Key Points
Ruxolitinib alleviates systemic inflammation and induces remission in refractory AOSD-MAS.
Ruxolitinib modulates neutrophil activation in MAS by broadly inhibiting JAK-STAT signaling.
Visual Abstract
Macrophage activation syndrome (MAS) is believed to be caused by inappropriate proliferation and activation of the mononuclear phagocytic system. Adult-onset Still disease (AOSD) is characterized by neutrophil activation and a cytokine storm, which can lead to the severe and potentially life-threatening complication of MAS. RNA sequencing revealed that neutrophils may play a distinct role and enhance the innate immunity of patients with AOSD with MAS (AOSD-MAS). In the CpG-induced secondary hemophagocytic lymphohistiocytosis (HLH) model, the depletion of neutrophils significantly reduced cytokine levels with effects comparable with monocyte depletion. Significant enrichment was observed in the type I/II interferon and JAK-STAT pathways in neutrophils from patients with AOSD-MAS. Treatment of 10 patients with refractory AOSD-MAS with ruxolitinib led to the resolution of inflammatory parameters and clinical symptoms. RNA sequencing and ex vivo assays confirmed that ruxolitinib suppressed aberrant NETosis and STAT3/STAT5 signaling. In vivo, PAD4 knockout further confirmed the pathogenic role of NETosis in a secondary HLH model. Moreover, the selective inhibition of STAT3 or STAT5 alleviated systemic inflammation. Ten functional variants were identified in genes related to the JAK-STAT pathway, however, their clinical relevance requires further validation. These findings suggest that ruxolitinib has the potential to facilitate disease remission in patients with refractory AOSD-MAS by broadly inhibiting JAK-STAT signaling and modulating neutrophil activation and NETosis.
Introduction
Macrophage activation syndrome (MAS) is a type of hemophagocytic lymphohistiocytosis (HLH) with mortality rates of 20% to 50%.1 The manifestation of MAS includes prolonged fever, hepatosplenomegaly, hemorrhagic manifestations, and neurologic dysfunction, which could potentially lead to multiple organ failure if not treated promptly. Adult-onset Still disease (AOSD), a rare polygenic systemic autoinflammatory disease, is one of the most common rheumatic diseases that can cause MAS.2 The management of MAS in AOSD is challenging, and high-dose corticosteroids, cyclosporine A, and supportive measures are first-line treatments. Interleukin-1 (IL-1) or IL-6 blockers may be used in cases of inadequate response, and etoposide is an option despite its long-term side effects. Notably, 64% of MAS cases do not achieve remission with glucocorticoid pulse therapy, and 24% do not respond to dexamethasone plus etoposide, highlighting the need for effective salvage therapies.3 The lack of consensus on the optimal treatment for refractory MAS secondary to AOSD (AOSD-MAS) underscores the necessity for new treatment strategies.
The pathophysiology of MAS involves an aberrant immune response that is caused by a cytokine storm with uncontrolled secretion of proinflammatory cytokines, such as interferon gamma (IFN-γ),4 IFN-α,5 and tumor necrosis factor (TNF).6 Our previous study demonstrated that neutrophil activation is a key contributor to the amplified inflammation in AOSD through the release of neutrophil extracellular traps (NETs), which are induced by many inflammatory mediators, including IFN-α.7,8 Thus, targeting neutrophil activation may lead to new therapeutic strategies for AOSD.
JAK inhibitors (JAKis) disrupt the signaling pathways of various cytokines.9 There have been anecdotal reports that JAKis, such as tofacitinib and baricitinib, are being used to treat refractory AOSD10 and leading to reduced IFN-induced neutrophil activation in patients with AOSD.8 In addition, ruxolitinib, a JAK1/2 inhibitor, reduced hypercytokinemia and organ damage by inhibiting IFN-γ signaling or other proinflammatory cytokine signaling in mouse models of HLH.11-13 In children with secondary HLH, ruxolitinib has helped to control disease activity and reduced the required glucocorticoid dosage.14 Given the shared pathophysiological features of HLH/MAS and AOSD, the broad mechanism of action of ruxolitinib, and its proven efficacy in treating HLH/MAS, ruxolitinib holds promise as an alternative therapeutic option for AOSD-MAS. In this study, we present our experience of treating 10 patients with refractory AOSD-MAS with ruxolitinib and our investigation on its effect in neutrophil function. In addition, we explored the broad inhibitory impact of ruxolitinib on the JAK-STAT signaling pathway.
Methods
Study population and diagnostic criteria
Patients with AOSD were recruited from the Department of Rheumatology and Immunology, Ruijin Hospital, Shanghai Jiao Tong University. An AOSD diagnosis was based on the Yamaguchi criteria after excluding infections, tumors, and other autoinflammatory or autoimmune diseases.15 Patients with AOSD-MAS were defined using the 2016 European League Against Rheumatism/American College of Rheumatology/Paediatric Rheumatology International Trials Organization (EULAR/ACR/PRINTO) classification criteria for MAS complicating systemic juvenile idiopathic arthritis (sJIA; JIA-MAS criteria).16 AOSD disease activity was measured using a modified Pouchot’s systemic score,17 and the HScore was determined as reported previously.18
The first cohort included 13 patients with AOSD (7 with MAS and 6 without MAS), and the clinical characteristics are presented in supplemental Table 1, available on the Blood website.
In the second cohort, 10 patients with refractory AOSD-MAS who were subsequently treated with ruxolitinib were enrolled, as shown in Table 1. The analysis time point was the time of ruxolitinib initiation and the 3rd, 5th, 8th, 10th, 12th, and 15th months after ruxolitinib initiation or the latest follow-up.
Baseline information of the patients with AOSD with MAS at enrollment
| No. . | Sex . | Age (y) . | Disease duration (mo) . | Previous treatments . | Last regimen before ruxolitinib . | Treatments after enrollment . | Follow-up (mo) . | Clinical evaluation . | Present pred dose (mg/d) . | RNA-seq . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 40 | 4 | Pred, CsA, VP16 | Pred | Pred 130 mg + ruxolitinib 10 mg twice daily | 12 | CR | 5 | Y |
| 2 | F | 64 | 35 | Pred, CsA, tofacitinib, VP16 | Pred + VP16 + CsA | Pred 65 mg + ruxolitinib 10 mg twice daily | 15 | CR | 5 | Y |
| 3 | F | 42 | 1 | Pred, CsA | Pred + VP16 + CsA | Pred 60 mg + CsA + ruxolitinib 10 mg twice daily | 15 | CR | 5 | N |
| 4 | F | 22 | 41 | Pred, CsA, LEF, tofacitinib, HCQ | Pred + VP16 + HCQ | Pred 300 mg + CsA + ruxolitinib 5 mg twice daily | 8 | CR | 12.5 | Y |
| 5 | F | 57 | 29 | Pred, HCQ, MTX | Pred + VP16 | Pred 260 mg + ruxolitinib 10 mg twice daily | 3 | CR | 40 | N |
| 6 | F | 43 | 1 | Pred, baricitinib, VP16, HCQ, tocilizumab | Pred + CsA + VP16 | Pred 50 mg + CsA + ruxolitinib 5 mg twice daily | 3 | PR | 25 | N |
| 7 | F | 40 | 5 | Pred, CsA, VP16 | Pred | Pred 200 mg + ruxolitinib 5 mg twice daily | 12 | Relapse at 8 months | 5 | N |
| 8 | F | 30 | 120 | Pred | Pred + VP16 | Pred 60 mg + CsA + ruxolitinib 5 mg twice daily | 10 | CR | 2.5 | N |
| 9 | F | 37 | 6 | Pred, baricitinib, VP16, HCQ, tocilizumab | Pred + CsA + VP16 | Pred 50 mg + CsA + ruxolitinib 5 mg twice daily | 4 | CR | 20 | N |
| 10 | F | 29 | 25 | Pred, MTX, tofacitinib, HCQ | Pred + CsA + VP16 | Pred 50 mg + CsA + ruxolitinib 5 mg twice daily | 12 | CR | 5 | N |
| No. . | Sex . | Age (y) . | Disease duration (mo) . | Previous treatments . | Last regimen before ruxolitinib . | Treatments after enrollment . | Follow-up (mo) . | Clinical evaluation . | Present pred dose (mg/d) . | RNA-seq . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 40 | 4 | Pred, CsA, VP16 | Pred | Pred 130 mg + ruxolitinib 10 mg twice daily | 12 | CR | 5 | Y |
| 2 | F | 64 | 35 | Pred, CsA, tofacitinib, VP16 | Pred + VP16 + CsA | Pred 65 mg + ruxolitinib 10 mg twice daily | 15 | CR | 5 | Y |
| 3 | F | 42 | 1 | Pred, CsA | Pred + VP16 + CsA | Pred 60 mg + CsA + ruxolitinib 10 mg twice daily | 15 | CR | 5 | N |
| 4 | F | 22 | 41 | Pred, CsA, LEF, tofacitinib, HCQ | Pred + VP16 + HCQ | Pred 300 mg + CsA + ruxolitinib 5 mg twice daily | 8 | CR | 12.5 | Y |
| 5 | F | 57 | 29 | Pred, HCQ, MTX | Pred + VP16 | Pred 260 mg + ruxolitinib 10 mg twice daily | 3 | CR | 40 | N |
| 6 | F | 43 | 1 | Pred, baricitinib, VP16, HCQ, tocilizumab | Pred + CsA + VP16 | Pred 50 mg + CsA + ruxolitinib 5 mg twice daily | 3 | PR | 25 | N |
| 7 | F | 40 | 5 | Pred, CsA, VP16 | Pred | Pred 200 mg + ruxolitinib 5 mg twice daily | 12 | Relapse at 8 months | 5 | N |
| 8 | F | 30 | 120 | Pred | Pred + VP16 | Pred 60 mg + CsA + ruxolitinib 5 mg twice daily | 10 | CR | 2.5 | N |
| 9 | F | 37 | 6 | Pred, baricitinib, VP16, HCQ, tocilizumab | Pred + CsA + VP16 | Pred 50 mg + CsA + ruxolitinib 5 mg twice daily | 4 | CR | 20 | N |
| 10 | F | 29 | 25 | Pred, MTX, tofacitinib, HCQ | Pred + CsA + VP16 | Pred 50 mg + CsA + ruxolitinib 5 mg twice daily | 12 | CR | 5 | N |
CsA, cyclosporine A; F, female; HCQ, hydroxychloroquine; LEF, leflunomide; M, male; N, no; MTX, methotrexate; Pred, prednisone; VP16, etoposide; Y, yes.
The third cohort contained a total of 195 patients with AOSD, including 41 cases of MAS (15 refractory and 26 nonrefractory), and their clinical characteristics are presented in supplemental Table 2.
The study was performed in accordance with the Declaration of Helsinki and principles of Good Clinical Practice and was approved by the institutional research ethics committee of Ruijin Hospital (2016-62; 2024-245), Shanghai, China. All participants provided informed consent. A flowchart of the study design is shown in supplemental Figure 1.
Outcome assessment
The outcome was evaluated at the 1st, 3rd, 5th, 10th, 12th, and 15th months. The effectiveness of treatment was defined as follows: complete remission (CR) was considered when all initial clinical manifestations and abnormal laboratory tests had resolved; partial remission (PR) was considered when all but 1 initial clinical manifestation or abnormal laboratory test had resolved; ineffective treatment was considered when ≥2 clinical manifestation or abnormal laboratory tests persisted. Relapse was assessed during the 12 months of treatment.
Sample collection
Human biologic samples were obtained under a protocol approved by the institutional research ethics committee of Ruijin Hospital (ID: 2016-62; 2024-245), Shanghai, China. Serum and plasma samples were collected and stored at −80°C immediately. Heparinized blood from patients with AOSD was collected and the neutrophils were isolated by density gradient centrifugation with PolymorphPrep (Axis-Shield, Dundee, United Kingdom).
RNA sequencing (RNA-seq)
The neutrophils isolated using gradient centrifugation were used for total RNA isolation. Messenger RNA (mRNA) was purified, fragmented, and converted to complimentary DNA. Complimentary DNA fragments underwent polymerase chain reaction (PCR) amplification, purification, denaturation, and circularization. Phi29 amplification produced DNA nanoballs that were loaded into a nanoarray to generate paired-end 150-base reads on the BGIseq500 platform. HISAT2 mapped the reads to the genome, Bowtie2 aligned reads to the reference coding gene set, and RNA-seq by expectation-maximization (RSEM) calculated gene expression levels. Differentially expressed genes (DEGs) with a log2(fold change) >1 and a P value <.05 were determined using DESeq2 (version 1.4.5). More details can be found in the supplemental Methods.
Mice
Wild-type (WT; C57BL/6N) mice were purchased from Vital River Laboratories (Beijing, China). Padi4–/– mice (catalog no. NM-KO-190334) were obtained from Shanghai Model organisms. Animals were matched for age and sex and used at 8 to 12 weeks of age. More details can be found in the supplemental Methods.
Plasma protein measurement
Olink Proteomics (Olink Proteomics, Uppsala, Sweden) was used to measure the levels of 92 inflammatory proteins in plasma. Specifically, the Olink Target 96 Inflammation Panel, which uses proximity extension assay technology, was employed. Normalized protein expression values, which represent protein expression levels on a log2 scale, were used to quantify the plasma protein expression levels with selection based on an elbow P value–rank plot, focusing on those with a P value <.05. Plasma free IL-18 was measured as described in Girard et al.19
Quantification of cell-free DNA and NET-DNA complexes
Serum cell-free DNA and NET-DNA complexes, including neutrophil elastase (NE)-DNA and myeloperoxidase (MPO)-DNA complexes, were quantified as previously described.20
NET detection and quantification
Isolated neutrophils were cultured in RPMI 1640 (Hyclone) supplemented with 10% fetal bovine serum for 3.5 hours at 37°C.
Details on the supernatant cell-free DNA quantification and NETosis fluorescence imaging can be found in the supplemental Methods.
Whole-exome sequencing (WES) and variant analysis
Exome sequencing of genomic DNA was performed with reads aligned to the human genome using Burrows-Wheeler Aligner (BWA). Variants were identified using Genome Analysis Toolkit, annotated with Variant Effect Predictor (VEP), and filtered. Pathogenicity was determined according to the American College of Medical Genetics and Genomics (ACMG) guidelines. More details can be found in the supplemental Methods.
JAK-STAT pathway gene list determination
Gene sets of the JAK-STAT pathway (ko: 04630) was curated based on the Kyoto Encyclopedia of Genes and Genomes database (https://www.genome.jp/kegg/). WES of DNA samples was conducted, and the data were analyzed by sequentially filtering out low-quality variants, common variants, synonymous variants, and benign or likely benign variants. Subsequently, the identified variants were confirmed by Sanger sequencing.
Cell culture and plasmid transfection
For the in vitro experiments, 293T cells (China Center for Type Culture Collection) were cultured at 37°C and 5% CO2 and grown in Dulbecco’s modified Eagle medium (Gibco) supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin. When the cells reached the appropriate confluence, they were transfected with WT or mutant PDGFRB, PDGFRA, IL7R, IL27RA, IL12RB1 (cotransfected with WT IL23R plasmid), IL11RA, or IL10RA overexpression plasmids using a calcium phosphate cell transfection kit (Yeasen, Shanghai, China) according to the manufacturer’s protocol. In addition, 293T cells were pretransfected with lentivirus-PIAS4-RNAi or lentivirus-SOCS4-RNAi, followed by transfection with WT or mutant PIAS4/SOCS4 overexpression plasmids. The empty vector was also transfected into the negative control group.
Western blotting
The transfected cells were lysed and the proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to membranes, blocked, and incubated with specific antibodies. Horseradish peroxidase–conjugated secondary antibodies were used, and the signals detected using enhanced chemiluminescence assays. More details can be found in the supplemental Methods.
Quantitative real-time PCR
The total RNA was extracted, reverse transcribed, and quantitative PCR was performed. The relative mRNA levels were normalized against ACTB (human) or Gapdh (mice) using specific primers. More details can be found in the supplemental Methods.
Statistical analysis
All statistical analyses were carried out using SPSS, version 22.0, software (SPSS Inc, Chicago, IL), GraphPad Prism, version 9.0.0, software (GraphPad Software, La Jolla, CA), and R Studio software (version 4.2.3). Quantitative data were expressed as the mean ± standard deviation. Data with a Gaussian distribution were analyzed using an unpaired 2-sided t test or a 1-way or 2-way analysis of variance, whereas nonparametric data were assessed using the Mann-Whitney U test or the Wilcoxon rank-sum test. P values <.05 were considered statistically significant.
Additional methods are available in the supplemental Methods.
Results
Bulk sequencing assessment of MAS neutrophil architecture
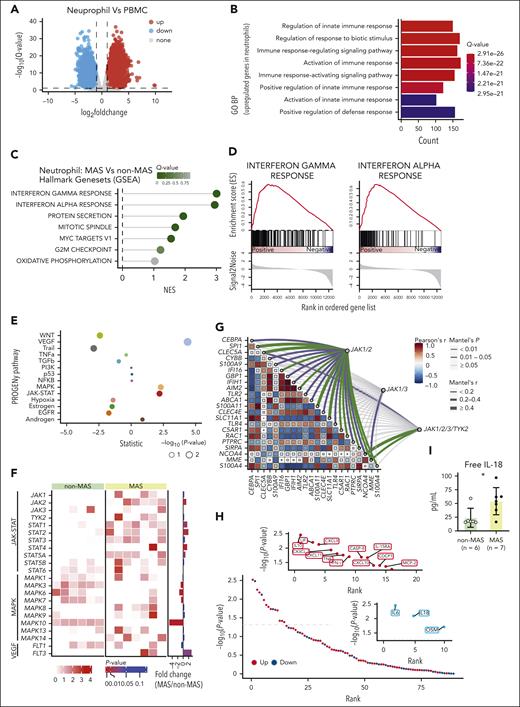
We conducted RNA-seq on paired neutrophils and peripheral blood mononuclear cells (PBMCs) obtained from 7 patients with AOSD-MAS (from cohort 1; Figure 1A). Gene Ontology analysis of the upregulated DEGs indicated that neutrophils may play a distinct and enhancing role in innate immunity when compared with PBMCs in AOSD-MAS (Figure 1B), suggesting that neutrophils may potentially contribute markedly to MAS pathogenesis. The murine model of secondary HLH displayed a significant increase in neutrophil and monocyte frequencies (supplemental Figure 2A), and in vivo experiments (supplemental Figure 2B) demonstrated that neutrophil depletion using anti-Ly6G antibody (α-Ly6G) led to significantly reduced cytokine levels, similar to the effects observed with monocyte depletion using clodronate liposomes (CL2MBP; supplemental Figure 2C-E). To further explore the role of neutrophils in AOSD-MAS pathogenesis and to identify potential therapeutic targets, RNA-seq data from 6 patients with AOSD without MAS (cohort 1) were used as controls. Using gene set enrichment analysis, we identified 7 significantly upregulated biologic processes terms. Among them, IFN-γ response and IFN-α response emerged as the most significantly indicated hallmark term (Figure 1C-D). When compared with the non-MAS controls, patients with MAS displayed greater activation of the JAK-STAT pathway and significantly increased expression levels of JAK2, STAT1, STAT2, and STAT4 in neutrophils (Figure 1E-F). A correlation analysis revealed a strong correlation between JAK1/JAK2 and genes involved in neutrophil function (Figure 1G). STAT1, activated by both type I and type II IFN via JAK1/2, showed significantly elevated phosphorylation at Tyr701 and Ser727 in neutrophils from patients with AOSD when compared with healthy controls with higher levels in patients with MAS than in those without MAS, although the latter did not reach statistical significance (supplemental Figure 3A). In addition, p-STAT1 (Ser727) levels were significantly positively correlated with p-STAT1 (Tyr701) levels only in patients with MAS (supplemental Figure 3B). Furthermore, inflammatory mediators associated with the JAK-STAT pathway, including IL-10, leukemia inhibitory factor (LIF), CXCL9, CXCL10, CXCL11, IFN-γ, and TNF, were elevated in patients with MAS, whereas the IL-6 and IL-18 levels were reduced (Figure 1H). Free IL-18 levels, however, were significantly elevated (Figure 1I). These data support the hypothesis that targeting JAK1/2 could be a promising therapeutic strategy for AOSD-MAS. In murine models of secondary HLH, combining α-Ly6G or CL2MBP with the JAK1/2 inhibitor ruxolitinib normalized the white blood cell (WBC) count and neutrophil and monocyte frequency (supplemental Figure 2B-C). Monocyte depletion, in combination with ruxolitinib, reduced the mRNA expression of Il6, Tnfa, and Il18 in liver tissue, whereas neutrophil depletion, combined with ruxolitinib, produced similar reductions (supplemental Figure 2E). These findings suggest that ruxolitinib exerts its therapeutic effects by modulating multiple immune cell populations, including neutrophils and monocytes, and by reducing inflammatory cytokine production. Furthermore, combination treatments (α-Ly6G or CL2MBP with ruxolitinib) led to a more pronounced reduction in serum CXCL9 and liver tissue Cxcl9 expression when compared with either treatment alone (supplemental Figure 2D-E). Overall, these findings demonstrate that ruxolitinib has broad immunomodulatory effects in MAS that affect both the neutrophils and monocytes.
Bulk sequencing assessment of MAS neutrophil architecture. (A) Volcano plot showing the DEGs in neutrophils vs PBMCs of patients with MAS. Red points represent upregulated genes, blue points represent downregulated genes. (B) GO analysis of the upregulated DEGs of neutrophils vs PBMCs. (C) GSEA of genes in neutrophils isolated from patients with AOSD with MAS vs patients with AOSD without MAS. (D) Gene set enrichment plots of target genes in the IFN-γ response (left) and IFN-α response (right). (E) PROGENy analysis of genes in neutrophils isolated from patients with AOSD with MAS vs patients with AOSD without MAS. (F) Comparison of the gene transcription levels involved in the JAK-STAT, MAPK, and VEGF pathways between the MAS and the non-MAS control group. (G) Mantel correlation analysis of the gene transcription levels related to neutrophil function and those related to JAK. (H) Olink analysis of the plasma inflammatory protein profile of the MAS group and the non-MAS control group. Shown are all tested proteins ranked by their P value. (I) Comparison of the plasma free IL-18 levels between the MAS and the non-MAS control group. ∗P < .05. BP, biological process; GSEA, gene set enrichment analysis; GO, Gene Ontology; NES, normalized enrichment score; PROGENy, Pathway RespOnsive GENes for activity inference; TGFβ, transforming growth factor β.
Bulk sequencing assessment of MAS neutrophil architecture. (A) Volcano plot showing the DEGs in neutrophils vs PBMCs of patients with MAS. Red points represent upregulated genes, blue points represent downregulated genes. (B) GO analysis of the upregulated DEGs of neutrophils vs PBMCs. (C) GSEA of genes in neutrophils isolated from patients with AOSD with MAS vs patients with AOSD without MAS. (D) Gene set enrichment plots of target genes in the IFN-γ response (left) and IFN-α response (right). (E) PROGENy analysis of genes in neutrophils isolated from patients with AOSD with MAS vs patients with AOSD without MAS. (F) Comparison of the gene transcription levels involved in the JAK-STAT, MAPK, and VEGF pathways between the MAS and the non-MAS control group. (G) Mantel correlation analysis of the gene transcription levels related to neutrophil function and those related to JAK. (H) Olink analysis of the plasma inflammatory protein profile of the MAS group and the non-MAS control group. Shown are all tested proteins ranked by their P value. (I) Comparison of the plasma free IL-18 levels between the MAS and the non-MAS control group. ∗P < .05. BP, biological process; GSEA, gene set enrichment analysis; GO, Gene Ontology; NES, normalized enrichment score; PROGENy, Pathway RespOnsive GENes for activity inference; TGFβ, transforming growth factor β.
Characteristics of patients with AOSD at disease onset and ruxolitinib initiation
Ten patients with refractory AOSD-MAS were treated with ruxolitinib, and their demographic and clinical characteristics are outlined in Table 1. The study cohort, with an average age of 40.4 years (range, 29-64), comprised 9 females and 1 male. The most common symptoms were fever, skin rash, sore throat, arthralgia, and pneumonia. Previous treatments include glucocorticoids, hydroxychloroquine, cyclosporine A, and methotrexate. At the time of enrollment, 6 patients had even received etoposide because of the development of MAS but had poor response. In this study, the patients received ruxolitinib at a dose of 10 mg twice daily at maximum.
Ruxolitinib treatment and improvement in the inflammatory parameters
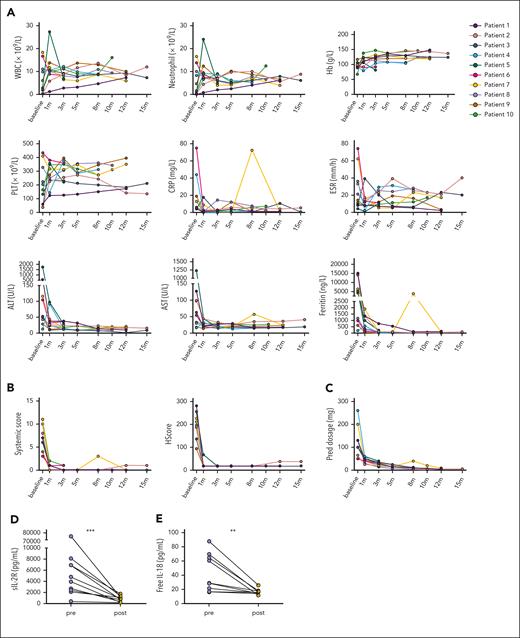
Before commencing ruxolitinib therapy, all patients manifested fever, 7 presented with skin rash, and 8 experienced arthralgia and/or sore throat. After the initiation of ruxolitinib and continuous treatment for 1 month, these patients exhibited rapid improvement. Among the 10 patients, 8 achieved CR with a reduced prednisone dosage, 1 achieved PR, and 1 experienced relapse when the prednisone dosage was reduced to 10 mg per day.
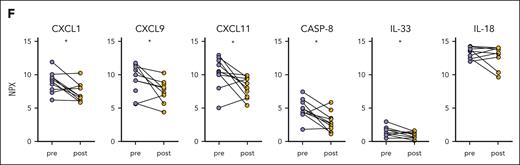
An evaluation of the efficacy of ruxolitinib was conducted during each visit and encompassed assessments of the clinical manifestations, laboratory tests, such as WBC count, neutrophil count, hemoglobin (Hb) concentration, platelet (PLT) count, C-reactive protein level, erythrocyte sedimentation rate, alanine transaminase level, aspartate transaminase level, and ferritin level, as well as glucocorticoids dosage adjustment (Figure 2A). A significant reduction in the systemic score and HScore was observed after 1 month following the initiation of ruxolitinib treatment (Figure 2B). The glucocorticoid dosage was rapidly decreased without flares, except in 1 case (Figure 2C). The levels of circulating soluble IL-2R and free IL-18 were also significantly decreased (Figure 2D-E). In addition, the cytokine levels associated with neutrophil activation, measured by Olink, including CXCL1, CXCL9, CXCL11, caspase-8, and IL-33, showed a significant decrease (Figure 2F). These findings suggest that ruxolitinib may be a promising therapeutic option for patients with AOSD-MAS.
Ruxolitinib treatment and its impact on alleviating the hyperinflammatory state. (A) Changes in the WBC count, neutrophil count, Hb concentration, PLT count, CRP level, ESR, ALT level, AST level, and ferritin level in patients with AOSD with MAS from baseline. (B) Changes in the systemic score and HScore from baseline. (C) Glucocorticoid-sparing effects of ruxolitinib administration. (D) Comparison of the serum sIL-2R levels after ruxolitinib and those before ruxolitinib. (E) Comparison of the plasma free IL-18 levels after ruxolitinib and those before ruxolitinib. (F) Comparison of the plasma inflammatory proteins levels measured using Olink after ruxolitinib and those before ruxolitinib. ∗P < .05; ∗∗P < .01. ALT, alanine transaminase; AST, aspartate transaminase; CASP-8, caspase-8; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; NPX, normalized protein expression; sIL-2R, soluble IL-2 receptor.
Ruxolitinib treatment and its impact on alleviating the hyperinflammatory state. (A) Changes in the WBC count, neutrophil count, Hb concentration, PLT count, CRP level, ESR, ALT level, AST level, and ferritin level in patients with AOSD with MAS from baseline. (B) Changes in the systemic score and HScore from baseline. (C) Glucocorticoid-sparing effects of ruxolitinib administration. (D) Comparison of the serum sIL-2R levels after ruxolitinib and those before ruxolitinib. (E) Comparison of the plasma free IL-18 levels after ruxolitinib and those before ruxolitinib. (F) Comparison of the plasma inflammatory proteins levels measured using Olink after ruxolitinib and those before ruxolitinib. ∗P < .05; ∗∗P < .01. ALT, alanine transaminase; AST, aspartate transaminase; CASP-8, caspase-8; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; NPX, normalized protein expression; sIL-2R, soluble IL-2 receptor.
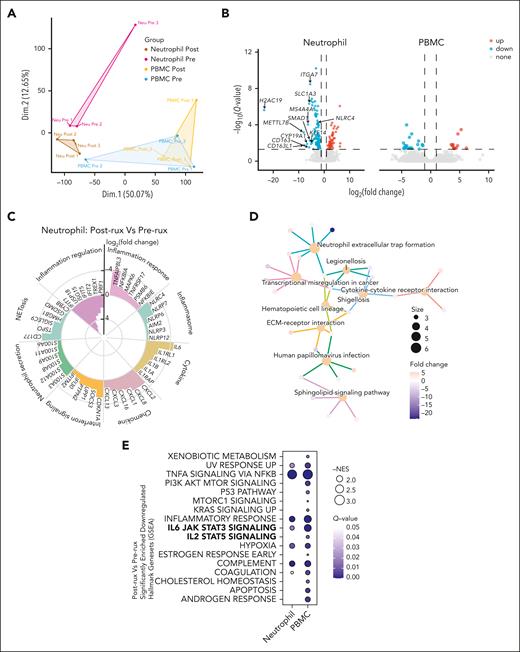
Effects of ruxolitinib on saving neutrophil dysfunction in proinflammatory process
The gene expression profiles of paired neutrophils and PBMCs from 3 patients with AOSD with MAS were analyzed using RNA-seq, both before and 3 months after initiating ruxolitinib treatment. A principal component analysis of bulk RNA-seq data sets from neutrophils and PBMCs revealed distinct clustering based on treatment status, underscoring the significant transcriptional changes (Figure 3A). We identified 160 DEGs in neutrophils, with 112 genes downregulated and 48 genes upregulated, compared with only 31 DEGs in PBMCs (Figure 3B). Genes that are associated with the inflammation response, inflammasome, cytokine, chemokine, IFN-I/II signaling, neutrophil secretion, NETosis, and inflammation regulation exhibited differences before and after treatment (Figure 3C). Kyoto Encyclopedia of Genes and Genomes enrichment analysis revealed that NET formation is among the significantly enriched biological processes (Figure 3D). To identify global changes in gene expression, gene set enrichment analysis was performed, revealing a significant reduction in IL-6/STAT3 signaling in both neutrophils and PBMCs, as well as a significant decrease in IL-2/STAT5 signaling in PBMCs (Figure 3E).
Impact of ruxolitinib on inflammatory signatures in neutrophils and PBMCs revealed by bulk RNA-seq. (A) Principal component analysis plots of bulk RNA-seq data sets of neutrophils and PBMCs in 3 patients with AOSD-MAS. (B) Volcano plot shows the DEGs in neutrophils and PBMCs with after ruxolitinib and before treatment. Red points represent upregulated genes, blue points represent downregulated genes. (C) Circular bar plot displaying the log2(fold change) of different genes involved in the inflammation response, inflammasome, cytokine, chemokine, IFN-I/II signaling, neutrophil secretion, NETosis, and inflammation regulation after ruxolitinib treatment. (D) Genes and Genomes (Kyoto Encyclopedia of Genes and Genomes) pathway enrichment analysis of DEGs in neutrophils after treatment. (E) GSEA analysis showing the significantly downregulated hallmark gene sets in neutrophils and PBMCs after ruxolitinib treatment. ECM, extracellular matrix; NES, normalized enrichment score.
Impact of ruxolitinib on inflammatory signatures in neutrophils and PBMCs revealed by bulk RNA-seq. (A) Principal component analysis plots of bulk RNA-seq data sets of neutrophils and PBMCs in 3 patients with AOSD-MAS. (B) Volcano plot shows the DEGs in neutrophils and PBMCs with after ruxolitinib and before treatment. Red points represent upregulated genes, blue points represent downregulated genes. (C) Circular bar plot displaying the log2(fold change) of different genes involved in the inflammation response, inflammasome, cytokine, chemokine, IFN-I/II signaling, neutrophil secretion, NETosis, and inflammation regulation after ruxolitinib treatment. (D) Genes and Genomes (Kyoto Encyclopedia of Genes and Genomes) pathway enrichment analysis of DEGs in neutrophils after treatment. (E) GSEA analysis showing the significantly downregulated hallmark gene sets in neutrophils and PBMCs after ruxolitinib treatment. ECM, extracellular matrix; NES, normalized enrichment score.
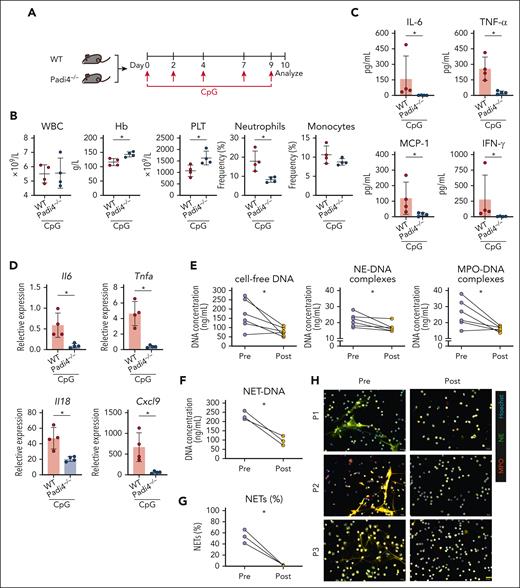
Contribution of ruxolitinib to the decreased formation of NETs in patients with AOSD with MAS
Although MAS has been linked to increased NETosis, its precise role in the pathology remains unclear. Using a CpG-induced murine model (Figure 4A), Padi4–/– (NETosis-deficient) mice exhibited improvements in their Hb levels, PLT counts, and neutrophil frequencies (Figure 4B). NETosis ablation also led to decreased serum levels of inflammatory cytokines, including IL-6, TNF-α, monocyte chemotactic protein-1 (MCP-1), IFN-γ, and CXCL9 (Figure 4C), as well as to reduced liver mRNA expression of Il6, Tnfa, Il18, and Cxcl9 (Figure 4D). To assess ruxolitinib’s potential to block NET formation in patients with AOSD-MAS, serum samples from treated individuals were analyzed and showed decreased circulating NETs markers, including cell-free DNA, MPO-DNA, and NE-DNA levels (Figure 4E). Moreover, the isolated neutrophils from 3 patients demonstrated reduced NETs formation ex vivo (Figure 4F-H). The in vitro assay also demonstrated that ruxolitinib had a direct effect on inhibiting IFN-α/γ–induced NET formation (supplemental Figures 5 and 6). These results indicate that ruxolitinib may inhibit NETosis, thereby contributing to its anti-inflammatory effects in AOSD-MAS.
Improvement of systemic inflammation after NETosis ablation in a secondary HLH model and evaluation of reduced NETs formation after ruxolitinib treatment. (A) Experimental design of the secondary HLH model in which WT or Padi4–/– C57BL/6N mice were injected with CpG on days 0, 2, 4, 7, and 9 with analysis conducted on day 10 after first injection. (B) Changes in the WBC counts, Hb concentration, PLT counts, and frequencies of neutrophils, monocytes, and lymphocytes. (C) Quantified serum levels of IL-6, TNF-α, MCP-1, and IFN-γ. (D) Liver mRNA expression of Il6, Tnfa, Il18, and Cxcl9. The data points represent individual mice from a single experiment. (E) Quantification of circulating NETs, including cell-free DNA, MPO-DNA, and NE-DNA, from serum. (F) Quantification of NET-DNA concentration from neutrophils isolated from 3 patients with AOSD with MAS. (G) Quantification of the percentage of NETs (NE, MPO, and Hoechst-labeled neutrophils/total neutrophils). (H) Representative micrographs showing the immunostainings for the NET marker NE and MPO in neutrophils. ∗P < .05. Scale bar, 20 μm. MPO, myeloperoxidase; NE, neutrophil elastase.
Improvement of systemic inflammation after NETosis ablation in a secondary HLH model and evaluation of reduced NETs formation after ruxolitinib treatment. (A) Experimental design of the secondary HLH model in which WT or Padi4–/– C57BL/6N mice were injected with CpG on days 0, 2, 4, 7, and 9 with analysis conducted on day 10 after first injection. (B) Changes in the WBC counts, Hb concentration, PLT counts, and frequencies of neutrophils, monocytes, and lymphocytes. (C) Quantified serum levels of IL-6, TNF-α, MCP-1, and IFN-γ. (D) Liver mRNA expression of Il6, Tnfa, Il18, and Cxcl9. The data points represent individual mice from a single experiment. (E) Quantification of circulating NETs, including cell-free DNA, MPO-DNA, and NE-DNA, from serum. (F) Quantification of NET-DNA concentration from neutrophils isolated from 3 patients with AOSD with MAS. (G) Quantification of the percentage of NETs (NE, MPO, and Hoechst-labeled neutrophils/total neutrophils). (H) Representative micrographs showing the immunostainings for the NET marker NE and MPO in neutrophils. ∗P < .05. Scale bar, 20 μm. MPO, myeloperoxidase; NE, neutrophil elastase.
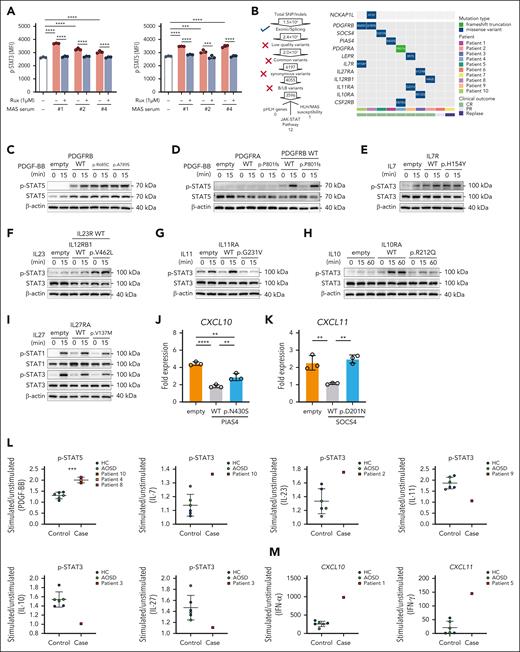
Identification and functional characterization of JAK-STAT pathway variants in patients with MAS
STAT3/STAT5 signaling was downregulated following ruxolitinib treatment (Figure 3E). To confirm that ruxolitinib effectively suppressed the downstream signaling, phosphorylation of STAT3 and STAT5 was assessed in WBCs from healthy donors that were pretreated in vitro with ruxolitinib, followed by stimulation with serum from 3 patients previously analyzed by RNA-seq. Ruxolitinib significantly attenuated STAT3 and STAT5 phosphorylation induced by the patient serum (Figure 5A). WES of DNA samples were analyzed from these 10 patients to identify HLH/MAS-related variants (from 2022 ACR/EULAR21) and variants in the JAK-STAT pathway, and we identified 1 variant in NCKAP1L (patient 8) and 12 variants in genes associated with the JAK-STAT pathway, including PDGFRB, SOCS4, PIAS4, PDGFRA, LEPR, IL17R, IL27RA, IL12RB1, IL11RA, and CSF2RB (Figure 5B). The Sanger sequencing chromatograms of these variants and their distribution across different locations and the key functional domains involved are shown in supplemental Figure 6. Expanding the cohort to 195 patients with AOSD, including 41 MAS cases (15 refractory and 26 nonrefractory), revealed a higher prevalence of JAK-STAT pathway variants in patients with MAS, particularly refractory MAS, however, this was not statistically significant (supplemental Figure 7A-B). Among 13 patients with MAS treated with ruxolitinib, all 10 with JAK-STAT variants achieved CR, whereas only 1 of 3 without such variants achieved PR and 2 experienced relapse (supplemental Figure 7C-E; supplemental Table 3). Although these variants were more common in complete responders, this association does not imply causation and requires further investigation.
WES of DNA samples and functional assays to identify variations in the JAK-STAT pathway. (A) The inhibitory effect of Rux on p-STAT3 and p-STAT5 in WBCs from healthy donors following stimulation with patient serum. The findings were confirmed in 3 independent experiments using cells from different donors. (B) Filtering method for pHLH, MAS/HLH susceptibility, and JAK-STAT pathway variation and specific variation of the MAS/HLH susceptibility and JAK-STAT pathway in 10 patients. (C-D) Representative immunoblot of STAT5 phosphorylation in transfected 293T cells treated with PBS or PDGF-BB (100 ng/mL) for 15 minutes. (E) Representative immunoblot of STAT3 phosphorylation in transfected 293T cells treated with PBS or IL-7 (100 ng/mL) for 15 minutes. (F) Representative immunoblot of STAT3 phosphorylation in transfected 293T cells treated with PBS or IL-23 (100 ng/mL) for 15 minutes. (G) Representative immunoblot of STAT3 phosphorylation in transfected 293T cells treated with PBS or IL-11 (100 ng/mL) for 15 minutes. (H) Representative immunoblot of STAT3 phosphorylation in transfected 293T cells treated with PBS or IL-10 (100 ng/mL) for 15 or 60 minutes. (I) Representative immunoblot of STAT1 and STAT3 phosphorylation in transfected 293T cells treated with PBS or IL-27 (100 ng/mL) for 15 minutes. (J) Quantitative PCR (qPCR) of CXCL10 in 293T cells transfected with WT or mutant PIAS4 plasmids and stimulated with IFN-γ (50 ng/mL) for 8 hours. (K) qPCR of CXCL11 in 293T cells transfected with WT or mutant SOCS4 plasmids and stimulated with IFN-α (1000 U/mL) for 8 hours. (L) The effect of activation of p-STAT3/5 on WBCs from patients and controls upon stimulation. (M) The effect of upregulation of CXCL10/CXCL11 in WBCs from patients and controls upon stimulation. ∗∗P < .01; ∗∗∗∗P < .0001. HC, healthy control; MFI, median fluorescence intensity; PBS, phosphate-buffered saline; Rux, ruxolitinib.
WES of DNA samples and functional assays to identify variations in the JAK-STAT pathway. (A) The inhibitory effect of Rux on p-STAT3 and p-STAT5 in WBCs from healthy donors following stimulation with patient serum. The findings were confirmed in 3 independent experiments using cells from different donors. (B) Filtering method for pHLH, MAS/HLH susceptibility, and JAK-STAT pathway variation and specific variation of the MAS/HLH susceptibility and JAK-STAT pathway in 10 patients. (C-D) Representative immunoblot of STAT5 phosphorylation in transfected 293T cells treated with PBS or PDGF-BB (100 ng/mL) for 15 minutes. (E) Representative immunoblot of STAT3 phosphorylation in transfected 293T cells treated with PBS or IL-7 (100 ng/mL) for 15 minutes. (F) Representative immunoblot of STAT3 phosphorylation in transfected 293T cells treated with PBS or IL-23 (100 ng/mL) for 15 minutes. (G) Representative immunoblot of STAT3 phosphorylation in transfected 293T cells treated with PBS or IL-11 (100 ng/mL) for 15 minutes. (H) Representative immunoblot of STAT3 phosphorylation in transfected 293T cells treated with PBS or IL-10 (100 ng/mL) for 15 or 60 minutes. (I) Representative immunoblot of STAT1 and STAT3 phosphorylation in transfected 293T cells treated with PBS or IL-27 (100 ng/mL) for 15 minutes. (J) Quantitative PCR (qPCR) of CXCL10 in 293T cells transfected with WT or mutant PIAS4 plasmids and stimulated with IFN-γ (50 ng/mL) for 8 hours. (K) qPCR of CXCL11 in 293T cells transfected with WT or mutant SOCS4 plasmids and stimulated with IFN-α (1000 U/mL) for 8 hours. (L) The effect of activation of p-STAT3/5 on WBCs from patients and controls upon stimulation. (M) The effect of upregulation of CXCL10/CXCL11 in WBCs from patients and controls upon stimulation. ∗∗P < .01; ∗∗∗∗P < .0001. HC, healthy control; MFI, median fluorescence intensity; PBS, phosphate-buffered saline; Rux, ruxolitinib.
To evaluate the functional impact of the identified genetic variants in these 10 patients, we conducted in vitro experiments using transfected 293T cells. The PDGFRB variants R685C and A789S exhibited constitutive STAT5 phosphorylation independent of ligand stimulation, a phenotype not observed with WT PDGFRB (Figure 5C). Furthermore, co-expression of the PDGFRA P801fs variant with WT PDGFRB enhanced STAT5 phosphorylation after platelet-derived growth factor-BB (PDGF-BB) stimulation when compared with WT PDGFRA co-expression (Figure 5D). The IL7R p.H154Y variant displayed increased responsiveness to IL-7 when compared with the WT (Figure 5E). In addition, cells that coexpressed IL12RB1 p.V462L and WT IL23R demonstrated enhanced STAT5 phosphorylation upon IL-23 stimulation (Figure 5F). IL11RA p.G231V and IL10RA p.R212Q altered STAT3 phosphorylation following IL-11 and IL-10 stimulation, respectively (Figure 5G-H). Conversely, IL27RA p.V137M significantly reduced STAT1 and STAT3 phosphorylation upon IL-27 stimulation when compared with WT IL27RA (Figure 5I). Clinically, the patient who carried IL27RA p.V137M had high titers of Epstein-Barr virus (EB) viral capsid antigen immunoglobulin G (IgG) and elevated EB IgM and was EB early antigen IgG negative and EB nuclear antigen IgG positive, suggesting a possible increased susceptibility to MAS triggered by EB caused by the IL27RA dysfunction. In addition, the PIAS4 p.N430S variant demonstrated reduced capability to suppress IFN-γ–induced CXCL10 expression when compared with WT PIAS4 (Figure 5J), correlating with elevated serum CXCL10 levels in the patient who harbored this variant. Similarly, the SOCS4 p.D201N variant led to diminished suppression of IFN-α2–induced CXCL11 expression relative to WT SOCS4, aligning with the elevated CXCL11 observed clinically (Figure 5K). Furthermore, WBCs from patients with MAS who carried these variants exhibited distinct p-STAT and CXCL signaling upon stimulation when compared with controls (Figure 5L-M).
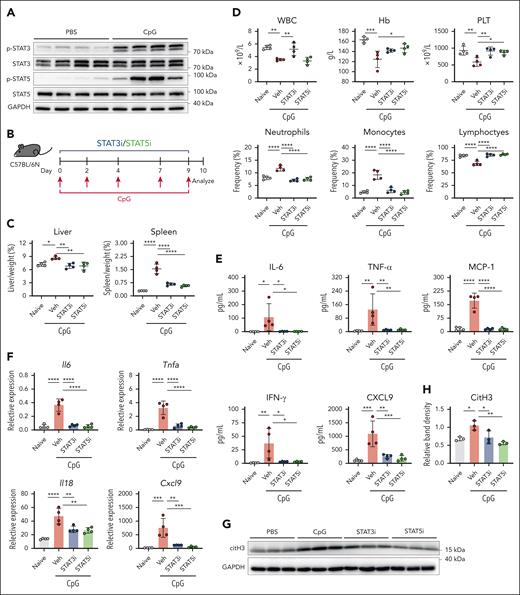
Selective STAT3 or STAT5 inhibition lessens disease manifestations in a model of secondary HLH
Increased phosphorylation levels of STAT3 and STAT5 were observed in liver tissue from the secondary HLH model (Figure 6A). To evaluate their pathogenic roles, mice were treated with STAT3-IN-1 (STAT3i), STAT5-IN-2 (STAT5i), or vehicle control (Figure 6B). Both inhibitors alleviated hepatosplenomegaly and improved the PLT counts and neutrophil, monocyte, and lymphocyte frequencies (Figure 6C-D). The STAT3i restored WBC counts, whereas the STAT5i improved Hb levels. In addition, inhibiting STAT3/STAT5 reduced the serum levels of IL-6, TNF-α, MCP-1, IFN-γ, and CXCL9 (Figure 6E) and decreased the liver mRNA expression levels of Il6, Tnfa, Il18, and Cxcl9 (Figure 6F). Furthermore, mice treated with STAT3i and STAT5i had reduced citrullinated histone 3 (citH3) expression in their livers (Figure 6G-H). These findings underscore the pathogenic role of STAT3 and STAT5 in secondary HLH.
Improvement in systemic inflammation after selective inhibition of STAT3 or STAT5 in the secondary HLH model. (A) Immunoblot analysis of phosphorylated STAT3 and STAT5 in liver tissue. (B) Experimental design for the secondary HLH model in which WT C57BL/6N mice were injected with CpG on days 0, 2, 4, 7, and 9 and with analysis conducted on day 10 after the first injection. (C) Liver and spleen weight normalized to body weight. (D) Changes in WBC counts, Hb concentration, PLT counts, and the frequencies of neutrophils, monocytes, and lymphocytes. (E) Quantified serum levels of IL-6, TNF-α, MCP-1, IFN-γ, and CXCL9. (F) Liver mRNA expression of Il6, Tnfa, Il18, and Cxcl9. The data points represent individual mice from a single experiment. (G) Immunoblot of the NET marker citH3 in the liver. (H) Densitometric analysis of citH3/GAPDH in panel G. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; Veh, vehicle.
Improvement in systemic inflammation after selective inhibition of STAT3 or STAT5 in the secondary HLH model. (A) Immunoblot analysis of phosphorylated STAT3 and STAT5 in liver tissue. (B) Experimental design for the secondary HLH model in which WT C57BL/6N mice were injected with CpG on days 0, 2, 4, 7, and 9 and with analysis conducted on day 10 after the first injection. (C) Liver and spleen weight normalized to body weight. (D) Changes in WBC counts, Hb concentration, PLT counts, and the frequencies of neutrophils, monocytes, and lymphocytes. (E) Quantified serum levels of IL-6, TNF-α, MCP-1, IFN-γ, and CXCL9. (F) Liver mRNA expression of Il6, Tnfa, Il18, and Cxcl9. The data points represent individual mice from a single experiment. (G) Immunoblot of the NET marker citH3 in the liver. (H) Densitometric analysis of citH3/GAPDH in panel G. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; Veh, vehicle.
Discussion
MAS represents the most life-threatening complication of AOSD with a poor prognosis, therefore necessitating aggressive treatment. The management of HLH/MAS often relies on high-dose corticosteroids, although there have been reports of fatalities among patients who received massive doses.22 Alternative treatments, such as IV immunoglobulin, cyclophosphamide, plasma exchange, and etoposide, have shown variable efficacy. As a typical cytokine storm syndrome, HLH/MAS presents opportunities for biologic interventions that target IL-1β, IL-6, and TNF. However, these approaches have limitations, particularly in patients with poor response or tolerance to biologics.23 Moreover, biologic agents that target TNF or IL-1 can enhance type I IFN and interferon-stimulated genes (ISGs) expressions, whereas targeting IL-1 or IL-6 may disrupt immune cell differentiation, thereby potentially exacerbating hyperinflammation through dysregulated IL-18 expression.24-26 IFN-γ is central to MAS pathology, and therapies that block the IFN-γ signaling pathway offer promising alternatives. Despite limited reports on the safety and efficacy of ruxolitinib in AOSD-MAS, our study explored its potential as a therapeutic option.
It is worth noting that among the subtypes of HLH, significant differences in neutrophil involvement have been observed.27 Unlike other HLH subtypes, patients with MAS typically maintained normal neutrophil counts during initial testing.27 Notably, an elevated neutrophil-to-lymphocyte ratio has been observed in patients with AOSD-MAS,28 potentially suggesting a pivotal role for neutrophils in MAS. MAS can be experimentally induced in mice through repeated administration of CpG with the hyperstimulation of innate immune cells being sufficient to trigger a cytokine storm.29 Secondary HLH models demonstrated elevated neutrophils and monocytes, and treatment with itacitinib significantly reduced the neutrophil counts, thereby improving survival and ameliorating clinical scores.30 In murine models of primary HLH (pHLH), the protective effect of ruxolitinib could be mimicked by transient treatment with IFN-γ neutralization and a neutrophil-depleting antibody.12 Recent evidence suggests that ruxolitinib reprograms myeloid cells, especially neutrophils.31 Moreover, previous studies have reported elevated NETosis markers in AOSD-MAS,32 whereas inhibition of NETosis or neutrophil depletion protected mice from tissue damage and hyperinflammation.20 Our findings further support the involvement of neutrophils in MAS with ruxolitinib demonstrating efficacy in reducing neutrophil activation and resolving inflammatory features in patients with refractory MAS. In addition, ruxolitinib inhibits STAT3 and STAT5 signaling, and selective inhibition of either STAT3 or STAT5 leads to the resolution of inflammation and a reduction in NETosis markers. These results highlight the potential of ruxolitinib to regulate neutrophil activity and provide therapeutic benefit in AOSD-MAS.
However, although our study focused on neutrophils, it is essential to consider the broader immune network in MAS, particularly the interplay between antigen-presenting cells and T/natural killer cells, which are central to MAS pathogenesis.33 In peripheral T-cell lymphoma with a follicular helper T-cell phenotype, hyperactivation of granulocytes is mediated by autoregulatory cytokine loops involving IFN-γ (from CD4+ T cells) and IL-6 (from activated granulocytes).34 These cytokine interactions activate the JAK-STAT pathway in both granulocytes and T cells, thereby fueling the inflammatory response and demonstrating the interaction between neutrophils and other cells. A recent study has suggested that monocytes may serve as a potential therapeutic target in AOSD and MAS.35 Ruxolitinib, for example, has demonstrated the ability to suppress numerous immune cell populations, including CD8+ T cells, monocytes, and neutrophils, in pHLH.12 Thus, further studies are needed to elucidate the distinct contributions of various immune cells to MAS pathogenesis and to clarify the differential impact of ruxolitinib on specific immune cell compartments.
We also observed that genes in the vascular endothelial growth factor (VEGF) and MAPK pathways were elevated in some individuals with MAS. VEGF, a marker of disease activity in AOSD, is closely linked to inflammatory cytokines and MAPK signaling.36-38 MAPK inhibitors are notably effective in Langerhans cell histiocytosis and provide rapid responses in patients who need urgent intervention.39 In 1 study, patients with Langerhans cell histiocytosis and HLH/MAS who were treated with dabrafenib showed prompt symptom resolution and lower toxicity than those treated with second-line chemotherapy with a significantly higher 4-year progression-free survival rate, especially because most patients failed to achieve HLH/MAS control after an induction treatment.40 In addition, MAPK signaling synergizes with IFN-γ to enhance ISG expression41 and induces p38 MAPK-dependent phosphorylation of STAT1 at Ser727.42 Hence, targeting VEGF or MAPK may represent a novel therapeutic approach for HLH/MAS.
Currently, >100 genes related to HLH have been identified, and 11 pathogenic genes are relatively well understood.43 However, why certain individuals with autoimmune conditions progress to MAS remains poorly understood. WES revealed overlap between MAS in sJIA and pHLH,44 yet, recent studies suggested that HLH-associated variants may influence the pathophysiology of sJIA even in the absence of MAS.45 This adds complexity to the underlying genetic backgrounds. In a study of pediatric HLH, variants linked to dysregulated immune activation or proliferation were predominantly observed in individuals with rheumatologic triggers.46 Furthermore, rheumatologic triggers were more prevalent in elder subjects, indicating a correlation between genetic findings, rheumatologic triggers, and disease onset.46 So, interpreting the detected variants involves complex and evolving considerations.21
Ruxolitinib has been successfully used in patients with gain-of-function STAT mutations that are associated with autoimmunity and immune dysregulation.47-51 In our study, we found 10 variants that affected protein function in 9 genes in the JAK-STAT pathway (PDGFRB, PDGFRA, SOCS4, PIAS4, IL7R, IL27RA, IL12RB1, IL11RA, and IL10RA), which were previously unrevealed in MAS (Table 2). However, AOSD is a rare disease, making AOSD-MAS even rarer, with an occurrence rate of only 12% to 14% among patients with AOSD.52,53 Only a few genetic studies have focused on AOSD-MAS, and the largest study to date that investigated gene variations in sJIA-MAS included only 32 participants.45 Similarly, our study was limited by a small cohort size, making it difficult to draw definitive conclusions about the role of JAK/STAT variants and their association with clinical outcomes. Validating these potential risk alleles for MAS would require significantly larger studies, and establishing their predictive value for JAKi responsiveness would demand even greater sample sizes. Given the complexity of the genetic influences in MAS, the potential benefit of ruxolitinib in patients with MAS based on their genotypes remains a topic of debate. Further functional studies and larger cohorts are required to establish their relevance. At this stage, ruxolitinib should still be considered a salvage therapy for refractory HLH/MAS based on clinical indications54 rather than genetic findings alone.
Presumed functions and clinical relevance of the identified JAK-STAT pathway genes in HLH/MAS
| Gene . | Cytokines . | Presumed functions and clinical relevance in HLH/MAS . |
|---|---|---|
| PDGFRA | PDGF | PDGF is crucial for cell proliferation, survival, differentiation, chemotaxis, and migration.55,56 |
| PDGFRB | PDGF | PDGF is crucial for cell proliferation, survival, differentiation, chemotaxis, and migration.55,56 PDGFRB fusion is linked to chronic myeloproliferative disorders.56 |
| SOCS4 | IFN | SOCS4 inhibits IFN signaling.57 |
| PIAS4 | IFN | PIAS4 inhibits IFN signaling.58 |
| IL7R | IL-7 | IL-7 is induced by IFN-γ signaling.59 IL-7/IL-7R–mediated signaling disorder is associated with immunopathology.60 IL7R deficiency is combined with severe combined immunodeficiency.61 IL7R mutational activation predicts sensitivity to JAK inhibition.62 |
| IL27RA | IL-27 | IL-27 plays a critical role in immunity to EBV, revealed by a recent study on IL-27RA deficiency.63 |
| IL12RB1 | IL-12, IL-23 | IL-23 is implicated in the pathogenesis of IBD.64 IL-23 is involved in neutrophilic inflammation.65 |
| IL11RA | IL-11 | IL-11 protects cells from IFN-γ induced damage.66 |
| IL10RA | IL-10 | IL-10 protects cells from IFN-γ induced damage.67 IL10RA mutation is known for its role in IBD.68 |
| Gene . | Cytokines . | Presumed functions and clinical relevance in HLH/MAS . |
|---|---|---|
| PDGFRA | PDGF | PDGF is crucial for cell proliferation, survival, differentiation, chemotaxis, and migration.55,56 |
| PDGFRB | PDGF | PDGF is crucial for cell proliferation, survival, differentiation, chemotaxis, and migration.55,56 PDGFRB fusion is linked to chronic myeloproliferative disorders.56 |
| SOCS4 | IFN | SOCS4 inhibits IFN signaling.57 |
| PIAS4 | IFN | PIAS4 inhibits IFN signaling.58 |
| IL7R | IL-7 | IL-7 is induced by IFN-γ signaling.59 IL-7/IL-7R–mediated signaling disorder is associated with immunopathology.60 IL7R deficiency is combined with severe combined immunodeficiency.61 IL7R mutational activation predicts sensitivity to JAK inhibition.62 |
| IL27RA | IL-27 | IL-27 plays a critical role in immunity to EBV, revealed by a recent study on IL-27RA deficiency.63 |
| IL12RB1 | IL-12, IL-23 | IL-23 is implicated in the pathogenesis of IBD.64 IL-23 is involved in neutrophilic inflammation.65 |
| IL11RA | IL-11 | IL-11 protects cells from IFN-γ induced damage.66 |
| IL10RA | IL-10 | IL-10 protects cells from IFN-γ induced damage.67 IL10RA mutation is known for its role in IBD.68 |
EBV, Epstein-Barr virus; IBD, inflammatory bowel disease; IL-7R, IL-7 receptor.
Several other limitations should be acknowledged. First, although we observed good tolerability of ruxolitinib based on our experience, it is necessary to obtain more long-term data to validate its overall safety profile, especially in the context of AOSD-MAS. Second, our findings need to be verified in well-designed trials to minimize potential bias associated with patient selection, the influence of previous and ongoing treatments, and the absence of randomization in the study design.
In summary, neutrophil activation via the JAK-STAT pathway seems to be an added contributor in refractory AOSD-MAS, and ruxolitinib emerged as a promising therapeutic option for these patients. Future studies should explore the broader immune landscape of MAS and validate the potential of ruxolitinib and other targeted therapies in larger patient cohorts to establish their role in clinical practice.
Acknowledgments
The authors thank all members of the Department of Rheumatology and Immunology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, for their valuable and critical comments.
This research was supported by the National Natural Science Foundation of China (grants 82471816, 82201969, and 82171769), Shanghai “Rising Stars of Medical Talents” Youth Development Program (grant RC20220021), Special Project for Clinical Research in the Health Industry of the Shanghai Municipal Health Commission (grant 20224Y0069), Shanghai Sailing Program (grants 22YF1425600 and 23YF1424600), Chenguang Program of Shanghai Education Development Foundation, Shanghai Municipal Education Commission (grant 22CGA14), Postdoctoral Research Foundation of China (grant 2023M732305), and Shanghai Pujiang Young Rheumatologists Training program (grants SPROG2109, SPROG2209, and SPROG2304).
Authorship
Contribution: Q.H., J.J., Y. Sun, and J.T. were responsible for study conception and design; Y.M., X. Chen, M.W., J.M., D.Z., L.C., Y.X., D.Y., H.S., H.L., X. Cheng, Y. Su, J.Y., H.C., Z.Z., T.L., C.Y., J.T., J.J., and Q.H. provided study materials or patients; Y.M., X. Chen, M.W., and J.J. collected and assembled the data; Y.M., X. Chen, and M.W. conducted the data analysis and interpretation; Y.M., X. Chen, J.J., and Q.H. wrote the manuscript; and all authors approved the final manuscripts and are accountable for all aspects of the work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Qiongyi Hu, Department of Rheumatology and Immunology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, No. 197 Ruijin Second Rd, Shanghai 200025, China; email: huqiongyi131@163.com; Jinchao Jia, Department of Rheumatology and Immunology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, No. 197 Ruijin Second Rd, Shanghai 200025, China; email: jiajinchao15@163.com; Yue Sun, Department of Rheumatology and Immunology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, No. 197 Ruijin Second Rd, Shanghai 200025, China; email: sy11953@rjh.com.cn; and Jialin Teng, Department of Rheumatology and Immunology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, No. 197 Ruijin Second Rd, Shanghai 200025, China; email: tengteng8151@sina.com.
References
Author notes
Y.M., X. Chen, and M.W. contributed equally to this study.
The data sets that support the conclusions of this article are available in the Genome Sequence Archive Human database (accession number HRA011417). The processed gene expression matrices have been deposited in OMIX (accession number OMIX009959). The accession codes of the published data used in this study are as follows: RNA sequencing data of neutrophils from 6 patients with adult-onset Still disease were downloaded from the Genome Expression Omnibus (accession number GSE150996).
Original data are available on request from the corresponding author, Qiongyi Hu (huqiongyi131@163.com).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal