In this issue of Blood, Pelzl et al compare the systemic immune landscape in patients with diffuse large B-cell lymphoma (DLBCL) when newly diagnosed to patients who are in durable complete remission (CR), to patients with active relapsed or refractory disease (R/R), and to healthy controls (HCs).1 The characterization of the immune landscape was based on a circulating immune phenotype, which included the quantity of monocytic myeloid-derived suppressor cells (M-MDSCs; defined as CD14+/HLA-DRlo) and of several T-lymphocyte subsets. Compared with HCs, patients with DLBCL have increased M-MDSCs; reduced naive, central memory, and regulatory T-cell populations; and increased polyclonal activated T central memory and terminal effector memory T cells. Functional assessment revealed decreased T-cell vaccine responses across patients with DLBCL. The finding that patients with DLBCL with active disease exhibit systemic myeloid inflammation and T-cell immune deficits is consistent with clinical experience and prior observations across multiple cancers.2
However, Pelzl et al also make the provocative observation that patients with DLBCL in CR, even years after completing treatment, showed minimal improvement in systemic immune dysfunction compared with newly diagnosed patients with active lymphoma. Patients in CR maintained high levels of circulating M-MDSCs that are functionally T-cell suppressive and harbored chronically activated T cells that produced reduced T-cell vaccine responses. Moreover, long-term gene expression differences and higher inflammatory cytokine expression were observed to affect both myeloid cells and T cells in patients in CR compared with HCs. The deficits observed in patients in CR were more similar to patients with active lymphoma than they were to HCs. Suggesting a conservation across cancers, systemic immune dysregulation was also noted in patients with active but untreated chronic lymphocytic leukemia, in patients with active or remitted acute myelogenous leukemia, and in patients with active or remitted breast cancer. The profile of immune deficits was similar between patients with active cancer or remission of the same tumor type and different between patients in remission from different cancers. Here, the authors posit that each cancer leaves a unique “imprint” on the immune system that remains long after the cancer is eradicated.
What is the cause of the long-lasting systemic inflammatory and immunodeficient state in these patients? Pelzl et al provide evidence for the role of the tumor as a driver. In an immunocompetent mouse model, injection of lymphoma cells caused recruitment of MDSCs and concomitant T-cell suppression within lymphoid organs. In addition, when comparing circulating M-MDSCs and T cells between patients in CR to those with active lymphoma, they observed higher levels of inflammation within the patients with active disease. On the other hand, no clinical tumor feature (cell of origin, bulky disease, or constituents of the international prognostic index) affected circulating M-MDSC or T-cell levels, and eradication of the tumor (as occurred in patients in CR) only minimally affected the phenotype of the circulating immune cell abnormalities over the long term. Therefore, questions remain as to the role of the tumor as the driver of the long-lasting systemic immune changes and whether individual patients with DLBCL may experience unique immune deficits depending on tumor mutational biology or histological subtype.
In patients with R/R DLBCL, it has been shown that the tumor microenvironment becomes progressively immunosuppressive with additional lines of therapy.3 Here, Pelzl et al identify that patients with R/R DLBCL have severe circulating T-cell deficits and exaggerated inflammatory profiles. Since patients with R/R DLBCL are typically treated with T-cell immunotherapies such as chimeric antigen receptor T-cell therapy and bispecific T-cell engagers, further work is needed to understand the underlying mechanism by which these systemic immune deficits occur and how they affect the quality of T cells that are the effectors of these therapies.
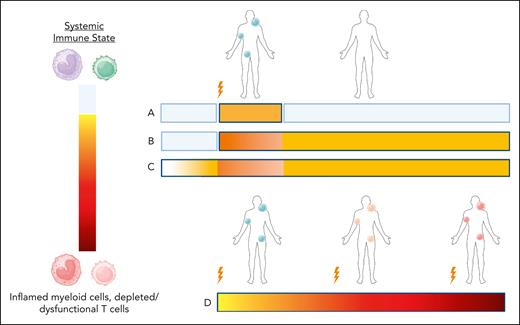
Collectively, it appears that current therapy, despite eradicating the tumor, does not address the functional changes within the immune system that may underlie cancer. Consider the possible models of the relationship between the systemic immune state, the development of the tumor, and eradication or relapse of the tumor (see figure). One model, which is not supported by the data of Pelzl et al, is that tumor onset initiates the immune dysfunction, which is then reset to normal following tumor eradication (model A in figure). An alternative model, favored by the data here, suggests that tumor onset initiates systemic immune dysfunction, but it imprints a “scar” on circulating immunity that is long-lasting (model B in figure). Teleologically from the perspective of a plastic immune system that adapts to the conditions of life, one might consider that the life-threatening cancer event convinces the immune system to adopt a more inflammatory posture than before, since the host was previously susceptible to the development of cancer. This new posture may be maladaptive or adaptive, and it may be fruitful to understand the systemic immune conditions under which relapse or infection is most common. However, the data also support the possibility that these immune changes represent a separate disease to the cancer itself (model C in figure). Here, the immune dysregulation may precede the development of the tumor and continue irrespective of tumor eradication. There may be a transient increase of inflammation during tumor growth and treatment, but over the long term the underlying immune dysregulation disease continues apace. In fact, the tumor may be a manifestation of this underlying immune disease. The links between inflammation and the aging process (“inflammaging”), and the finding that many normal tissues tolerate vast numbers of DNA mutations in the absence of malignancy, are supportive of this model.4,5 Finally, patients with multiple relapses experience progressive immune dysfunction both systemically and in the tumor microenvironment, possibly due to progression of the underlying immune disease process, or due to treatment, tumor-resistance mechanisms, or a combination of all of these (model D in figure).
Models of systemic immunity before and after lymphoma diagnosis. Depictions of the systemic immune state in patients who attain durable CR (models A-C) or patients with multiple relapses (model D). The lightning symbol indicates lymphoma diagnosis or relapse. In models A and B, patients have normal systemic immunity until the tumor diagnosis, at which point systemic immune dysregulation is caused by the tumor. Model A, where tumor response results in reversion to normal systemic immunity over time, is not supported by the data of Pelzl et al. In model B, tumor-driven dysregulation of systemic immunity leaves a long-lasting imprint. In model C, systemic immune dysregulation precedes lymphomagenesis, and tumor clearance has minimal effects on this chronic process. Model D depicts progressive worsening of the systemic dysregulation associated with multiple relapses and treatment. Figure created with Biorender.com.
Models of systemic immunity before and after lymphoma diagnosis. Depictions of the systemic immune state in patients who attain durable CR (models A-C) or patients with multiple relapses (model D). The lightning symbol indicates lymphoma diagnosis or relapse. In models A and B, patients have normal systemic immunity until the tumor diagnosis, at which point systemic immune dysregulation is caused by the tumor. Model A, where tumor response results in reversion to normal systemic immunity over time, is not supported by the data of Pelzl et al. In model B, tumor-driven dysregulation of systemic immunity leaves a long-lasting imprint. In model C, systemic immune dysregulation precedes lymphomagenesis, and tumor clearance has minimal effects on this chronic process. Model D depicts progressive worsening of the systemic dysregulation associated with multiple relapses and treatment. Figure created with Biorender.com.
In summary, this new study by Pelzl et al identifies that patients with DLBCL and other cancers have long-term systemic immune dysregulation despite tumor remission. This consists of persistent myeloid inflammation and T-cell dysfunction, with specific deficits unique to the individual cancer type. Future work is needed to understand the relationship between these deficits and carcinogenesis, relapse, infection, and response to T-cell therapies.
Conflict-of-interest disclosure: M.D.J. reports consultancy/advisory for Kite/Gilead and Novartis and research funding (to institution) from Kite/Gilead, Lilly, and Incyte. J.C.-L. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal