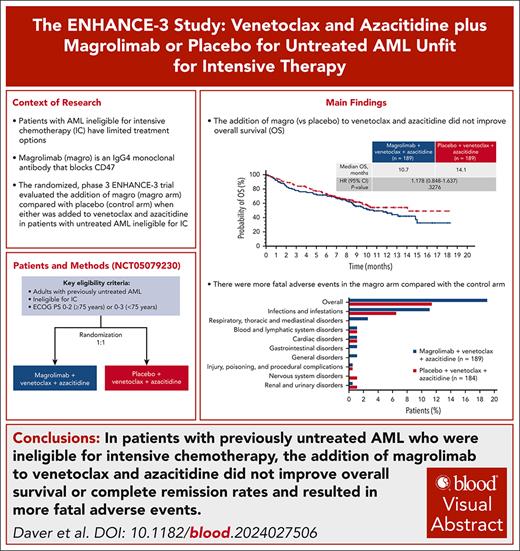
Key Points
Addition of magrolimab to venetoclax and azacitidine did not improve OS in patients with untreated AML ineligible for IC.
This study highlights the challenges of improving outcomes in AML and can help inform future development of anti-CD47 therapies.
Visual Abstract
Patients with acute myeloid leukemia (AML) ineligible for intensive chemotherapy (IC) have limited treatment options. The phase 3 ENHANCE-3 study aimed to determine whether magrolimab (magrolimab arm) was superior to placebo (control arm) when either was combined with venetoclax and azacitidine. Adults with previously untreated AML who were ineligible for IC were randomized to receive magrolimab (1 mg/kg on days 1 and 4, 15 mg/kg on day 8, 30 mg/kg on days 11 and 15, then weekly for 5 weeks, and then every 2 weeks) or placebo, venetoclax (100 mg on day 1, 200 mg on day 2, and 400 mg daily thereafter), and azacitidine (75 mg/m2 days 1-7) in 28-day cycles. The primary end point was overall survival (OS); key secondary end points included complete remission (CR) rate and safety. After randomization of 378 patients, the trial was stopped at a prespecified interim analysis owing to futility. At final analysis, with median follow-up of 7.6 months (magrolimab arm) vs 7.4 months (control arm), median OS was 10.7 vs 14.1 months (hazard ratio, 1.178; 95% confidence interval, 0.848-1.637). The CR rate within 6 cycles was 41.3% vs 46.0%. Addition of magrolimab to venetoclax and azacitidine resulted in more fatal adverse events (19.0% vs 11.4%), primarily driven by grade 5 infections (11.1% vs 6.5%) and respiratory events (2.6% vs 0%). There were similar incidences of any-grade infections, febrile neutropenia, and neutropenia between arms. These results highlight the difficulty in improving outcomes for patients with AML who were ineligible for IC. This trial was registered at www.clinicaltrials.gov as #NCT05079230.
Introduction
Intensive chemotherapy (IC) continues to form the backbone of most first-line treatment strategies for medically fit patients with newly diagnosed, previously untreated acute myeloid leukemia (AML).1 However, patients may not be candidates for this approach owing to age or comorbidities because AML primarily affects older individuals aged 69 years or older.2 In real-world practice, only approximately half of patients with AML receive first-line treatment with IC.3
For patients who are ineligible for IC, venetoclax paired with a hypomethylating agent (HMA; eg, azacitidine) is a standard-of-care frontline treatment option.4 Although combining venetoclax and HMAs has improved outcomes, most patients do not achieve long-term survival. In the phase 3 VIALE-A trial comparing venetoclax and azacitidine with azacitidine alone in chemotherapy-ineligible, previously untreated AML, the venetoclax/azacitidine arm showed a median overall survival (OS) of 14.7 months and an estimated 2-year OS rate of 37.5%; in comparison, the azacitidine control arm had a median OS of 9.6 months and an estimated 2-year OS rate of 16.9%.5,6 Outcomes are worse for AML with adverse cytogenetic profiles and/or the presence of specific gene mutations (eg, TP53, N/K-RAS, FLT3-ITD).7-9
Novel combination therapies based on effective agents that can be safely combined with venetoclax and azacitidine may help to improve outcomes in this population. The monoclonal antibody magrolimab targets CD47, an antiphagocytic signal present on cancer cells.10,11 In preclinical studies, magrolimab combined with venetoclax and azacitidine enhanced phagocytosis and promoted cancer cell death more than venetoclax and azacitidine alone.11,12 In early-stage clinical studies, magrolimab combined with azacitidine or venetoclax/azacitidine demonstrated encouraging efficacy and an acceptable safety profile, even in patients with TP53 mutations.13,14
ENHANCE-3, a randomized phase 3 study, was designed to determine whether magrolimab plus venetoclax and azacitidine was superior to placebo plus venetoclax and azacitidine in patients with previously untreated AML ineligible for IC. Herein, we present results from the ENHANCE-3 final analysis.
Methods
Patients
Previously untreated patients with histologically confirmed AML per 2016 World Health Organization criteria and who were ineligible for IC (including a standard cytarabine and anthracycline induction regimen) owing to age or comorbidity were included in the study. Ineligibility for IC was defined as being (1) ≥75 years of age or (2) 18 to 74 years of age with ≥1 of the following comorbidities: an Eastern Cooperative Oncology Group performance status (ECOG PS) score of 2 or 3, diffusing capacity of the lungs for carbon monoxide ≤65% or forced expiratory volume in 1 second ≤65%, left ventricular ejection fraction ≤50%, baseline creatinine clearance ≥30 mL/min to <45 mL/min, hepatic disorder with total bilirubin >1.5× upper limit of normal, or any other comorbidity that the investigator judged to be incompatible with IC. Patients ≥75 years of age needed an ECOG PS score of 0 to 2. All patients were required to have hemoglobin ≥9 g/dL before treatment (which could be achieved with red blood cell [RBC] transfusions). Patients were excluded if they received previous anti-AML therapy (eg, HMAs, low-dose cytarabine, and/or venetoclax). Full eligibility criteria are in the protocol (supplemental Appendix, available on the Blood website).
Study design and treatments
In this phase 3, randomized, double-blind, placebo-controlled, multicenter study, eligible patients were randomly assigned 1:1 to receive magrolimab plus venetoclax and azacitidine (magrolimab arm) or placebo plus venetoclax and azacitidine (control arm) (supplemental Figure 1). Randomization was stratified by age (<75 or ≥75 years), genetic risk group per European LeukemiaNet (ELN) 2017 criteria (favorable/intermediate, adverse, or unknown), and geographic region (United States or ex-United States).
Treatment was administered in 28-day cycles. Magrolimab 1 mg/kg priming doses were administered IV on days 1 and 4. After priming, magrolimab was escalated to 15 mg/kg on day 8, then 30 mg/kg on days 11 and 15, and then weekly for 5 doses; 30 mg/kg maintenance doses were then administered every 2 weeks beginning 1 week after the fifth weekly dose. All patients received oral venetoclax 100 mg on day 1, 200 mg on day 2, and 400 mg on day 3 and daily thereafter. Dose interruptions of venetoclax were allowed to manage myelosuppression after marrow remission. All patients received subcutaneous or intravenous azacitidine 75 mg/m2 on days 1 to 7 or on days 1 to 5 and days 8 and 9. Patients continued study treatment until disease progression, relapse, loss of clinical benefit (investigator determination), unacceptable toxicities, or other study discontinuation criteria were met.
To mitigate anemia (a known effect of anti-CD47 therapy), documented hemoglobin ≥9 g/dL (based on complete blood count results) before each of the first 2 doses of magrolimab or placebo was required. Hemoglobin monitoring was also required 3 to 6 hours after the initiation of the first and second doses of magrolimab or placebo, with RBC transfusion when clinically appropriate. Dose modifications/delays were permitted to manage anemia and other treatment-emergent adverse events (TEAEs). Patients with prolonged (2-4 weeks) delays of magrolimab or placebo doses were required to undergo repriming. Patients who had treatment delays owing to cytopenia after achieving complete remission (CR) could continue scheduled magrolimab/placebo even if azacitidine/venetoclax was delayed for count recovery.
The study (ClinicalTrials.gov identifier: NCT05079230) was conducted in accordance with the International Conference on Harmonisation Good Clinical Practice guidelines, the Declaration of Helsinki, and local authority requirements. Institutional review boards and independent ethics committees approved the study at each site. All patients provided a written informed consent before study entry.
Procedures
Clinical response assessments were conducted by investigators using ELN 2017 recommendations for AML and 2003 and 2006 International Working Group criteria with modifications.15-17 Bone marrow (BM) assessments (including aspirate and/or core/trephine biopsy) were required for response evaluation. In addition, BM specimens were used for biomarker studies, minimal residual disease (MRD) monitoring, exploratory evaluations, and biobanking. MRD testing using a flow cytometry–based assay (lower level of detection = 0.1%) with a leukemia-associated immunophenotype approach was performed by a central laboratory (Labcorp); MRD negativity was defined as AML blast <0.1%. BM aspirate for response assessment (including MRD) and cytogenetics was collected at screening; on day 28 (±1 week for cycles 1 and 2) of cycles 1, 2, 4, and 6; and then every 3 cycles thereafter (response/cytogenetics only). Conventional cytogenetics were tested per institutional standards.
Safety evaluation included TEAE incidence, clinical laboratory test findings, physical examination, and vital signs measurements. TEAEs were documented and classified using the Medical Dictionary for Regulatory Activities, and severities of toxicities were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events v5.0.
Outcomes
The primary end point was OS, measured from the date of randomization to the date of death from any cause.
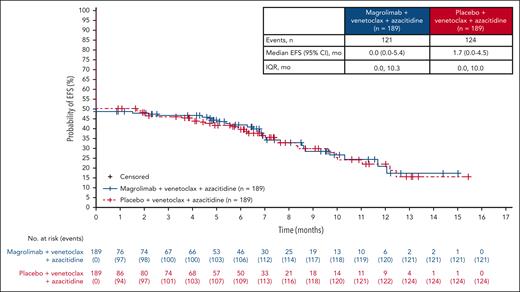
Secondary end points were the rate of CR, rate of CR + CR with partial hematologic recovery (CRh), duration of CR, duration of CR + CRh, rate of CR without MRD, and rate of CR/CRh without MRD. These response end points were assessed as the proportion of patients who achieved the relevant response category within 6 treatment cycles. Patients without a response assessment were assigned a best overall response of “No assessment.” Other secondary end points included event-free survival (EFS; time from randomization to the earliest of relapse from CR, failure to achieve CR within 6 cycles, or death), RBC transfusion independence conversion rate, and the incidence of TEAEs and clinical laboratory abnormalities during the study. In addition, the composite CR rate (CR, CRh, or CR with incomplete hematologic recovery) was analyzed.
Exploratory analyses
Targeted sequencing on the biomarker-evaluable population (BEP) was performed at 2 central laboratories (Therapy Acceleration Laboratory and MRC Molecular Haematology Unit, Oxford University; HOVON, Erasmus MC Cancer Institute) on DNA isolated from pretreatment BM or peripheral blood mononuclear cells of 244 patients for whom samples from diagnosis were sent. Variants from 37 genes were reported at a minimum variant allele frequency of 1%. OS was analyzed according to subgroups per the molecular signature developed for OS risk stratification after treatment with venetoclax/azacitidine as published by Döhner et al.8 This molecular signature stratifies patients by NRAS, KRAS, FLT3-ITD, and TP53 into 3 distinct median OS groups: lower-benefit (TP53 mutations), intermediate-benefit (wild-type TP53; NRAS, KRAS, and/or FLT3-ITD mutations), and higher-benefit (all other patients).8
Statistical analysis
All randomized patients (intent-to-treat [ITT] population) were included in efficacy analyses; patients who received ≥1 dose of any study treatment were included in safety analyses. For the primary end point of OS, it was estimated that 303 events among 432 patients would provide 86.4% power to detect a hazard ratio (HR) of 0.7 at a 1-sided significance level of 0.025. Two interim analyses for OS were planned: (1) when ∼121 deaths (40% of the expected 303 deaths) had occurred and (2) when ∼227 deaths (75% of the expected 303 deaths) had occurred. Both interim analyses included planned nonbinding futility analyses, with futility boundaries determined by a gamma beta–spending function with parameter −5 (first interim analysis, futility boundary of HR = 1.1; second interim analysis, futility boundary of HR = 0.9). The final analysis was planned to occur after all patients completed the study.
For OS, the HR between treatment arms and its 95% confidence interval (CI) was estimated using the Cox proportional hazards regression model stratified by the stratification factors at randomization. The median OS and EFS and their 95% CIs were estimated using the Kaplan-Meier method.
For response rates, comparisons between treatment arms were made using the Cochran-Mantel-Haenszel test, and the odds ratio (OR) comparing the 2 arms was adjusted for the stratification factors at randomization. The Kaplan-Meier method was used to estimate median (95% CI) duration.
Results
Patients
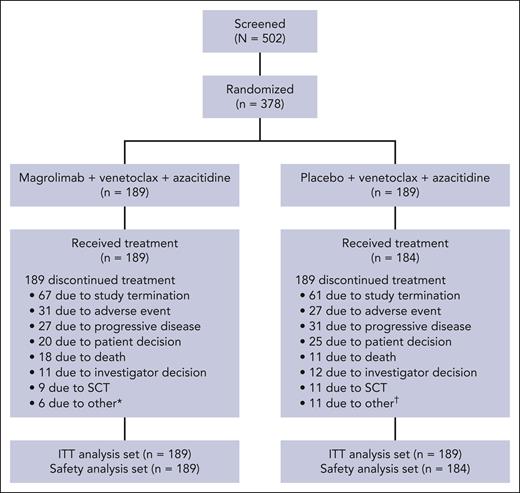
From 12 July 2022 to 5 September 2023, a total of 378 patients (189 in each arm) were randomized (Figure 1). Of these, 189 patients in the magrolimab arm received treatment and 184 patients in the control arm received treatment. Study termination, TEAEs, and disease progression were the most common reasons for discontinuing magrolimab or placebo in both arms. After the study, 5.8% of patients in the magrolimab arm went on to receive allogeneic stem cell transplantation vs 6.9% in the control arm.
CONSORT diagram. ∗Lack of efficacy (n = 4), protocol violation (n = 1), and initiation of anti-AML therapy (n = 1). †Lack of efficacy (n = 6) and patient never exposed to study drug (n = 5). CONSORT, Consolidated Standards of Reporting Trials; SCT, stem cell transplantation.
CONSORT diagram. ∗Lack of efficacy (n = 4), protocol violation (n = 1), and initiation of anti-AML therapy (n = 1). †Lack of efficacy (n = 6) and patient never exposed to study drug (n = 5). CONSORT, Consolidated Standards of Reporting Trials; SCT, stem cell transplantation.
Baseline characteristics were generally well balanced between treatment arms (Table 1). The median age was 75 years in both arms. Overall, 72 patients (38.1%) in the magrolimab arm and 75 (39.7%) in the control arm were aged <75 years but met comorbidity criteria for IC ineligibility; most of these patients had an ECOG PS score of 2 or 3. The proportion of patients with an ECOG PS score ≥2 was less for magrolimab vs control arms (37.6% vs 44.4%). Of patients in the ITT with known TP53 status based on local laboratory data, the TP53 mutation rate was similar between treatment arms (37/124 [29.8%] and 35/120 [29.2%] patients in the magrolimab and control arms, respectively), which is marginally higher than would be expected.18,19 Notably, more patients in the magrolimab arm had adverse genetic risk (ELN 2017 criteria) than patients in the control arm (44.4% vs 35.4%).
Patient demographics and disease characteristics
| Characteristic . | Magrolimab, venetoclax, and azacitidine (n = 189) . | Placebo, venetoclax, and azacitidine (n = 189) . |
|---|---|---|
| Age, median (range), y | 75 (43-88) | 75 (28-91) |
| ≥75, n (%) | 96 (50.8) | 98 (51.9) |
| Male, n (%) | 116 (61.4) | 106 (56.1) |
| Race, n (%) | ||
| White | 130 (68.8) | 130 (68.8) |
| Asian | 26 (13.8) | 25 (13.2) |
| Black | 3 (1.6) | 2 (1.1) |
| Other | 4 (2.1) | 2 (1.1) |
| Not available | 26 (13.8) | 30 (15.9) |
| Geographic region, n (%) | ||
| United States | 46 (24.3) | 47 (24.9) |
| Ex-United States | 143 (75.7) | 142 (75.1) |
| 2017 ELN genetic risk group,∗n (%) | ||
| Favorable/intermediate | 73 (38.6) | 88 (46.6) |
| Adverse | 84 (44.4) | 67 (35.4) |
| TP53 mutation,† n/N (%) | 37/124 (29.8) | 35/120 (29.2) |
| ECOG PS, n (%) | ||
| 0 | 35 (18.5) | 35 (18.5) |
| 1 | 83 (43.9) | 70 (37.0) |
| 2 | 66 (34.9) | 73 (38.6) |
| 3 | 5 (2.6) | 11 (5.8) |
| BM blast count, n (%) | ||
| <30% | 89 (47.1) | 87 (46.0) |
| ≥30% to <50% | 40 (21.2) | 38 (20.1) |
| ≥50% | 50 (26.5) | 51 (27.0) |
| Missing | 10 (5.3) | 13 (6.9) |
| Patients <75 y with ≥1 comorbidity, n (%) | 72 (38.1) | 75 (39.7) |
| ECOG PS score of 2 or 3 | 52 (27.5) | 62 (32.8) |
| Diffusing capacity of the lung ≤65% or FEV1 ≤65% | 18 (9.5) | 18 (9.5) |
| LVEF ≤50% | 8 (4.2) | 5 (2.6) |
| Total bilirubin >1.5× ULN | 4 (2.1) | 3 (1.6) |
| Other comorbidity‡ | 26 (13.8) | 27 (14.3) |
| Characteristic . | Magrolimab, venetoclax, and azacitidine (n = 189) . | Placebo, venetoclax, and azacitidine (n = 189) . |
|---|---|---|
| Age, median (range), y | 75 (43-88) | 75 (28-91) |
| ≥75, n (%) | 96 (50.8) | 98 (51.9) |
| Male, n (%) | 116 (61.4) | 106 (56.1) |
| Race, n (%) | ||
| White | 130 (68.8) | 130 (68.8) |
| Asian | 26 (13.8) | 25 (13.2) |
| Black | 3 (1.6) | 2 (1.1) |
| Other | 4 (2.1) | 2 (1.1) |
| Not available | 26 (13.8) | 30 (15.9) |
| Geographic region, n (%) | ||
| United States | 46 (24.3) | 47 (24.9) |
| Ex-United States | 143 (75.7) | 142 (75.1) |
| 2017 ELN genetic risk group,∗n (%) | ||
| Favorable/intermediate | 73 (38.6) | 88 (46.6) |
| Adverse | 84 (44.4) | 67 (35.4) |
| TP53 mutation,† n/N (%) | 37/124 (29.8) | 35/120 (29.2) |
| ECOG PS, n (%) | ||
| 0 | 35 (18.5) | 35 (18.5) |
| 1 | 83 (43.9) | 70 (37.0) |
| 2 | 66 (34.9) | 73 (38.6) |
| 3 | 5 (2.6) | 11 (5.8) |
| BM blast count, n (%) | ||
| <30% | 89 (47.1) | 87 (46.0) |
| ≥30% to <50% | 40 (21.2) | 38 (20.1) |
| ≥50% | 50 (26.5) | 51 (27.0) |
| Missing | 10 (5.3) | 13 (6.9) |
| Patients <75 y with ≥1 comorbidity, n (%) | 72 (38.1) | 75 (39.7) |
| ECOG PS score of 2 or 3 | 52 (27.5) | 62 (32.8) |
| Diffusing capacity of the lung ≤65% or FEV1 ≤65% | 18 (9.5) | 18 (9.5) |
| LVEF ≤50% | 8 (4.2) | 5 (2.6) |
| Total bilirubin >1.5× ULN | 4 (2.1) | 3 (1.6) |
| Other comorbidity‡ | 26 (13.8) | 27 (14.3) |
FEV1, forced expiratory volume during the first second; LVEF, left ventricular ejection fraction; ULN, upper limit of normal.
Per electronic data capture using ELN 2017 criteria; 32 patients in the magrolimab arm and 34 in the control arm each had missing or unknown risk status.
Sixty-five patients in the magrolimab group and 69 in the control group had missing or unknown TP53 mutation status.
Any other comorbidity that the investigator judged to be incompatible with IC and was approved by the sponsor’s medical monitor before study enrollment.
Efficacy
OS in the ITT population
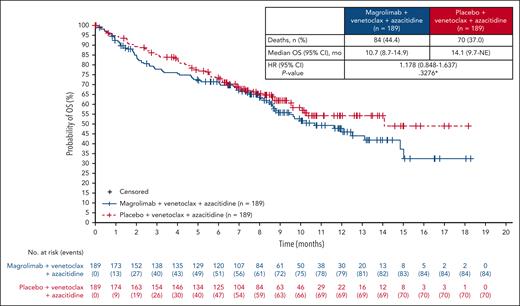
At the preplanned interim analysis (cutoff date, 15 December 2023), the median follow-up for OS was 5.2 and 5.6 months in the magrolimab and placebo arms, respectively. At this time, 68 (36.0%) and 58 (30.7%) deaths occurred in the magrolimab and placebo arms, respectively. The median OS was 11.7 months (95% CI, 8.6 to not estimable [NE]) in the magrolimab arm and 10.4 months (95% CI, 9.4 to NE) in the placebo arm (supplemental Figure 2). The OS crossed the prespecified futility boundary (HR = 1.1) with an HR of 1.173 (95% CI, 0.819-1.679), and the study was stopped early.
After the discontinuation of all patients from study treatment, a final analysis was performed. At data cutoff (data finalization date, 22 May 2024), with a median (range) follow-up of 7.62 months (0.03-18.33 months) in the magrolimab arm and 7.36 months (0.03-18.23) in the control arm, 84 deaths (44.4%) had occurred in the magrolimab arm, and 70 deaths (37.0%) had occurred in the control arm (Figure 2). The median (95% CI) OS was 10.7 months (8.7-14.9) in the magrolimab arm vs 14.1 months (9.7 to NE) in the control arm (HR, 1.178; 95% CI, 0.848-1.637; stratified 2-sided P = .3276). The Kaplan-Meier estimates of the OS rates at 1 year were 48.3% (magrolimab arm) and 54.5% (control arm).
Other efficacy end points
Key secondary efficacy results are presented in Table 2. The CR rate (95% CI) within 6 cycles of treatment was 41.3% (34.2-48.6) in the magrolimab arm and 46.0% (38.8-53.4) in the control arm (OR, 0.856; 95% CI, 0.560-1.307). The median (95% CI) duration of CR was 9.4 months (6.3 to NE) and 8.1 months (5.7 to NE), and the median (interquartile range) time to CR was 1.26 months (0.95-2.53) and 1.87 months (0.95-2.60) in the magrolimab and control arms, respectively. The composite CR rate was 67.2% and 68.8%, respectively. CR without MRD was achieved by 21.7% and 20.1% of patients, respectively. OS by MRD status and response (CR or composite CR) is shown in supplemental Figure 3. Overall, MRD negativity was obtained in 46.0% of patients (95% CI, 38.8-53.4) in the magrolimab arm and 36.5% (95% CI, 29.6-43.8) in the control arm (OR, 1.507; 95% CI, 0.991-2.291) at any time in the study. The median OS in patients without MRD was 14.9 months vs not reached in magrolimab vs control arms (HR, 1.907; 95% CI, 0.932-3.903). The median OS in patients with MRD was 8.0 vs 8.8 months in magrolimab vs control arms (HR, 1.296; 95% CI, 0.875-1.919). The EFS in the ITT population is shown in Figure 3.
Response outcomes within 6 cycles of treatment
| Outcome∗ . | Magrolimab, venetoclax, and azacitidine (n = 189) . | Placebo, venetoclax, and azacitidine (n = 189) . |
|---|---|---|
| CR rate, % (95% CI) | 41.3 (34.2-48.6) | 46.0 (38.8-53.4) |
| Stratified OR (95% CI) | 0.856 (0.560-1.307) | |
| Median duration of CR, months (95% CI) | 9.4 (6.3-NE) | 8.1 (5.7-NE) |
| Median time to CR, months (IQR) | 1.26 (0.95-2.53) | 1.87 (0.95-2.60) |
| CR without MRD rate, % (95% CI) | 21.7 (16.0-28.3) | 20.1 (14.6-26.5) |
| Stratified OR (95% CI) | 1.154 (0.690-1.932) | |
| CR + CRh rate, % (95% CI) | 47.6 (40.3-55.0) | 53.4 (46.1-60.7) |
| Stratified OR (95% CI) | 0.826 (0.545-1.251) | |
| Composite CR rate,† % (95% CI) | 67.2 (60.0-73.8) | 68.8 (61.7-75.3) |
| Outcome∗ . | Magrolimab, venetoclax, and azacitidine (n = 189) . | Placebo, venetoclax, and azacitidine (n = 189) . |
|---|---|---|
| CR rate, % (95% CI) | 41.3 (34.2-48.6) | 46.0 (38.8-53.4) |
| Stratified OR (95% CI) | 0.856 (0.560-1.307) | |
| Median duration of CR, months (95% CI) | 9.4 (6.3-NE) | 8.1 (5.7-NE) |
| Median time to CR, months (IQR) | 1.26 (0.95-2.53) | 1.87 (0.95-2.60) |
| CR without MRD rate, % (95% CI) | 21.7 (16.0-28.3) | 20.1 (14.6-26.5) |
| Stratified OR (95% CI) | 1.154 (0.690-1.932) | |
| CR + CRh rate, % (95% CI) | 47.6 (40.3-55.0) | 53.4 (46.1-60.7) |
| Stratified OR (95% CI) | 0.826 (0.545-1.251) | |
| Composite CR rate,† % (95% CI) | 67.2 (60.0-73.8) | 68.8 (61.7-75.3) |
Medians are based on Kaplan-Meier estimates. Durations of response and times to response are calculated for patients who achieved the indicated response category.
CRi, complete remission with incomplete hematologic recovery; IQR, interquartile range.
All rates are within 6 cycles of treatment and were calculated using the ITT population as the denominator.
Composite CR includes CR, CRi, or CRh.
Biomarker analyses
Patient demographics and baseline characteristics for the 244 patients in the BEP with targeted sequencing data (magrolimab, n = 120; control, n = 124) were generally consistent with those in the ITT population (supplemental Table 1). The most common mutations (based on centralized data from the BEP) in the magrolimab and control arms were TET2 (28.3% and 28.2%), DNMT3A (25.0% and 21.8%), and TP53 (22.5% and 30.6%) (supplemental Figure 4); oncoplots are shown in supplemental Figure 5. In the BEP, the TP53 variant allele frequency was similar between the magrolimab and control arms (median, 40% vs 39%; mean, 38.75% vs 42.3%; P = .46). Based on the 4-gene signature identified from experience with venetoclax/azacitidine, 22.5% of patients in the magrolimab arm were allocated to the lower-benefit group (TP53 mutations), 18.3% to the intermediate-benefit group (wild-type TP53; NRAS, KRAS, and/or FLT3-ITD mutations), and 59.2% to the higher-benefit group (all other patients). In the control arm, these percentages were 30.6%, 21.0%, and 48.4%, respectively.
Stratification by this molecular signature did not reveal any OS benefit in the magrolimab arm. The median OS in the magrolimab arm was not reached in the higher-benefit group, 9.8 months in the intermediate-benefit group, and 7.4 months in the lower-benefit group (supplemental Figure 6A). The median OS in the control arm was not reached in the higher-benefit group, not reached in the intermediate-benefit group, and 6.9 months in the lower-benefit group (supplemental Figure 6B). The median OS was not reached in both arms for patients with wild-type TP53 AML.
Safety
A total of 373 patients were included in the safety analysis (189 in the magrolimab arm; 184 in the control arm). The median (range) duration of exposure was 22.0 weeks (0.1-74.1) for magrolimab and 21.4 weeks (0.1-72.4) for placebo. In the magrolimab arm, 86 patients (45.5%) were exposed to magrolimab for ≥24 weeks (≥6 cycles); in the control arm, 78 patients (42.4%) were exposed to placebo for ≥24 weeks. Relative dose intensities are summarized in the supplemental Appendix.
Overall, 188 patients (99.5%) in the magrolimab arm and 184 (100%) in the control arm experienced a TEAE; grade ≥3 TEAEs were reported by 97.4% and 97.3% of patients, respectively (Table 3). The most commonly reported grade ≥3 TEAEs (≥15%) were similar in both arms and were all hematologic: neutropenia (62.4% and 60.3%, respectively), anemia (43.4% and 26.1%), thrombocytopenia (36.0% and 44.6%), and febrile neutropenia (33.3% and 38.6%) (Table 4).
Safety summary
| Patients, n (%) . | Magrolimab, venetoclax, and azacitidine (n = 189) . | Placebo, venetoclax, and azacitidine (n = 184) . |
|---|---|---|
| Any TEAE | 188 (99.5) | 184 (100) |
| Related to any study drug | 177 (93.7) | 162 (88.0) |
| Any grade ≥3 TEAE | 184 (97.4) | 179 (97.3) |
| Related to any study drug | 160 (84.7) | 144 (78.3) |
| Any serious TEAE | 138 (73.0) | 134 (72.8) |
| Related to any study drug | 87 (46.0) | 72 (39.1) |
| Any TEAE leading to discontinuation of any study drug | 36 (19.0) | 30 (16.3) |
| Leading to discontinuation of magro/PBO | 32 (16.9) | 29 (15.8) |
| Leading to discontinuation of AZA | 25 (13.2) | 25 (13.6) |
| Leading to discontinuation of VEN | 29 (15.3) | 26 (14.1) |
| Any TEAE leading to dose reduction of any study drug | 37 (19.6) | 30 (16.3) |
| Leading to dose reduction of magro/PBO | 3 (1.6) | 1 (0.5) |
| Leading to dose reduction of AZA | 25 (13.2) | 18 (9.8) |
| Leading to dose reduction of VEN | 24 (12.7) | 13 (7.1) |
| Any TEAE leading to dose delay or missed dose of any study drug | 110 (58.2) | 114 (62.0) |
| Any TEAE leading to drug interruption of any study drug | 140 (74.1) | 132 (71.7) |
| Leading to drug interruption of magro/PBO | 71 (37.6) | 59 (32.1) |
| Leading to drug interruption of AZA | 77 (40.7) | 91 (49.5) |
| Leading to drug interruption of VEN | 128 (67.7) | 124 (67.4) |
| Any TEAE leading to death | 36 (19.0) | 21 (11.4) |
| All deaths | 84 (44.4) | 69 (37.5) |
| Within 30 days of the first study drug | 13 (6.9) | 8 (4.3) |
| Within 60 days of the first study drug | 28 (14.8) | 18 (9.8) |
| Patients, n (%) . | Magrolimab, venetoclax, and azacitidine (n = 189) . | Placebo, venetoclax, and azacitidine (n = 184) . |
|---|---|---|
| Any TEAE | 188 (99.5) | 184 (100) |
| Related to any study drug | 177 (93.7) | 162 (88.0) |
| Any grade ≥3 TEAE | 184 (97.4) | 179 (97.3) |
| Related to any study drug | 160 (84.7) | 144 (78.3) |
| Any serious TEAE | 138 (73.0) | 134 (72.8) |
| Related to any study drug | 87 (46.0) | 72 (39.1) |
| Any TEAE leading to discontinuation of any study drug | 36 (19.0) | 30 (16.3) |
| Leading to discontinuation of magro/PBO | 32 (16.9) | 29 (15.8) |
| Leading to discontinuation of AZA | 25 (13.2) | 25 (13.6) |
| Leading to discontinuation of VEN | 29 (15.3) | 26 (14.1) |
| Any TEAE leading to dose reduction of any study drug | 37 (19.6) | 30 (16.3) |
| Leading to dose reduction of magro/PBO | 3 (1.6) | 1 (0.5) |
| Leading to dose reduction of AZA | 25 (13.2) | 18 (9.8) |
| Leading to dose reduction of VEN | 24 (12.7) | 13 (7.1) |
| Any TEAE leading to dose delay or missed dose of any study drug | 110 (58.2) | 114 (62.0) |
| Any TEAE leading to drug interruption of any study drug | 140 (74.1) | 132 (71.7) |
| Leading to drug interruption of magro/PBO | 71 (37.6) | 59 (32.1) |
| Leading to drug interruption of AZA | 77 (40.7) | 91 (49.5) |
| Leading to drug interruption of VEN | 128 (67.7) | 124 (67.4) |
| Any TEAE leading to death | 36 (19.0) | 21 (11.4) |
| All deaths | 84 (44.4) | 69 (37.5) |
| Within 30 days of the first study drug | 13 (6.9) | 8 (4.3) |
| Within 60 days of the first study drug | 28 (14.8) | 18 (9.8) |
AZA, azacitidine; Magro, magrolimab; PBO, placebo; VEN, venetoclax.
TEAEs in ≥15% of patients in either arm
| Patients, n (%) . | Magrolimab, venetoclax, and azacitidine (n = 189) . | Placebo, venetoclax, and azacitidine (n = 184) . | ||
|---|---|---|---|---|
| Overall . | Grade ≥3 . | Overall . | Grade ≥3 . | |
| Any TEAE | 188 (99.5) | 184 (97.4) | 184 (100) | 179 (97.3) |
| Neutropenia∗ | 119 (63.0) | 118 (62.4) | 115 (62.5) | 111 (60.3) |
| Anemia† | 97 (51.3) | 82 (43.4) | 64 (34.8) | 48 (26.1) |
| Thrombocytopenia‡ | 79 (41.8) | 68 (36.0) | 91 (49.5) | 82 (44.6) |
| Diarrhea | 77 (40.7) | 10 (5.3) | 67 (36.4) | 1 (0.5) |
| Constipation | 72 (38.1) | 0 | 76 (41.3) | 1 (0.5) |
| Febrile neutropenia | 70 (37.0) | 63 (33.3) | 73 (39.7) | 71 (38.6) |
| Nausea | 66 (34.9) | 3 (1.6) | 60 (32.6) | 3 (1.6) |
| Pyrexia | 60 (31.7) | 4 (2.1) | 53 (28.8) | 3 (1.6) |
| Hypokalemia | 50 (26.5) | 16 (8.5) | 56 (30.4) | 11 (6.0) |
| Decreased appetite | 40 (21.2) | 5 (2.6) | 30 (16.3) | 6 (3.3) |
| Blood bilirubin increased | 36 (19.0) | 14 (7.4) | 14 (7.6) | 6 (3.3) |
| Fatigue | 35 (18.5) | 8 (4.2) | 36 (19.6) | 6 (3.3) |
| Infusion-related reaction | 35 (18.5) | 9 (4.8) | 16 (8.7) | 3 (1.6) |
| Vomiting | 31 (16.4) | 1 (0.5) | 40 (21.7) | 5 (2.7) |
| Edema peripheral | 27 (14.3) | 0 | 39 (21.2) | 1 (0.5) |
| Pneumonia | 29 (15.3) | 19 (10.1) | 26 (14.1) | 22 (12.0) |
| COVID-19 | 25 (13.2) | 7 (3.7) | 28 (15.2) | 6 (3.3) |
| Patients, n (%) . | Magrolimab, venetoclax, and azacitidine (n = 189) . | Placebo, venetoclax, and azacitidine (n = 184) . | ||
|---|---|---|---|---|
| Overall . | Grade ≥3 . | Overall . | Grade ≥3 . | |
| Any TEAE | 188 (99.5) | 184 (97.4) | 184 (100) | 179 (97.3) |
| Neutropenia∗ | 119 (63.0) | 118 (62.4) | 115 (62.5) | 111 (60.3) |
| Anemia† | 97 (51.3) | 82 (43.4) | 64 (34.8) | 48 (26.1) |
| Thrombocytopenia‡ | 79 (41.8) | 68 (36.0) | 91 (49.5) | 82 (44.6) |
| Diarrhea | 77 (40.7) | 10 (5.3) | 67 (36.4) | 1 (0.5) |
| Constipation | 72 (38.1) | 0 | 76 (41.3) | 1 (0.5) |
| Febrile neutropenia | 70 (37.0) | 63 (33.3) | 73 (39.7) | 71 (38.6) |
| Nausea | 66 (34.9) | 3 (1.6) | 60 (32.6) | 3 (1.6) |
| Pyrexia | 60 (31.7) | 4 (2.1) | 53 (28.8) | 3 (1.6) |
| Hypokalemia | 50 (26.5) | 16 (8.5) | 56 (30.4) | 11 (6.0) |
| Decreased appetite | 40 (21.2) | 5 (2.6) | 30 (16.3) | 6 (3.3) |
| Blood bilirubin increased | 36 (19.0) | 14 (7.4) | 14 (7.6) | 6 (3.3) |
| Fatigue | 35 (18.5) | 8 (4.2) | 36 (19.6) | 6 (3.3) |
| Infusion-related reaction | 35 (18.5) | 9 (4.8) | 16 (8.7) | 3 (1.6) |
| Vomiting | 31 (16.4) | 1 (0.5) | 40 (21.7) | 5 (2.7) |
| Edema peripheral | 27 (14.3) | 0 | 39 (21.2) | 1 (0.5) |
| Pneumonia | 29 (15.3) | 19 (10.1) | 26 (14.1) | 22 (12.0) |
| COVID-19 | 25 (13.2) | 7 (3.7) | 28 (15.2) | 6 (3.3) |
Includes neutropenia and decreased neutrophil count.
Includes anemia and decreased hemoglobin.
Includes thrombocytopenia and decreased platelet count.
Discontinuations and dose modifications are presented in Table 3. The percentages of patients who discontinued any study drug owing to TEAEs were 19.0% in the magrolimab arm and 16.3% in the control arm. TEAEs led to magrolimab discontinuation in 16.9% of patients; TEAEs leading to magrolimab discontinuation in >1 patient were sepsis (2.6%), anemia (2.1%), pneumonia (2.1%), and infusion-related reactions (2.1%). The proportion of patients who had a dose reduction in any study drug was 19.6% in the magrolimab arm and 16.3% in the control arm. Drug interruptions owing to neutropenia, which are common for venetoclax, occurred in 43.4% (magrolimab arm) and 46.2% of patients (control arm).
There were more treatment-related serious TEAEs (46.0% and 39.1%) and any TEAE leading to death (19.0% and 11.4%) in the magrolimab arm than in the control arm (Table 3). The rate of grade 5 infections was higher in the magrolimab arm than in the control arm (11.1% vs 6.5%), which included higher rates of pneumonia (3.7% vs 2.2%) and sepsis (6.9% vs 3.3%) (supplemental Table 2). Five patients (2.6%) in the magrolimab arm had grade 5 respiratory failure vs none in the control arm. Time to onset of grade 5 pneumonia and/or respiratory failure was generally variable. There was a similar incidence of febrile neutropenia and neutropenia between arms (Table 4); rates of any-grade infections (70.9% vs 69.6%) and nonfatal pneumonia were also similar (11.6% vs 12.0%). Mortality at 30 days was 6.9% in the magrolimab arm and 4.3% in the control arm; mortality at 60 days was 14.8% and 9.8%, respectively. In the magrolimab arm, 21 of 28 deaths within 60 days of first study drug were because of AEs; in the control arm, 11 of 18 deaths within 60 days were because of AEs.
Discussion
ENHANCE-3 was the first randomized phase 3 trial to evaluate an anti-CD47 therapy in frontline AML for patients ineligible for IC and the first randomized trial in this setting to use venetoclax and azacitidine as a comparator since the United States accelerated approval of this combination in 2018. In this study, the addition of magrolimab to venetoclax and azacitidine did not improve OS, and the study was stopped early because the prespecified futility boundary for OS was crossed at an interim analysis. After study termination, the final OS analysis showed that the HR for OS trended toward favoring the control arm. Other key secondary efficacy end points assessed, including CR rate, also did not demonstrate benefit in adding magrolimab to venetoclax/azacitidine.
ENHANCE-3 was designed based on early-phase clinical data showing encouraging clinical activity.13,14 In a single-arm phase 1/2 study of magrolimab plus venetoclax and azacitidine for the treatment of newly diagnosed patients with AML who were ineligible for IC, the 1-year OS rates for patients with TP53-mutant (53%) or wild-type TP53 AML (83%) were both numerically >1-year OS rate observed with magrolimab in the ENHANCE-3 study (48%), despite the former study including a very-high-risk cohort.13 In contrast, the median OS in the control arm of ENHANCE-3 was consistent with that reported in the venetoclax and azacitidine arm in the phase 3 VIALE-A trial (14.1 vs 14.7 months).5,6
The addition of magrolimab to venetoclax and azacitidine resulted in more deaths, including early deaths within 60 days of treatment initiation. Early mortality with magrolimab, venetoclax, and azacitidine in ENHANCE-3 was more frequent than previously observed in the phase 1/2 study of this combination (60-day rate, 14.8% vs 0%).13 In contrast, early mortality was less frequent with venetoclax and azacitidine in ENHANCE-3 vs VIALE-A (30-day rate, 4% vs 7%). AEs were the most common reason for early deaths in ENHANCE-3. Fatal TEAEs at any time during the study were also more common with the addition of magrolimab to venetoclax and azacitidine.
The increased incidence of fatal TEAEs seemed to be driven by grade 5 infections and respiratory failure. Although CD47 loss was shown to protect against infections in preclinical models,20-23 serious infections have been reported in clinical studies of magrolimab. In patients with newly diagnosed AML treated with magrolimab and azacitidine in a phase 1b study, fatal events of pneumonia and sepsis were reported.14 The randomized phase 3 ENHANCE study showed that infections were the most common TEAE with fatal outcome for magrolimab and azacitidine in frontline higher-risk myelodysplastic syndromes.24
The reasons why the addition of magrolimab to venetoclax and azacitidine resulted in more fatal infections in ENHANCE-3 are unclear, especially because there were similar incidences of any-grade infections (including nonfatal pneumonia), febrile neutropenia, neutropenia, and drug interruptions owing to neutropenia (suggesting similar duration of neutropenia) between treatment arms. The protocol encouraged, but did not mandate, the use of infection prophylaxis per local standard of care for patients with prolonged neutropenia or patients at risk. It is unknown whether the incidence of serious infections would have been lower had infection prophylaxis been mandated. There may be a need for standardized prophylaxis to avoid negative outcomes; this is an important consideration to assess in future frontline AML trials, especially among older/unfit patients.
Anemia is an on-target effect of magrolimab and was, as expected, more frequent in the magrolimab arm than in the control arm (any grade, 51.3% vs 34.8%; grade ≥3, 43.4% vs 26.1%). Anemia was manageable in ENHANCE-3 using a dose priming/maintenance strategy and required hemoglobin monitoring after the initial magrolimab doses (with RBC transfusion when clinically appropriate) given that only 4 patients discontinued magrolimab owing to anemia.
A higher proportion of patients in the magrolimab arm had adverse genetic risk per ELN 2017 criteria vs the control arm at final analysis (44.4% vs 35.4%), which may have affected efficacy outcomes. Given that genetic risk was a predetermined stratification factor at randomization, genetic risk strata were balanced at the time of randomization with “unknown” as an option that could be selected at enrollment. Genetic risk categories were updated in the database used for final analysis if additional results became available after randomization, allowing for random shifts in the strata. Although adverse genetic risk has been shown to be an independent predictor of shorter OS in AML,25 some studies indicate that ELN 2017 risk groups are not accurate for patients receiving nonintensive therapy.8,18 Analyses of OS by the more recently proposed 4-gene molecular signature, which has been validated for patients with AML treated with venetoclax and azacitidine, did not reveal any benefit in the magrolimab arm.
Compared with other studies with similar populations, the ENHANCE-3 BEP (assessed centrally with targeted sequencing) was enriched for TP53 mutations (magrolimab arm, 22.5%; control arm, 30.6%). By contrast, in the phase 3 VIALE-A registration study, 23% of patients in the venetoclax and azacitidine arm had TP53 mutations,6 and in the phase 3 ASTRAL-1 study of guadecitabine in previously untreated elderly patients with AML unfit for intensive therapy (N = 604), <20% had TP53 mutations.18 Similarly, in a real-world study of newly diagnosed patients who received nonintensive treatment (N = 654), TP53 mutations were detected in 13% of patients.26 The high proportion of TP53-mutated AML and adverse ELN genetic risk in the magrolimab and control arms may have affected outcomes.
As expected, our OS analyses using the 4-gene molecular signature previously identified for venetoclax and azacitidine showed that patients in the lower-benefit group (TP53 mutated) had worse outcomes than those without TP53 mutations in both treatment arms, highlighting the continuing unmet needs in this population. Although this molecular signature has been previously validated to segregate patients treated with venetoclax and HMAs into 3 risk groups with distinct survival,8 we did not observe any OS differentiation between the intermediate- and higher-benefit groups (non-TP53 mutation harboring groups) for patients in the ENHANCE-3 control arm. This may have been because of the limited follow-up and sample size in the ENHANCE-3 biomarker subset population. There is also ongoing research into improving AML risk prediction.19,27
In conclusion, the ENHANCE-3 study demonstrated that the addition of magrolimab to venetoclax and azacitidine did not improve OS or CR rates and resulted in more fatal TEAEs driven by grade 5 infections in patients with previously untreated AML who were ineligible for IC. These results highlight the challenges of improving outcomes for patients with AML who are not candidates for intensive treatment. ENHANCE-3 is one of the largest prospective data sets of patients with AML ineligible for IC, and it provides important information and benchmarks to guide future development of anti-CD47 therapies and trial designs involving venetoclax and azacitidine in this setting.
Acknowledgments
The authors thank the patients and their caregivers and families and the study investigators for their participation and commitment to this clinical study.
The ENHANCE-3 study was funded by Gilead Sciences, Inc. Medical writing assistance was provided by Ashfield MedComms, an Inizio Company, and was funded by Gilead Sciences, Inc.
Authorship
Contribution: N.D., P.V., and G.S.G.M. conceived and designed the study; N.D., P.V., G.H., H.D., S.M., J.N., C.P., C.M.C., P.M., R.N., P.F., J.E., S.-J.W., A.D.V., J.M., P.J.M.V., and G.S.G.M. performed the data acquisition and patient recruitment; L.J., M.D., K.L., S.K., and K.C. collected and assembled the data; and all authors analyzed and interpreted the data and drafted and revised the manuscript.
Conflict-of-interest disclosure: N.D. reports research funding from AbbVie, Aptose, Arcellx, Astellas, AstraZeneca, Bristol Myers Squibb (BMS), Caribou, Daiichi Sankyo, Fate Therapeutics, Forty Seven, Genentech, Gilead, Immunogen, Incyte, Karyopharm, Kite, Novartis, NovImmune, Pfizer, Servier, Shattuck Labs, and Vincerx and advisory/consulting for AbbVie, Agios, Aptose, Arcellx, Astellas, AvenCell, BMS, Caribou Biosciences, Daiichi Sankyo, Forty Seven, Genentech, Gilead, Immunogen, Jazz, Kite, Novartis, Pfizer, Roche, Servier, Shattuck Labs, Syndax, Trillium, and Vincerx. P.V. reports advisory roles or travel/accommodation from AbbVie, Auron Therapeutics, ImmunoGen, Jazz Pharmaceuticals, Kura, Pfizer, Rigel, Servier, Stemline/Menarini, and Takeda; reports honoraria from AbbVie, Astellas, Celgene, Daiichi Sankyo, Jazz Pharmaceuticals, and Pfizer; reports speakers’ bureau participation with AbbVie, Astellas, Gilead, and Servier; reports research funding to institution from BMS/Celgene; reports stock or ownership in Auron Therapeutics and Yellowstone Biosciences; and reports patents for flow cytometric detection of leukemic stem cells. G.H. reports advisory board participation for Servier and Astellas. H.D. reports advisory role for AbbVie, Agios, Amgen, Astellas, AstraZeneca, Berlin-Chemie, BMS, Celgene, Gilead, Janssen, Jazz, Novartis, Servier, Stemline, and Syndax and research funding from AbbVie, Agios, Amgen, Astellas, Bristol Myers Squibb, Celgene, Jazz Pharmaceuticals, Kronos Bio, Novartis, and Pfizer. C.P. reports consulting/advisory roles at AbbVie, Astellas, Novartis, BMS, Menarini, Stemline, Blueprint Medicines, Incyte, GlaxoSmithKline (GSK), Amgen, Pfizer, and Janssen and personal fees from Astellas, Novartis, Pfizer, and Amgen. S.M. reports advisory/consulting roles for Amgen, BMS, and Pfizer. G.S.G.M. reports research funding to institution from Gilead, Xencor, Zentalis, Beigene, Lilly/OXO, Oryzon Genomics, Merck, and Schrodinger and consulting fees/honoraria from Amgen, Beigene, Gilead, Pfizer, BMS, Rigel, Stemline Therapeutics, Cardinal Health, Servier, DAVA Oncology, Aptitude Health, Curio Science, and Autolus Therapeutics. J.N. reports advisory/consulting for AbbVie, Amgen, Astellas, Novartis, Pfizer, Roche, and Takeda and travel grants from Amgen and Janssen. P.M. reports advisory board participation for AbbVie, Astellas, BMS/Celgene, Daiichi Sankyo, Incyte, Janssen, Karyopharm, Novartis, Pfizer, Sanofi, Servier, Syndax, and Teva; consultancy for Astellas, Agios, BMS/Celgene Forma Therapeutics, Glycomimetics, and Tolero Pharmaceuticals; speakers bureau participation for AbbVie, BMS/Celgene, Daiichi Sankyo, Incyte, Janssen, Novartis, Pfizer, Sanofi, Servier, and Teva; and research support from AbbVie, Astellas, BMS/Celgene, Daiichi Sankyo, Janssen, Karyopharm, Novartis, Pfizer, and Teva. A.D.V. reports research funding from Gilead; speaker honoraria for AbbVie, Gilead, Johnson & Johnson, and Servier; and travel grants from AbbVie, Gilead, and Servier. J.M. reports research funding from AbbVie and Gilead. C.M.C. reports advisory board participation for AbbVie, BeiGene, GSK, and Johnson & Johnson; speakers bureau for AbbVie, Amgen, AstraZeneca, Johnson & Johnson, and Roche; travel grants from AbbVie, Johnson & Johnson, Kite, and Roche; mentoring for GSK; and training support from BMS and Johnson & Johnson. R.N. reports advisory/consulting for Janssen. L.J., M.D., K.L., S.K., and K.C. are employees of Gilead Sciences Inc. The remaining authors declare no competing financial interests.
Correspondence: Naval Daver, Leukemia Research Alliance Program, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; email: ndaver@mdanderson.org.
References
Author notes
Gilead Sciences shares anonymized individual patient data upon request or as required by law or regulation with qualified external researchers based on their submitted curriculum vitae and reflecting no conflict of interest. The request proposal must also include a statistician. Approval of such requests is at Gilead Science’s discretion and is dependent on the nature of the request, the merit of the research proposed, the availability of the data, and the intended use of the data. Data requests should be sent to datarequest@gilead.com.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal