In this issue of Blood, Peyron et al1 present exciting data regarding a potential new therapeutic approach for von Willebrand disease (VWD). In particular, they demonstrate that a bispecific nanobody can be used to increase endogenous plasma von Willebrand factor (VWF) levels by bridging it to albumin. In a murine type 1 VWD model, this bispecific nanobody produced a twofold increase in plasma VWF antigen (VWF:Ag) levels that was sustained for many days (see figure).
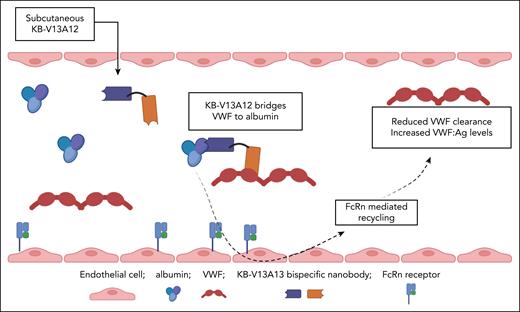
Bispecific nanobody KB-V13A12 bridges VWF to albumin and consequently attenuates normal VWF clearance in vivo, leading to a significant increase in endogenous plasma VWF levels. Figure created with BioRender.com. O'Donnell J.S. (2025) https://app.biorender.com/illustrations/689efc486cb58b1325e4a189.
Bispecific nanobody KB-V13A12 bridges VWF to albumin and consequently attenuates normal VWF clearance in vivo, leading to a significant increase in endogenous plasma VWF levels. Figure created with BioRender.com. O'Donnell J.S. (2025) https://app.biorender.com/illustrations/689efc486cb58b1325e4a189.
VWD represents the most common inherited bleeding disorder.2 Type 1 VWD is characterized by a partial quantitative reduction in plasma VWF:Ag and accounts for ∼75% of cases.2,3 Although it is almost 100 years since Erik von Willebrand described the index case, which involved fatal menorrhagia in a 14-year adolescent girl with type 3 VWD, recent studies have highlighted that heavy menstrual bleeding (HMB) remains a major clinical problem in women with type 1 VWD.4 Despite the clinical burden and impaired quality of life associated with type 1 VWD, particularly in female patients, treatment options remain limited to mainly desmopressin or VWF-containing factor concentrates. The lack of progress in developing novel therapeutics for type 1 VWD contrasts with the astonishing innovative progress over recent years in hemophilia A and B treatment.5
In their article, Peyron et al present compelling data regarding a novel approach to increasing VWF levels in type 1 VWD. Because endogenous albumin has an extended plasma half-life due to the neonatal Fc receptor (FcRn)–recycling pathway, the authors hypothesized that linking endogenous VWF to albumin in the plasma could extend the in vivo half-life of VWF. In brief, they developed a bispecific nanobody named KB-V13A12. This comprised 1 nanobody with high affinity for VWF coupled to another nanobody with high affinity for albumin. Importantly, the VWF nanobody was directed toward the VWF-D’D3 region (residues His1114-Val1155 and His1176-Pro1197) and had no functional effect on either factor VIII (FVIII) or glycoprotein Ibα interactions. Pharmacokinetic studies in mice demonstrated that after a single subcutaneous 5 mg/kg bolus injection, KB-V13A12 plasma levels peaked at 24 hours and remained detectable for up to 14 days. Pertinently, KB-V13A12 plasma concentrations also remained in molar excess over VWF levels throughout this extended period.
In a murine model of human type 1 VWD (hVWD1; baseline VWF:Ag level, ∼12 U/dL),6 Peyron et al demonstrated that a single subcutaneous dose of KB-V13A12 (at doses of 2, 5, or 25 mg/kg) significantly increased plasma VWF:Ag and VWF collagen-binding activity levels approximately twofold. In keeping with the elevation in VWF levels, plasma FVIII:C levels were also increased up to 1.5-fold after KB-V13A12 treatment. Importantly, the higher the dose of KB-V13A12 administered, the longer the increase in murine plasma VWF levels was sustained. For example, even 14 days after a single 25 mg/kg dose of KB-V13A12, plasma VWF:Ag levels remained significantly increased compared to baseline (19.1 ± 4.3 U/dL vs 12.6 ± 1.5 U/dL; P = .002). From a mechanistic perspective, the ratio of plasma VWF-propeptide to VWF:Ag ratio (a surrogate marker for VWF clearance in vivo)7 was significantly reduced after KB-V13A12 administration, suggesting that the increase in plasma VWF levels was predominantly due to delayed VWF clearance. Moreover, consistent with an albumin-mediated prolongation in VWF half-life, KB-V13A12 treatment of FcRn-deficient mice was not associated with any alteration in VWF clearance kinetics. Based upon their exciting in vitro and in vivo data, Peyron et al proceeded to study the hemostatic effects of KB-V13A12 (5 mg/kg administered 3 days before injury) in managing saphenous vein bleeding in hVWD1 mice. Interestingly, the number of clots was significantly increased and maximal bleeding time reduced after KB-V13A12 pretreatment. These data are consistent with McCluskey et al who recently reported that KB-V13A12 also significantly diminished blood loss after tail clip injury in hVWD1 mice.6
The findings presented by Peyron et al undoubtedly represent an important advance in the search for novel therapeutics for patients with VWD. Clinical trials in patients with type 1 VWD will be necessary to develop upon these exciting in vitro and murine data. Nevertheless, it is interesting to consider the potential clinical implications of these initial findings. First, it seems likely that this strategy has the potential to increase plasma VWF levels in patients with VWD by prolonging the half-life of endogenous VWF via albumin-mediated FcRn recycling. Second, if the approximate twofold increase in murine VWF levels after KB-V13A12 treatment is replicated in human patients, this treatment would likely be most useful for patients with mild-to-moderate partial quantitative VWF deficiency (ie, type 1 VWD and low VWF). Third, KB-V13A12 treatment could offer advantages over desmopressin. For example, the potential to induce increases in plasma VWF:Ag levels that are sustained over many days, would be an asset compared to desmopressin. Furthermore, KB-V13A12 is not associated with fluid restriction, hyponatremia, or vascular spasm, which limit desmopressin use in some patients with VWD. Fourth, for type 1 patients with very low endogenous VWF:Ag levels, coadministration of KB-V13A12 might be useful to extend the half-life of VWF in plasma-derived or recombinant concentrates. Consequently, clinicians involved in providing care for patients with VWD will await the human trial data regarding the safety and efficacy of KB-V13A12 with great interest.
In terms of novel VWD therapeutics under development, 2 others also aim to increase plasma VWF levels by inhibiting the clearance of endogenous VWF. BT200 is a PEGylated aptamer targeted to the VWF-A1 domain.8,9 Recent human studies have shown that it increases endogenous plasma VWF:Ag and FVIII levels in patients with VWD.8 In contrast to KB-V13A12, BT200 does not promote FcRn recycling but rather inhibits macrophage LRP1–mediated VWF clearance.9 In addition, preliminary data regarding an investigational monovalent antibody (HMB-002; Hemab Therapeutics) were presented at the recent 2025 International Society on Thrombosis and Haemostasis congress.10 Although the mechanism(s) remains unclear, HMB-002 binds to the VWF-CK domain and prolongs in vivo VWF half-life in vivo. For the VWD community of patients, families, and treaters, we appear to be entering an exciting age. What a shame it has taken so long!
Conflict-of-interest disclosure: J.S.O. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal