRAFV600E/WT and BRAFWT/WT iPSCs derived cells: at day 7 of the protocol, culture medium is either switched to monocyte differentiation medium to generate CD14+ iMonocytes or kept as medium B to generate CD34+ iHPCs. For both culture conditions, cells are harvested at day 12 and either sorted for CD34+ expression (iHPCs) or into CD14+ and CD14− fractions (iMonocytes). The separate fractions are cultured in 96-well plates with MAPKis or their respective DMSO/untreated controls for 48 hours. Cells are analyzed and counted via FACS. (B) Flow cytometry analysis of all living cell numbers for CD34+ BRAFV600E/WT iHPCs normalized to DMSO after 48 hours of treatment with different MAPKis (n = 4 for all drugs except for trametinib and cobimetinib which was n = 3). Dotted line equaling 1 indicates DMSO. (C) Flow cytometry analysis of numbers of all living cells normalized to DMSO for BRAFV600E/WT and BRAFWT/WT cells sorted at day 12 of monocytic differentiation into CD14+ and CD14− fractions then treated for 48 hours with different MAPKis (n = 6 for BRAFV600E/WT CD14− cells, n = 5 for BRAFV600E/WT CD14+ cells, and n = 4 for all BRAFWT/WT cells). Dotted line equaling 1 indicates DMSO. (D) CD14− fractions from BRAFV600E/WT samples were isolated at day 12 of monocytic differentiation and then treated for 48 hours with different MAPKis. Four different populations based on expression of CD14 or CD1C (same scheme as Figure 1H) were defined in flow cytometry and their numbers normalized to DMSO are shown (n = 6). Dotted line equaling 1 indicates DMSO. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001. Panels B-C include replicates from patient-derived iPSCs as well as ShiPS-MIFF3 iPSCs cell line. For the statistical analysis of panels B-D, log-transformed ratios of cell numbers in drugs/DMSO were used. A mixed multivariate model was used with different inhibitors (belvarafenib, tovorafenib, encorafenib, dabrafenib, trametinib, and cobimetinib) mutation status (V600E or WT) and fraction (CD14+, CD14−, or CD34+) as fixed effects. Pairwise post-hoc testing was carried out using the Tukey method. (E) UMAP of integrated scRNA-seq data set comprising 16 502 cells generated from fraction sorted as shown in supplemental Figure 8A. For the annotation, the same labels as in Figure 2 were used. (F-G) Bar plots showing the distribution of cell clusters and cell cycle phases among treated samples. (H) Dot plots showing DEGs (adjusted P value <.05, log2-fold change of less than −0.5 or >0.5) between V600E vs WT samples (left, data set from Figure 2), belvarafenib- vs DMSO-treated cells (center), or dabrafenib- vs DMSO-treated cells (right) among the same clusters. A positive log2-fold change indicates higher expression in V600E vs WT or in treatment vs DMSO. Transcripts that are upregulated in BRAFV600E/WT vs BRAFWT/WT cells are downregulated upon treatment and vice versa. Dots for gene/cluster combinations, which are not significant, are not shown. (I) GSEA shows pathways that are positively (NES > 0) or negatively enriched (NES < 0) in treated vs DMSO cells. Some ontology terms have a similar score between dabrafenib- and belvarafenib-treated cells whereas others are only enriched in 1 of the 2 drugs or are negatively enriched for 1 inhibitor but positively enriched for the other. The letter in brackets indicates the database of each ontology/pathway of the heat map: Reactome_2022 (R), GO_Biological_Process_2021 (G), MSigDB_Hallmark_2020 (M), KEGG_2021_Human (K), TRRUST_Transcription_Factors_2019 (TR), and Azimuth_Cell_Types_2021 (A). (J) Heat map highlighting selected regulons, analyzed using the SCENIC40 package, that are present in either belvarafenib-, dabrafenib-, or DMSO-treated samples.
Effect of MAPKis on cell numbers and single-cell transcriptome of different populations of iPSC-derived myelomonocytic cells. (A) Schematic of drug treatments on BRAFV600E/WT and BRAFWT/WT iPSCs derived cells: at day 7 of the protocol, culture medium is either switched to monocyte differentiation medium to generate CD14+ iMonocytes or kept as medium B to generate CD34+ iHPCs. For both culture conditions, cells are harvested at day 12 and either sorted for CD34+ expression (iHPCs) or into CD14+ and CD14− fractions (iMonocytes). The separate fractions are cultured in 96-well plates with MAPKis or their respective DMSO/untreated controls for 48 hours. Cells are analyzed and counted via FACS. (B) Flow cytometry analysis of all living cell numbers for CD34+ BRAFV600E/WT iHPCs normalized to DMSO after 48 hours of treatment with different MAPKis (n = 4 for all drugs except for trametinib and cobimetinib which was n = 3). Dotted line equaling 1 indicates DMSO. (C) Flow cytometry analysis of numbers of all living cells normalized to DMSO for BRAFV600E/WT and BRAFWT/WT cells sorted at day 12 of monocytic differentiation into CD14+ and CD14− fractions then treated for 48 hours with different MAPKis (n = 6 for BRAFV600E/WT CD14− cells, n = 5 for BRAFV600E/WT CD14+ cells, and n = 4 for all BRAFWT/WT cells). Dotted line equaling 1 indicates DMSO. (D) CD14− fractions from BRAFV600E/WT samples were isolated at day 12 of monocytic differentiation and then treated for 48 hours with different MAPKis. Four different populations based on expression of CD14 or CD1C (same scheme as Figure 1H) were defined in flow cytometry and their numbers normalized to DMSO are shown (n = 6). Dotted line equaling 1 indicates DMSO. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001. Panels B-C include replicates from patient-derived iPSCs as well as ShiPS-MIFF3 iPSCs cell line. For the statistical analysis of panels B-D, log-transformed ratios of cell numbers in drugs/DMSO were used. A mixed multivariate model was used with different inhibitors (belvarafenib, tovorafenib, encorafenib, dabrafenib, trametinib, and cobimetinib) mutation status (V600E or WT) and fraction (CD14+, CD14−, or CD34+) as fixed effects. Pairwise post-hoc testing was carried out using the Tukey method. (E) UMAP of integrated scRNA-seq data set comprising 16 502 cells generated from fraction sorted as shown in supplemental Figure 8A. For the annotation, the same labels as in Figure 2 were used. (F-G) Bar plots showing the distribution of cell clusters and cell cycle phases among treated samples. (H) Dot plots showing DEGs (adjusted P value <.05, log2-fold change of less than −0.5 or >0.5) between V600E vs WT samples (left, data set from Figure 2), belvarafenib- vs DMSO-treated cells (center), or dabrafenib- vs DMSO-treated cells (right) among the same clusters. A positive log2-fold change indicates higher expression in V600E vs WT or in treatment vs DMSO. Transcripts that are upregulated in BRAFV600E/WT vs BRAFWT/WT cells are downregulated upon treatment and vice versa. Dots for gene/cluster combinations, which are not significant, are not shown. (I) GSEA shows pathways that are positively (NES > 0) or negatively enriched (NES < 0) in treated vs DMSO cells. Some ontology terms have a similar score between dabrafenib- and belvarafenib-treated cells whereas others are only enriched in 1 of the 2 drugs or are negatively enriched for 1 inhibitor but positively enriched for the other. The letter in brackets indicates the database of each ontology/pathway of the heat map: Reactome_2022 (R), GO_Biological_Process_2021 (G), MSigDB_Hallmark_2020 (M), KEGG_2021_Human (K), TRRUST_Transcription_Factors_2019 (TR), and Azimuth_Cell_Types_2021 (A). (J) Heat map highlighting selected regulons, analyzed using the SCENIC40 package, that are present in either belvarafenib-, dabrafenib-, or DMSO-treated samples.
To characterize pathways downstream of MAPK-pathway inhibition, we sequenced CD14+ and/or CD1c+ cells after 24 hours of belvarafenib, dabrafenib, or DMSO treatment (Figure 4E; supplemental Figure 8A-B). Belvarafenib-treated cells showed a reduced proportion in the MΦs MKI67 high cluster and G2M/S phase, suggesting that belvarafenib might inhibit proliferation (Figure 4F-G; supplemental Figure 8C). Overall, belvarafenib induced stronger transcriptomic changes than dabrafenib (7255 vs 4236 DEGs), with large differences among cell clusters (supplemental Figure 8D). MAPK-pathway inhibition switched off the transcriptomic changes induced by the BRAFV600E mutation: genes that were significantly upregulated in BRAFV600E/WT vs BRAFWT/WT were instead downregulated by MAPKi treatment and vice versa (Figure 4H), including inflammatory cytokines IL1B, CCL2, and CXCL8, as well as LCH markers CD36, MMP9, and, importantly, CD207. Gene set enrichment analysis indicated that treated cells were less inflammatory and better antigen-presenting cells by upregulating MHC-2–related transcripts (supplemental Figure 8E), highlighting that MAPK-pathway inhibition might lead to a rapid reversal of LCH-associated pathologic features and restore normal cellular functionality. Importantly, several MAPK-pathway members (BRAF, RAF1, MAP2K1, and DUSP6) were differentially expressed (supplemental Figure 8F), underscoring that inhibitor-induced paradoxical activation of the MAPK-pathway might occur in LCH as described in other cancers.67-69 Notably, characteristic gene set enrichment analysis terms were drug specific or even differentially enriched between inhibitors (Figure 4I; supplemental Figure 8G): dabrafenib positively enriched for G2M checkpoints and E2F targets, whereas belvarafenib did the opposite, in line with their different efficacy in reducing cell numbers. Similarly, certain regulons were expressed exclusively in samples treated with either belvarafenib (NFKB2) or dabrafenib (KLF4; Figure 4J).
iPSC-derived BRAFV600E/WT iHPCs give rise to iMGLs and drive inflammation and neurodegeneration-associated signatures
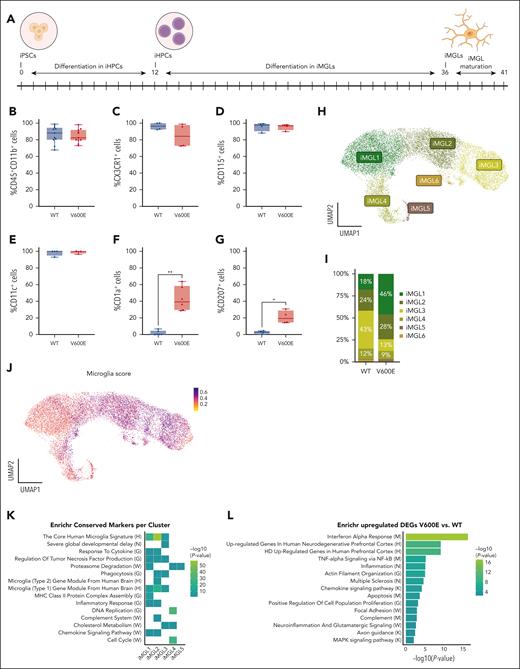
Neurodegeneration in LCH might be caused by mutated PBMCs that infiltrate the brain, differentiate into MΦs,70,71 and cause tissue damage.24 To test this hypothesis, we differentiated CD34+ BRAFV600E/WT iHPCs and their isogenic controls into iPSC-derived iMGLs72 (Figure 5A). After 5 days of maturation, both BRAFV600E/WT and BRAFWT/WT iMGLs expressed CD45, CD11b, CX3CR1, CD115, and CD11c72-74 (Figure 5B-E; supplemental Figure 9A-G). However, BRAFV600E/WT adhered better to the surface, spread out more widely, and resulted in double the cell numbers on average than BRAFWT/WT iMGLs (data not shown). Interestingly, a population of mutated iMGLs expressed CD1a and CD207 together with the microglia markers (Figure 5F-G), suggesting that BRAFV600E regulates the expression of key LCH markers in cell types not conventionally associated with LCH lesions.
Differentiation of iMGLs from patient-derived iPSCs. (A) Schematic of differentiation of human patient-derived BRAFV600E/WT iPSCs into microglia. iPSCs were first differentiated into iHPCs for 12 days and then further differentiated into iMGLs for 24 days, followed by 4 to 10 days of iMGL maturation (B-E) Bar plot showing the differentiation efficiency of iHPCs toward iMGLs at day 5 of iMGL maturation of 4 independent experiments (except for panel B in which n = 11) determined by expression of lineage markers CD11b and CD45 (B), fractalkine receptor CX3CR1 (C), CD115 (macrophage colony-stimulating factor receptor) (D), and CD11c (E). (F) Bar plot showing the expression of LCH surface markers CD1a and CD207 (G) via flow cytometry of 6 or 4 independent experiments, respectively. Paired parametric Student t test was used when variance was significant between groups, ∗P < .05, ∗∗P < .01. (H) UMAP of 16 818 single living cells sequenced for scRNA-seq; 5 samples (3 × BRAFV600E/WT and 2 × BRAFWT/WT) were collected at day 5 of iMGL maturation. Unsupervised clustering identified 6 iMGL clusters named iMGL1-6. (I) Bar plot showing the distribution of BRAFV600E/WT and BRAFWT/WT cells in the different clusters. Frequencies of <2% are not shown in the bar plot. (J) A microglia marker score was calculated based on the expression of 12 genes (AIF1, C1QA, CSF1R, CD74, C3, CX3CR1, MERTK, P2RY12, TREM2, TYROBP, ITGAM, and ITGAX), commonly used as microglia marker genes. (K) Enrichr64 analysis for specific markers per cluster of the iMGL data set and (L) for genes upregulated in BRAFV600E/WT iMGLs in comparison to BRAFWT/WT iMGLs. Letter in brackets in panels K-L indicates the database for each ontology/pathway of the heat map or bar plot, respectively: WikiPathway 2023 (W), HDSigDB Human 2021 (H), DisGeNET (N), GO_Biological_Process_2021 (G), MSigDB_Hallmark_2020 (M), and KEGG_2021_Human (K).
Differentiation of iMGLs from patient-derived iPSCs. (A) Schematic of differentiation of human patient-derived BRAFV600E/WT iPSCs into microglia. iPSCs were first differentiated into iHPCs for 12 days and then further differentiated into iMGLs for 24 days, followed by 4 to 10 days of iMGL maturation (B-E) Bar plot showing the differentiation efficiency of iHPCs toward iMGLs at day 5 of iMGL maturation of 4 independent experiments (except for panel B in which n = 11) determined by expression of lineage markers CD11b and CD45 (B), fractalkine receptor CX3CR1 (C), CD115 (macrophage colony-stimulating factor receptor) (D), and CD11c (E). (F) Bar plot showing the expression of LCH surface markers CD1a and CD207 (G) via flow cytometry of 6 or 4 independent experiments, respectively. Paired parametric Student t test was used when variance was significant between groups, ∗P < .05, ∗∗P < .01. (H) UMAP of 16 818 single living cells sequenced for scRNA-seq; 5 samples (3 × BRAFV600E/WT and 2 × BRAFWT/WT) were collected at day 5 of iMGL maturation. Unsupervised clustering identified 6 iMGL clusters named iMGL1-6. (I) Bar plot showing the distribution of BRAFV600E/WT and BRAFWT/WT cells in the different clusters. Frequencies of <2% are not shown in the bar plot. (J) A microglia marker score was calculated based on the expression of 12 genes (AIF1, C1QA, CSF1R, CD74, C3, CX3CR1, MERTK, P2RY12, TREM2, TYROBP, ITGAM, and ITGAX), commonly used as microglia marker genes. (K) Enrichr64 analysis for specific markers per cluster of the iMGL data set and (L) for genes upregulated in BRAFV600E/WT iMGLs in comparison to BRAFWT/WT iMGLs. Letter in brackets in panels K-L indicates the database for each ontology/pathway of the heat map or bar plot, respectively: WikiPathway 2023 (W), HDSigDB Human 2021 (H), DisGeNET (N), GO_Biological_Process_2021 (G), MSigDB_Hallmark_2020 (M), and KEGG_2021_Human (K).
We further characterized 5 iMGL samples (3 BRAFV600E/WT and 2 BRAFWT/WT) via scRNA-seq and identified 6 iMGL clusters (Figure 5H-I; supplemental Figure 10A). iMGL1 and iMGL5 are mostly found in BRAFV600E/WT samples, whereas iMGL3 and 6 were predominantly composed of BRAFWT/WT cells. iMGL2 and iMGL4 are approximately evenly distributed. iMGL1, iMGL4, ad iMGL5 contain CD1A/CD207 expressing cells (supplemental Figure 9H). Based on published data72-77 we compiled a microglia core signature using 12 genes (supplemental Figure 9I). Results indicate that the transcriptome of both BRAFV600E/WT and BRAFWT/WT iMGLs is similar to primary microglia cells (Figure 5J). Enrichr64 analysis of the specific cluster markers (Figure 5K) showed that iMGL1 and iMGL2 were significantly enriched for genes associated with both inflammation and microglia. iMGL1 through 3 were enriched for transcripts related to microglia fate and neurodegenerative disease, and iMGL3 was associated with fat metabolism. iMGL4 showed an overrepresentation of genes connected to neurological deficits and cell cycle. To understand how BRAFV600E changes signaling pathways, we compared those enriched in BRAFV600E/WT with BRAFWT/WT iMGLs (Figure 5L; supplemental Figure 10B-C). BRAFV600E/WT iMGLs showed enrichment for terms associated with inflammation and neurodegenerative disease. Furthermore, pathways involved in cell adhesion were significantly enriched, consistent with the enlarged and more adherent morphology of mutated cells.
Collectively, flow cytometry and transcriptomic data indicate that although both iPSC-derived BRAFV600E/WT and BRAFWT/WT iMGLs recapitulate features of primary human microglia, the mutation induces a proinflammatory phenotype, which might be linked to neurodegeneration.
BRAFV600E/WT iMGLs lead to neurodegeneration
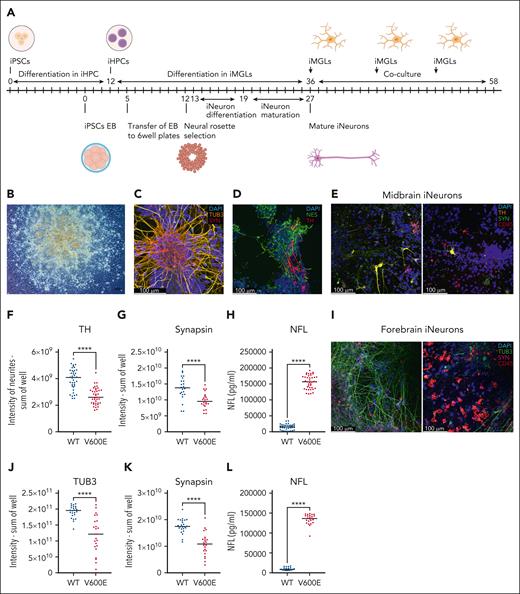
Finally, we assessed whether BRAFV600E/WT iMGLs, as suggested by our scRNA-seq data, could recapitulate features of the neurodegeneration observed in patients with LCH. We differentiated BRAFWT/WT iPSCs into neuronal cultures78 (Figure 6A-B) containing a pure population of class 3 β-tubulin (TUB3) neurons that coexpressed synapsin (Figure 6C). Midbrain neuron cultures additionally included nestin and tyrosine hydroxylase (TH)-positive dopaminergic neurons79,80 (Figure 6D).
BRAFV600E/WT iMGLs lead to neurodegeneration of iNeurons. (A) Schematic of differentiation of human patient-derived iPSCs into neuronal precursor cells and further forebrain iNeurons is shown, for midbrain iNeurons cells are kept 1 additional week in maturation medium. On day 36 of iMGL differentiation iMGLs were added to iNeurons on day 27 or day 34 of forebrain or midbrain iNeuron differentiation, respectively. Fresh iMGLs were repeatedly added to iNeurons. (B) At day 8 of differentiation neural rosettes are visible. Original magnification: 10×, scale bar: 100 μm. Image was acquired on an Axio Vert.A1 at room temperature. (C) Mature forebrain iNeurons on day 28 after embryoid body formation. Immunofluorescence staining for TUB3 (yellow, Alexa Fluor 488), synapsin (red, Alexa Fluor 568), and DAPI (4′,6-diamidino-2-phenylindole; blue). (D) Mature midbrain iNeurons on day 35 after embryoid body formation. Immunofluorescence staining for nestin (green, Alexa Fluor 488), TH (red, Alexa Fluor 568), and DAPI (blue). (E) Immunofluorescence staining of TH (yellow, Alexa Fluor 568), synapsin (green, Alexa Fluor 488), CD45 (red, Alexa Fluor647), and DAPI (blue): left panel, midbrain iNeurons with WT patient-derived iMGLs; right panel, with mutant patient iMGLs. For panels C-E, pictures were acquired using a Leica SP8 with 40× water objective. (F) Quantification of TH expression in neurite segments of midbrain iNeurons as sum of intensity per well from 5 independent experiments. At least 7 wells per experiment were acquired. (G) Quantification of synapsin expression in midbrain iNeurons as sum of intensity of a well from 3 independent experiments. At least 7 wells per experiment were acquired. (H) Quantification of released NFL in pg/mL from midbrain iNeurons cocultured with either WT or mutated iMGLs from 3 independent experiments. At least 7 wells were analyzed per experiment. NFL was measured using an enzyme-linked immunosorbent assay (ELISA) assay and the supernatants were diluted 1:45. (I) Immunofluorescence staining of coculture of human patient-derived iPSC forebrain iNeurons with iMGLs, synapsin (magenta, Alexa Fluor 568), TUB3 (green, Alexa Fluor 488), CD45 (red, Alexa Fluor 647), and DAPI (blue); left panel, forebrain iNeurons with WT iMGLs; right panel, with mutant iMGLs. (J) Quantification of TUB3 and (K) synapsin expression as sum of intensity of a well in forebrain iNeurons from 3 independent experiments. At least 7 wells per experiment were acquired. (L) Quantification of released NFL in pg/mL from forebrain iNeurons in coculture with BRAFV600E/WT or BRAFWT/WT iMGLs, from 3 independent experiments. At least 7 wells were analyzed per experiment. NFL was measured using an ELISA assay and the supernatants were diluted 1:45. Unpaired parametric Student t test was used with Welch correction when variance was significant between groups, ∗∗∗∗P < .0001. All results presented in this figure were obtained using human patient-derived iPSC iNeurons and iMGLs.
BRAFV600E/WT iMGLs lead to neurodegeneration of iNeurons. (A) Schematic of differentiation of human patient-derived iPSCs into neuronal precursor cells and further forebrain iNeurons is shown, for midbrain iNeurons cells are kept 1 additional week in maturation medium. On day 36 of iMGL differentiation iMGLs were added to iNeurons on day 27 or day 34 of forebrain or midbrain iNeuron differentiation, respectively. Fresh iMGLs were repeatedly added to iNeurons. (B) At day 8 of differentiation neural rosettes are visible. Original magnification: 10×, scale bar: 100 μm. Image was acquired on an Axio Vert.A1 at room temperature. (C) Mature forebrain iNeurons on day 28 after embryoid body formation. Immunofluorescence staining for TUB3 (yellow, Alexa Fluor 488), synapsin (red, Alexa Fluor 568), and DAPI (4′,6-diamidino-2-phenylindole; blue). (D) Mature midbrain iNeurons on day 35 after embryoid body formation. Immunofluorescence staining for nestin (green, Alexa Fluor 488), TH (red, Alexa Fluor 568), and DAPI (blue). (E) Immunofluorescence staining of TH (yellow, Alexa Fluor 568), synapsin (green, Alexa Fluor 488), CD45 (red, Alexa Fluor647), and DAPI (blue): left panel, midbrain iNeurons with WT patient-derived iMGLs; right panel, with mutant patient iMGLs. For panels C-E, pictures were acquired using a Leica SP8 with 40× water objective. (F) Quantification of TH expression in neurite segments of midbrain iNeurons as sum of intensity per well from 5 independent experiments. At least 7 wells per experiment were acquired. (G) Quantification of synapsin expression in midbrain iNeurons as sum of intensity of a well from 3 independent experiments. At least 7 wells per experiment were acquired. (H) Quantification of released NFL in pg/mL from midbrain iNeurons cocultured with either WT or mutated iMGLs from 3 independent experiments. At least 7 wells were analyzed per experiment. NFL was measured using an enzyme-linked immunosorbent assay (ELISA) assay and the supernatants were diluted 1:45. (I) Immunofluorescence staining of coculture of human patient-derived iPSC forebrain iNeurons with iMGLs, synapsin (magenta, Alexa Fluor 568), TUB3 (green, Alexa Fluor 488), CD45 (red, Alexa Fluor 647), and DAPI (blue); left panel, forebrain iNeurons with WT iMGLs; right panel, with mutant iMGLs. (J) Quantification of TUB3 and (K) synapsin expression as sum of intensity of a well in forebrain iNeurons from 3 independent experiments. At least 7 wells per experiment were acquired. (L) Quantification of released NFL in pg/mL from forebrain iNeurons in coculture with BRAFV600E/WT or BRAFWT/WT iMGLs, from 3 independent experiments. At least 7 wells were analyzed per experiment. NFL was measured using an ELISA assay and the supernatants were diluted 1:45. Unpaired parametric Student t test was used with Welch correction when variance was significant between groups, ∗∗∗∗P < .0001. All results presented in this figure were obtained using human patient-derived iPSC iNeurons and iMGLs.
We then added BRAF-mutated or WT iMGLs to the mature iNeurons and cocultivated them for up to 21 days. During this period, we observed a reduction in iNeurons cocultivated with mutated vs WT microglia. To assess neuronal damage, cells were stained with TUB3 and synapsin for forebrain, and with TH and synapsin for midbrain iNeurons (Figure 6E,I). The presence of mutated vs WT iMGLs caused a reduction of TH staining by an average of 32% in patient-derived iNeurons (Figure 6F) and by 42% in ShiPS-miFF3–derived iNeurons (supplemental Figure 11). Synapsin intensity decreased by 29% to 47%, depending on the neuronal cell type (Figure 6G,K). Furthermore, the presence of mutated iMGLs led to a reduction in TUB3 intensity by 38% (patient-derived iMGLs, Figure 6J) and 65% (ShiPS-miFF3–derived iMGLs) compared with WT controls. In summary, mutated iMGLs caused a reduction of neuronal markers in all conditions tested.
NFL, a biomarker for neuroaxonal damage, is elevated in patients with ND-CNS-LCH.36,81,82 We tested whether NFL levels increased when iNeurons were cultured with BRAFV600E/WT iMGLs: indeed, BRAFV600E/WT iMGLs lead to 10 to 27-fold higher release of NFL from iNeurons than BRAFWT/WT iMGLs (Figure 6H,L; supplemental Figure 11), indicating that BRAFV600E is the key factor causing iMGLs to become proinflammatory and directly induce neuronal damage in our in vitro model.
Discussion
We developed an in vitro LCH model based on isogenic iPSCs pairs, which constitutes a defined and virtually unlimited source of BRAFV600E/WT cells. This new model overcomes limitations of mouse models or human cord blood progenitors, such as restricted hematopoietic potential and interdonor variability. Our model successfully generates cells phenotypically and transcriptionally similar to lesional LCH cells, representing, to our knowledge, the first report of human iPSC-derived Langerin+ cells. The mutated progenitors rapidly differentiate into CD14+ monocytes, from which a subpopulation subsequently develops into CD1a+/CD207+ iLCH cells, consistent with the hypothesis of monocytes/DC3 as the LCH cell of origin.19 Although we also identify a CD14− iLCH population (supplemental Figure 1J) phenotypically similar to DC2 described in the same study, both seem to derive from CD14+ progenitors in our data. We hypothesize that this BRAFV600E-induced phenotype results from the modulation of TFs such as JUND, CEBPB, and PPARG. These TFs guide iHPCs first toward the myeloid lineage and later to differentiate into monocytes and CD1a+/CD207+ monocyte-derived cells that exhibit an inflammatory phenotype. iPSC-derived iLCH cells contain a proliferating subgroup expressing MKI67 similar to proliferative cell subsets found in LCH biopsies.65 This observation might warrant a reevaluation of the current hypothesis that senescence is the main driver of LCH,24,26,29 and the cell type specificity of senescent-associated transcripts should be considered.83 Although BRAFV600E/WT iPSC-derived myeloid cells upregulate a few senescence-associated secretory phenotype genes84 such as IL1B, TNF, CXCL8, and MMP9, we consider this overexpression part of the hyperinflammatory LCH phenotype rather than a sign of senescence. This is because these transcripts are physiologically enriched in monocytic cells and LCs. We propose that the upregulation of inflammatory markers early in myelopoiesis in mutated cells reflects that in vivo circulating monocytes and BM progenitors significantly contribute to systemic inflammation in high-risk patients. This paradigm, viewing lesional cells as the “tip of the iceberg” in LCH, is supported by our data on BRAFV600E/WT cells with multiple differentiation end points; not every mutated cell acquired CD207 expression. Notably, BRAFV600E/WT cells strongly upregulated CCL2, previously described as the only factor, among 20 analyzed, correlating with disease activity score of a patient.22
Neurodegeneration represents another disease manifestation not directly caused by lesional CD1a+/CD207+ cells but other mutated cell types. Our model strongly suggests that BRAF-mutated iHPCs differentiate into iMGLs that damage neurons. Interestingly, recent preprints suggest that in different diseases such as Alzheimer and LCH, neuroinflammatory microglial clones expressing MAPK-activating variants may contribute to neurodegeneration.33,85 Moreover, clonal mutations found in microglia and PBMCs of patients with Alzheimer disease, suggest that circulating mutated cells might migrate to the brain and differentiate into cells resembling microglia.86 The implication of PBMCs as progenitors underscores the necessity for therapeutic strategies that eradicate the mutated clone rather than simply achieving clinical remission, as demonstrated by retrospective analyses87 showing that patients who received the RAFi vemurafenib have high risk of developing neurodegeneration. Consequently, the use of MAPKis as monotherapy should be reevaluated to favor combinations of MAPKi with chemotherapy.
Furthermore, our results indicate that MAPKi sensitivity increases as cells differentiate, with CD34+ progenitors being least affected, consistent with clinical evidence that inhibitors alone cannot eradicate the disease. We observed significant differences between drugs: the pan-RAFi belvarafenib elicited direct cytotoxicity, inhibited cellular proliferation, and modulated more downstream genes than the RAFi dabrafenib. Despite their differences, both compounds also rapidly downregulated the expression of inflammatory molecules, consistent with clinical evidence,12,88 and reverted the expression of LCH-associated genes, including surface markers CD36, CD207, and ITGAX. We therefore hypothesize that LCH cells might “mask” themselves as WT cells after therapy. The upregulation of MHC class 2–related genes compared with DMSO could be attributed to the upregulation of CIITA and RFX3, master regulators of MHC class 2 expression and negatively modulated by MAPK-pathway activation.89 However, the finding that MAPK-pathway related genes were upregulated after treatment implies that cells can quickly revert to an inflammatory phenotype if therapy is discontinued, explaining why some patients develop signs of hyperinflammation upon cessation of RAFi.22,90
In conclusion, our model shows that introducing BRAFV600E in iPSCs is sufficient to recapitulate the myelomonocytic skewing observed in LCH; suggests that lesional cells originate from the CD14+ population; and provides, to our knowledge, the first direct evidence that human BRAFV600E/WT iMGLs contribute to neurodegeneration. Alongside our findings that MAPKis target different cell types with varying potency, we believe our iPSC-LCH model significantly advances the understanding of the disease and provides new perspectives on the clinical treatment of patients.
Acknowledgments
The authors thank Jennifer Volz, Sofia Aligianni, Elisabeth Stein, and Cindy Horenburg from the Stem Core Cell Facility at the Vienna Biocenter for their support with the reprogramming of the patient sample. The authors thank Christoph Bock and his team at the Biomedical Sequencing Facility at CeMM Research Center for Molecular Medicine of the Austrian Academy of Sciences in Vienna for their support with library preparation and sequencing of the single-cell sequencing experiments. The authors are grateful to Dieter Printz and Anna Maria Husa from the fluorescence-activated cell sorting core facility at the Children's Cancer Research Institute for their expertise with cell sorting and flow cytometry. The authors are also grateful to Anestis Tsakiridis (University of Sheffield, Sheffield, United Kingdom) for providing the ShiPS-MIFF3 cell line. The authors thank Eleni Tomazou and Christoph Dotter of the Children's Cancer Research Institute for their help with the whole-exome sequencing analysis of the patient-derived iPSCs lines; and Sören Mai, Karin Nebral, Kathrin Liszt, and Stefan Köhrer from LabDia in Vienna for having performed the karyotypic and genetic quality control of the iPSCs. The graphical abstract and the schematics in Figures 1, 3, 4, 5, and 6 were created with BioRender.com.
The authors thank the Histiocytosis Association, the Biedermann family, and the Austrian Fonds zur Förderung der Wissenschaftlichen Forschung (DOI 10.55776/P37332) for funding this study. The study was also supported by the Alex's Lemonade Stand Foundation for Childhood Cancer (ALSF 20-17258) and the Austrian Research Promotion Agency (FFG) project 7940628 533 (Danio4Can).
Authorship
Contribution: G.A., R.S., and C.H. conceived the study and carried out the experiments; S.K.E. conceived and performed bioinformatics analysis; P.B.S.-W. and W.v.M. carried out experiments and performed bioinformatics analysis; F.H. and C.H. further contributed to the bioinformatics analysis and provided conceptual input during the early phases of the project; U.P. carried out the statistical analysis of the flow cytometry and toxilight data for experiments with MAPKis; M.R. and S.T.-M. helped with the iPSC experiments; C.S. performed live cell imaging experiments of iMGL-iNeuron cocultures at the Operetta system and helped with the subsequent image analysis, and M.D. helped with image analysis data interpretation; I.S.-K. provided stainings of patient samples; and all authors contributed to writing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Caroline Hutter, St. Anna Childrens Cancer Research Institute, Zimmermannplatz 10, 1090 Vienna, Austria; email: caroline.hutter@ccri.at; and Sebastian K. Eder, Department of Pediatrics and Adolescent Medicine, St. Anna Children’s Hospital, Medical University of Vienna, Kinderspitalgasse 6, 1090 Vienna, Austria; email: sebastian.eder@ccri.at.
References
Author notes
G.A. and R.S. contributed equally to this study.
S.K.E. and C.H. contributed equally to this study.
Single-cell RNA sequencing processed data are available via Gene Expression Omnibus, accession no. GSE270891.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal