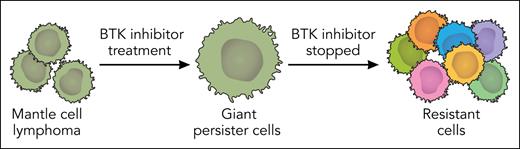
In this issue of Blood, Wang et al1 have identified drug-tolerant persister cells, which they call giant cells, in patients with mantle cell lymphoma (MCL) receiving the noncovalent Bruton tyrosine kinase (BTK) inhibitor pirtobrutinib. Using xenografts and organoids derived from patients with MCL, they found that these giant cells were also present upon treatment with covalent BTK inhibitors, venetoclax, and an anti-ROR1 monoclonal antibody, suggesting a more generalizable resistance mechanism. When the drugs were removed from the system, the cells returned to their usual size and proliferated (see figure). This change in cell size in response to drug exposure is potentially regulated by a metabolic switch in the tricarboxylic acid cycle. Acetyl coenzyme A (acetyl-CoA) controls this switch and could be modulated by inhibiting adenosine triphosphate-citrate lyase (ACL), an enzyme that produces acetyl-CoA from citrate. In addition, there was a shift of the malate-aspartate shuttle activity between the mitochondria and cytoplasm in the giant cells that was dependent on the expression of GOT2, the gene that encodes glutamate-oxaloacetate transaminase 2.
BTK inhibitor–tolerant persister cells in MCL lead to resistance. Wang et al showed that MCL cells exposed to BTK inhibitors become giant persister cells that proliferate and gain resistance to therapies when the BTK inhibitors are stopped. The giant-cell state is controlled by a metabolic switch and could offer an opportunity to intervene in MCL therapeutic resistance. Professional illustration by Somersault18:24.
BTK inhibitor–tolerant persister cells in MCL lead to resistance. Wang et al showed that MCL cells exposed to BTK inhibitors become giant persister cells that proliferate and gain resistance to therapies when the BTK inhibitors are stopped. The giant-cell state is controlled by a metabolic switch and could offer an opportunity to intervene in MCL therapeutic resistance. Professional illustration by Somersault18:24.
BTK inhibitor resistance is a significant clinical challenge in MCL for several reasons. One reason is the heterogeneity of responses to BTK inhibitors in MCL. For example, only ∼52% to 58% of patients responded to pirtobrutinib in a phase 1/2 trial2,3 compared with ∼82% of those with chronic lymphocytic leukemia (CLL) who responded to pirtobrutinib.4 Another reason is that the cause of BTK inhibitor resistance in MCL has not been as well studied or understood as in other diseases, such as CLL. In CLL, mutations in BTK and PLCG2, the gene that encodes phospholipase C gamma 2, the downstream substrate of BTK, account for ∼80% of cases of covalent BTK inhibitor resistance.5,6 Specifically, in pirtobrutinib-resistant CLL, novel non-C481 mutations accounted for most of the emerging resistance and were seen in 7 of 9 patients, whereas the remaining 2 had PLCG2 mutations.7 These mutations are rarely seen in patients with MCL, suggesting resistance to BTK inhibitors in MCL could either be intrinsic or through a nongenetic mechanism. Divergent responses of patients with MCL to BTK inhibitors may be partially explained by intrinsic sensitivity in some patients due to mutations that activate the B-cell receptor driven classical nuclear factor κB (NF-κB) vs intrinsic resistance in other patients with mutations that drive noncanonical NF-κB.8 Here, Wang et al describe a reversible BTK inhibitor–resistant persister state that was not associated with any genetic mutations and was dependent on the continued pressure of the drug as well as a metabolically driven switch in cell state. The exact mechanism by which pirtobrutinib or any of the other therapies induced these changes was not revealed and remains an outstanding question. However, whole exome sequencing did not reveal any mutations in BTK or other obvious resistance pathways, and the reversible morphologic changes suggest a nongenetic cause.
Given that these drug-tolerant persister cells morphologically transform into giant cells, these should be easily independently validated or confirmed by other groups as being present in resistant patient samples. Through prospective studies, giant cell detection could then be tested as a marker for potential pending resistance if seen early; however, this would need to be paired with a biomarker of the metabolic cell state, such as high ACL or GOT2 levels. The authors also raise an intriguing possibility of targeting the giant cells before they reprogram to stave off resistance. However, even though they were able to reverse the giant cell state through ACL inhibition, they did not show that the cells had become resensitized to BTK inhibition or other therapy. Furthermore, giant cells upon drug removal and then reexposure became resistant to therapies to which they were previously sensitive, suggesting that the giant cell state is transitional in the development of more stable resistance. Therefore, a window to intervene may be short and would require further preclinical evidence before moving into patient studies. Another potential intriguing question would be whether pirtobrutinib-resistant giant MCL cells cause resistance to BTK degraders, which have been used to overcome pirtobrutinib resistance in CLL.9 An additional question would be whether giant cells are unique to MCL or also seen in other types of lymphoma resistant to BTK inhibitors. Nonetheless, this work could mark the start of a new era of understanding resistance to BTK inhibitors in MCL, moving beyond genetic alterations. More research is needed to understand what triggers giant cell formation and any liabilities this creates that could be leveraged for more effective combination therapies. This could allow BTK inhibitor therapy to be more effective in a broader swath of patients with MCL and for a longer response duration among those who do respond to treatment.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal