In this issue of Blood, Takashima et al show that reduced STAT1 abundance in SRSF2-mutant myelodysplastic syndrome (MDS) cells confers protection against interferon (IFN)-driven cell suppression relative to normal wild-type (WT) cells.1 Importantly, the authors demonstrate that treatment with the proteasome inhibitor bortezomib in vitro increases STAT1 abundance and sensitizes SRSF2-mutant cells to IFN. These findings provide potential rationale for using bortezomib therapy in SRSF2-mutated MDS, which is characterized by poor outcomes.
It has been several years since the initial demonstration that inflammatory signaling and innate immune signaling play a critical role in promoting MDS through the differential effects of cytokines on malignant cells relative to WT cells.2 More recently, the deleterious effects of increased inflammation in clonal hematopoiesis have been noted to be attenuated in DNMT3A-mutant and TET2-mutant compared with WT hematopoietic stem cells from the same patient.3 In a mouse bone marrow failure model IFN-γ blockade promotes reemergence of myeloid activity.4 Interestingly, transformation from MDS to acute myeloid leukemia is associated with a reduced IFN-γ gene expression signature but no difference in levels of IFN-γ itself,4 suggesting that malignant myeloid cells resist the effects of IFN-γ. However, the mechanism by which this occurs has been unclear.
Takashima et al, using an induced pluripotent stem cell (iPSC) model derived from a patient with MDS with an SRSF2 P95L mutation, show that SRSF2-mutant hematopoietic stem progenitor cells (HSPCs) derived from these iPSCs have reduced IFN-γ and IFN-α gene expression signatures compared with isogenic iPSC-derived WT HSPCs. The key mediator of IFN signaling, STAT1, was found to be diminished in SRSF2-mutant HSPCs compared with WT HSPCs. The authors confirmed that IFN suppresses colony formation of WT iPSC-HSPCs, but not SRSF2-mutant iPSC-HSPCs, where clonogenic activity is already reduced at baseline. Consistent with the IFN effect being mediated by STAT1, knockdown of STAT1α or STAT1β reversed the decrease in clonogenic activity induced by IFN treatment in WT HSPCs, while overexpression of STAT1 allowed suppression of colony formation in SRSF2-mutant iPSC-HSPCs in response to IFN-γ, but not IFN-α (see figure). Although the authors suggest that the altered STAT1 level in MDS is due to differential polyadenylation site usage by mutant SRSF2, definitive studies are required to confirm this hypothesis.
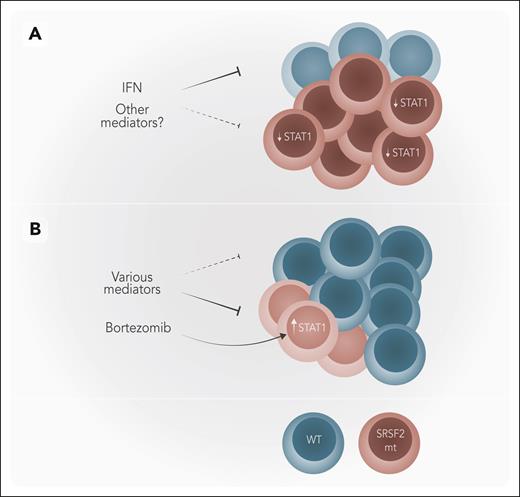
Model for the role of bortezomib in SRSF2-mutated MDS. (A) In SRSF2-mutant (mt) MDS, IFN only suppresses WT cells with SRSF2-mt cells being spared as they have lower levels of STAT1. (B) Bortezomib upregulates STAT1 in SRSF2-mt cells rendering them sensitive to IFN, allowing a relative expansion of WT cells, which are not affected by bortezomib. Normal WT hematopoietic cells are depicted in blue and SRSF2-mt cells in burgundy. Professional illustration by Somersault18:24.
Model for the role of bortezomib in SRSF2-mutated MDS. (A) In SRSF2-mutant (mt) MDS, IFN only suppresses WT cells with SRSF2-mt cells being spared as they have lower levels of STAT1. (B) Bortezomib upregulates STAT1 in SRSF2-mt cells rendering them sensitive to IFN, allowing a relative expansion of WT cells, which are not affected by bortezomib. Normal WT hematopoietic cells are depicted in blue and SRSF2-mt cells in burgundy. Professional illustration by Somersault18:24.
Although the authors limited their studies to IFN as the mediator of differential repression of WT HSPCs over SRSF2-mutant HSPCs, STAT1 transduces signals through various cytokines and other mediators. In addition to IFN, gene set expression analysis in this article revealed differential responses related to TNF signaling via NF-κB, complement, interleukin-2 (IL-2) and IL-6 signaling, all of which play a role in MDS and can use STAT1 to transduce signals.5 Thus, the implications of reduced expression of STAT1 caused by mutant SRSF2 may extend beyond IFN signaling to more generalized repression of STAT1-transmitted signals and broader modulation of inflammatory signaling.
Given the centrality of STAT1 signaling in SRSF2-mutant cells, the authors used the knowledge that bortezomib can restore STAT1 levels in mutant cells6 to examine whether restoration of STAT1 in SRSF2-mutant HSPCs would reinstate sensitivity to IFN. They confirmed that bortezomib did in fact increase STAT1 protein levels resulting in IFN-γ suppressing clonogenic activity of SRSF2-mutant iPSC-HSPCs, while not impacting WT iPSC-HSPCs. As the iPSC-HSPCs were derived from a single patient with MDS with an SRSF2 P95L mutation, the authors tested the effect of bortezomib on primary MDS samples as well. In the presence of bortezomib, IFN suppressed clonogenic activity of SRSF2-mutant cells, which were not affected by IFN alone. In contrast, MDS cells without SRSF2 mutations were already sensitive to IFN-mediated suppression, and bortezomib did not enhance this suppressive activity. However, responses in primary SRSF2-mutant MDS samples were quite variable with some samples showing no response.
From the clinical perspective, it will be important to understand which patients with MDS with SRSF2 mutations are likely to respond. For instance, it may be that the cytokine milieu, or other constituents of the marrow microenvironment, modulate the bortezomib response. This article does not address which STAT1-associated factors are actually present or increased in their model system or in patients with SRSF2 mutations. Although mutations in some myeloid genes (eg, TET2, JAK2, DNMT3A) are known to activate inflammatory signals, the specific inflammatory effects associated with SRSF2-mutant MDS are not as clear-cut.7 One study showed no association of SRSF2 mutations with inflammatory cytokines or chemokines in patients with MDS, but lipopolysaccharide stimulation of a cell line with an introduced SRSF2-mutant increased IL-6 and NF-κB reporter activity.8,9 STAT1 has been reported to shift TNF signaling away from activation of NF-κB to triggering an apoptotic pathway, implicating an alternate mechanism of action for STAT1 reconstitution by bortezomib.10 It is also not clear whether the STAT1-activating cytokines are elaborated from malignant HSPCs, their downstream progeny, or the microenvironment. The impact of coexisting mutations may also play a role in determining bortezomib response, either through the stimulation of cooperating cytokines or independent of inflammation. Future studies to elucidate these details will be useful to understand which patients might respond to bortezomib or whether alternative therapeutic strategies would be more fruitful.
It will need to be shown that the bortezomib response in vitro translates to activity in patients. Although it seems premature to proceed to a clinical trial immediately, this study provides impetus for preclinical studies to determine the context in which bortezomib will be beneficial for patients with SRSF2-mutated MDS.
Conflict-of-interest disclosure: A.K. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal