Abstract
This report examines the effects on hematopoietic regeneration of pegylated recombinant human megakaryocyte growth and development factor (PEG-rHuMGDF ) (2.5 μg/kg/d) alone and in combination with recombinant human granulocyte colony stimulating factor (rHu-GCSF ) (10 μg/kg/d) for 21 days in rhesus macaques receiving intense marrow suppression produced by single bolus injections of hepsulfam (1.5 g/m2). In six hepsulfam-only control animals thrombocytopenia (platelet count <100 × 109/L) was observed between days 12 and 25 (nadir 39 ± 20 × 109/L on day 17), and neutropenia (absolute neutrophil count <1 × 109/L) occurred between days 8 and 30 (nadir 0.167 ± 0.120 × 109/L on day 15). PEG-rHuMGDF (2.5 μg/kg/d) injected subcutaneously into four animals from day 1 to day 22 following hepsulfam administration produced trough serum concentrations of 1.9 ± 0.2 ng/mL and increased the platelet count twofold over basal prechemotherapy levels (856 ± 594 × 109/L v baseline of 416 ± 88 × 109/L; P = .01). PEG-rHuMGDF alone also shortened the period of posthepsulfam neutropenia from 22 days to 12 days (P = .01), although the neutropenic nadir was not significantly altered (neutrophil count 0.224 ± 0.112 × 109/L v 0.167 ± 0.120 × 109/L; P < .3). rHu-GCSF (10 μg/kg/d) injected subcutaneously into four animals from day 1 to day 22 following hepsulfam administration produced trough serum concentrations of 1.4 ± 1.1 ng/mL, and reduced the time for the postchemotherapy neutrophil count to attain 1 × 109/L from 22 days to 4 days (P = .005). The postchemotherapy neutropenic nadir was 0.554 ± 0.490 × 109neutrophils/L (P = .3 v hepsulfam-only control of 0.167 ± 0.120 × 109/L). However, thrombocytopenia of <100 × 109 platelets/L was not shortened (persisted from day 12 to day 25), or less severe (nadir of 56 ± 32 × 109 platelets/L on day 14; P = .7 compared with untreated hepsulfam animals). The concurrent administration of rHu-GCSF (10 μg/kg/d) and PEG-rHuMGDF (2.5 μg/kg/d) in four animals resulted in postchemotherapy peripheral platelet counts of 127 ± 85 × 109/L (P = .03 compared with 39 ± 20 × 109/L for untreated hepsulfam alone, and P = .02 compared with 856 ± 594 × 109/L for PEG-rHuMGDF alone), and shortened the period of neutropenia <1 × 109/L from 22 days to 4 days (P = .8 compared with rHu-GCSF alone). Increasing PEG-rHuMGDF to 10 μg/kg/d and maintaining the 21-day schedule of coadministration with rHu-GCSF (10 μg/kg/d) in another four animals produced postchemotherapy platelet counts of 509 ± 459 × 109/L (P < 10−4compared with untreated hepsulfam alone, and P = .04 compared with 2.5 μg/kg/d PEG-rHuMGDF alone), and 4 days of neutropenia. Coadministration of rHu-GCSF and PEG-rHuMGDF did not significantly alter the pharmacokinetics of either agent. The administration of PEG-rHuMGDF (2.5 μg/kg/d) from day 1 through day 22 and rHu-GCSF (10 μg/kg/d) from day 8 through day 22 in six animals produced peak postchemotherapy platelet counts of 747 ± 317 × 109/L (P < 10−4 compared with untreated hepsulfam alone, and P = .7 compared with PEG-rHuMGDF alone), and maintained the neutrophil count < 3.5 × 109/L (P = .008 v rHu-GCSF therapy alone). Thus, both thrombocytopenia and neutropenia are eliminated by initiating daily PEG-rHuMGDF therapy on day 1 and subsequently adding daily rHu-GCSF after 1 week in the rhesus model of hepsulfam marrow suppression. This improvement in platelet and neutrophil responses by delaying the addition of rHu-GCSF to PEG-rHuMGDF therapy demonstrates the importance of optimizing the dose and schedule of cytokine combinations after severe myelosuppressive chemotherapy.
NEUTROPENIC FEVER and thrombocytopenic bleeding complicate high-dose myelosuppressive cancer chemotherapy. Antibiotics and the granulocyte lineage-dominant hematopoietic growth factor, rHu-GCSF or granulocyte-macrophage colony-stimulating factor (GM-CSF ), significantly improve outcomes for patients who are at risk of developing neutropenic fever.1-3 While platelet transfusions protect these patients from thrombocytopenic bleeding, platelet transfusional therapy becomes complicated by limited availability, transmission of infectious diseases, sensitization and possible refractoriness to repeated platelet transfusions.4-6 The availability of recombinant megakaryocyte lineage-dominant hematopoietic growth factors (Mpl-ligands),7-12 permits an assessment of alternatively substituting amplified platelet production for prophylactic platelet transfusions in cancer patients receiving myeloablative chemotherapy. Before evaluating this strategy in patients, we examined the effects of combining granulocyte-dominant rHu-GCSF with megakaryocyte-dominant Mpl-ligands on hematopoietic recovery in a clinically relevant nonhuman primate model of severe chemotherapeutic myelosuppression.
Mpl-ligands induce hematopoietic stem cells to undergo megakaryocytic differentiation, proliferation, and endoreduplication,13-16 thereby expanding the formation of megakaryocyte cytoplasmic substrate destined for fragmentation and release as functional circulating platelets.7-10,16-19 The regulatory role of native Mpl-ligand, thrombopoietin, in the process of platelet production is evident from the capacity of excess soluble Mpl-receptor to neutralize both the megakaryocyte colony-stimulating activity and platelet-elevating activity in thrombocytopenic plasma.7,8,10,15 Mice engineered for deletion of the Mpl-receptor20 or endogenous thrombopoietin21 exhibit platelet counts about one sixth of normal without affecting other blood cell counts. In addition, circulating levels of Mpl-ligand in patients with postchemotherapy thrombocytopenia are elevated, and decrease after the peripheral platelet concentrations return toward normal by platelet transfusions or marrow recovery.10 15 In this study, the effects of PEG-rHuMGDF alone and in combination with rHu-GCSF, are investigated in a model of chemotherapy-induced intense myelosuppression in rhesus macaques.
MATERIALS AND METHODS
Animals studied.A total of 32 rhesus monkeys (26 males and 6 females) weighing 5 to 9 kg were used for this study. All procedures were approved by the Institutional Animal Care and Use Committee and conducted in accordance with Federal guidelines (Guide for the Care and Use of Laboratory Animals. National Institutes of Health, Bethesda, MD, NIH Publ. No. 86-23).
Study design.The effects of injecting PEG-rHuMGDF (2.5 μg/kg/d) subcutaneously each day for 21 days was assessed in four normal control animals without associated hepsulfam administration. Hepsulfam, a myelosuppressive agent,22-24 was given to the remaining 28 animals by intravenous bolus injections. The hepsulfam animals were allocated to six different treatment groups. These animals (except for group 6) received daily subcutaneous injections from day 1 through day 22 consisting of: (1) saline controls (n = 6); (2) PEG-rHuMGDF (n = 4); (3) rHu-GCSF (n = 4); (4) concurrent PEG-rHuMGDF (2.5 μg/kg/d) plus rHu-GCSF (10 μg/kg/d) (n = 4); (5) concurrent PEG-rHuMGDF (10 μg/kg/d) plus rHu-GCSF (10 μg/kg/d) (n = 4); and (6) PEG-rHuMGDF (2.5 μg/kg/d) on day 1 through day 22, and rHu-GCSF(10 μg/kg/d) beginning on day 8 and continuing through day 22 (n = 6).
Enrofloxacin (2.5 mg/kg as Batril; Mobay Corp, Shawnee, KS) was administered by intramuscular injection twice daily beginning on day 1 and continuing through at least day 21 until the neutrophil count was >500/μL for two successive determinations. Fluconazol (5 mg/kg as Diflucan; Pfizer, Brooklyn, NY) was given orally once daily. Blood was obtained for complete blood counts by femoral vein puncture before hepsulfam administration and three times weekly thereafter (Monday, Wednesday, and Friday) for 6 weeks. Blood for serum chemistry determinations was drawn at baseline and at the end of cytokine administration. Bone marrow aspirates were obtained before hepsulfam administration and 3, 7, 14, and 21 days later.
Reagents.Hepsulfam (sulfamic acid diester, NSC 329680)23 was a gift from the Division of Cancer Treatment, National Cancer Institute, Bethesda, MD. The lyophilized drug was solubilized in diluent containing 10% (vol/vol) ethanol and 40% (vol/vol) propylene glycol in 0.05 mol/L phosphate buffer (pH 7.4), and then diluted in 150 mL of normal saline. Hepsulfam (1.5 g/m2) was administered over 30 minutes by single bolus intravenous infusion.
PEG-rHuMGDF is a truncated molecule including the N-terminal of human c-Mpl ligand, in this case having 163 amino acids, which is expressed in Escherichia coli and derivatized with polyethylene glycol on the N-terminus by reductive alkylation.10 25 PEG-rHuMGDF was formulated in an aqueous buffer, sterilized by filtration, and provided by Amgen Inc, Thousand Oaks, CA. Dosing was based on protein weight. rHu-GCSF was provided as filgrastim by Amgen Inc.
Laboratory procedures.Peripheral platelet counts, mean platelet volumes, red blood cell counts, total white blood cell counts, and neutrophil counts were determined in whole blood collected every other day in Na2EDTA (2 mg/mL) using Serono/Baker model 9000 whole blood analyzer (Allentown, PA).26 27 The neutrophil counts were determined from electronic leukocyte counts and manual 500-cell leukocyte differential counts on Wright-Giemsa-stained peripheral blood films. The baseline mean blood cell counts were 416 ± 88 × 109/L for platelets, 5.69 ± 0.49 × 1012/L for erythrocytes, 10.2 ± 3.4 × 109/L for leukocytes, and 4.5 ± 2.1 × 109/L for neutrophils.
Serum concentrations of PEG-rHuMGDF and thrombopoietin (TPO) were determined using an enzyme-linked immunosorbent assay (ELISA) involving an initial polyclonal antibody capture procedure followed by enzyme product formation and determination.16,28 Serum levels of exogenous and endogenous G-CSF were also determined using ELISA involving initial antibody capture procedure and subsequent enzyme product formation and determination.1 29
Megakaryocyte number, size, and ploidy were measured by flow cytometry using a previously reported method for multiparameter correlative marrow analysis with a single-argon-ion-laser FACScan analyzer (Becton Dickinson, San Jose, CA).28,30-32 Cell DNA in aspirated marrow was stained with propidium iodide, and surface membrane receptors were analyzed with antibodies labeled with fluorescein and phycoerythrin. Megakaryocytes expressing platelet glycoprotein (GP) IIb/IIIa were enumerated in relation to the nucleated erythroid precursors expressing glycophorin A.6 Measurements of megakaryocyte diameters were based on the time-of-flight principle, ie, time required for a cell in suspension to pass through a focused light beam.30 33 Megakaryocytes were selected on the basis of their distinct immunofluorescence at levels above that of control cells labeled with an unrelated monoclonal antibody (MoAb). In each sample, 2,000 to 3,000 megakaryocytes were analyzed. Flow cytometry was performed using FACScan (Becton Dickinson). Bone marrow aspirates were obtained baseline and after 3, 7, 14, and 21 days of treatment.
Estimates of marrow megakaryocyte mass were used to represent the marrow substrate giving rise to circulating platelets, and was calculated as the product of megakaryocyte numbers and mean megakaryocyte volumes.6,16,18 28 Normal rhesus marrow values (n = 16) averaged: megakaryocyte diameter of 39 μm (range, 21 μm for 2N to 56 μm for 64N cells), volume of 30.1 ± 3.60 × 103 f L, and megakaryocyte number of 6.36 ± 1.41 × 106 megas/kg, giving a total megakaryocyte mass of 19.5 ± 6.62 × 1010 f L/kg. The normal modal ploidy was 16N.
Steady-state platelet mass turnover (platelet concentration multiplied by mean platelet volume and divided by platelet life span and by platelet recovery) was used to estimate the rate at which viable platelet mass was delivered to the peripheral blood.16,18,28 To measure platelet survival time, autologous platelets were labeled with 111In-oxine using the method described previously34; the labeled platelets functioned normally.35 Platelet survival time, ie, the average time in circulation, was then calculated using computer least-squares fitting of the raw data to a gamma-function modeling program.34 Baseline rhesus platelet lifespan (n = 10) was 5.5 ± 0.6 days. The recovery of labeled platelets in the circulation at equilibrium was estimated by extrapolating the survival curve to time zero and estimating the blood volume (70 mL/kg) using the formula: recovery in the circulation = total circulating platelet radioactivity/total platelet radioactivity injected × 100 (%). Basal platelet recovery in rhesus was 85% ± 4%. Platelet mass turnover, a measure of steady state platelet production and destruction, was calculated by the formula: platelet mass turnover rate (f L plats/μL/d) = platelet count/μL multiplied by mean platelet volume (f L) and divided by platelet survival time (days) and proportion of 111In-platelets recovered immediately after intravenous infusion. Platelet mass turnover in normal rhesus (n = 10) was 5.17 ± 1.27 × 105 f L plats/μL/d.
Marrow biopsies were obtained from the proximal femur before hepsulfam treatment, and after 3 weeks of cytokine therapy. The biopsies were processed for paraffin sectioning and stained for morphologic evaluation by light microscopy.
Data analysis.Data were analyzed using SIGMA STAT (Jandel Scientific Software, San Rafael CA). Comparisons between two groups were performed using the two-tailed Student's t-test, unless the data were not distributed randomly, in which case nonparametric analyses were performed. Analysis of variance (ANOVA) was used to compare values for a particular group at various time points.36 Unless otherwise stated, variance about the mean is given as ± 1 standard deviation (SD).
RESULTS
Effects of PEG-rHuMGDF on platelet production in normal rhesus controls.Daily subcutaneous injections of PEG-rHuMGDF (2.5 μg/kg/d) produced serum trough levels of 1.60 ± 0.19 ng/mL (P < 10−4 compared with endogenous Mpl-ligand levels of <100 pg/mL). The corresponding peripheral platelet counts increased fivefold, attaining peak levels at 2 weeks of 2,197 ± 249 × 109/L (Fig 1, left and Table 1; P = .001 v baseline of 416 ± 88 × 109/L). During the period of induced thrombocytosis, the mean platelet volume (MPV) decreased from the baseline value of 8.3 ± 1.0 f L to 7.2 ± 0.9 f L (P = .01). Platelet ultrastructure was normal (data not shown), and platelet lifespan averaged 132 ± 7 hours (P = .68 compared with the mean baseline value of 130 ± 14 hours). PEG-rHuMGDF (2.5 μg/kg/d) increased platelet mass turnover sixfold, from 5.2 ± 1.3 to 32.7 ± 1.5 × 105 f L plats/μL/d (P < 10−4). After discontinuing therapy, platelet counts fell to baseline values and MPVs normalized within 10 days (Fig 1). The peripheral neutrophil counts were not significantly altered from baseline values (Fig 1, right; P > .2), and peripheral erythrocyte counts and volumes were not changed from baseline (P > .5).
Effects of PEG-rHuMGDF on hematopoiesis in untreated controls. PEG-rHuMGDF (2.5 μg/kg/d) increased the concentration of circulating platelets sixfold by 2 weeks. The platelet count normalized within 10 days after stopping therapy (left). Peripheral leukocyte, neutrophil, or erythrocyte counts did not change significantly during PEG-rHuMGDF administration (right).
Effects of PEG-rHuMGDF on hematopoiesis in untreated controls. PEG-rHuMGDF (2.5 μg/kg/d) increased the concentration of circulating platelets sixfold by 2 weeks. The platelet count normalized within 10 days after stopping therapy (left). Peripheral leukocyte, neutrophil, or erythrocyte counts did not change significantly during PEG-rHuMGDF administration (right).
Effects of PEG-rHuMGDF on Marrow Megakaryocytes and Platelet Production in Normal Rhesus Macaques
| Duration PEG-rHu MGDF Therapy (2.5 μg/kg/d) . | Megakaryocytes . | Platelets . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| . | Ratio to NE (×103)* . | No. . | Volume (×103 fL) . | Mass . | Increase (×N) . | Concentration (×109/L) . | Volume (fL) . | Mass turnover . | Increase (×N) . |
| . | . | (×106 megas/kg) . | . | (×1010 fL/kg) . | . | . | . | (×105 fL plats/μL/d) . | . |
| Baseline | 1.20 ± 0.26 | 6.36 ± 1.41 | 30.1 ± 3.60 | 19.5 ± 6.62 | 1.0 | 416 ± 88 | 8.3 ± 1.0 | 5.17 ± 1.27 | 1.0 |
| 3 d | 2.21 ± 0.84 | 11.7 ± 4.45 | 57.2 ± 3.39 | 67.4 ± 29.5 | 3.4 | 474 ± 108 | 8.0 ± 0.5 | 7.95 ± 1.64 | 1.5 |
| 7 d | 6.25 ± 2.73 | 33.2 ± 14.6 | 48.6 ± 1.44 | 160 ± 68.6 | 8.2 | 1,084 ± 153 | 7.6 ± 0.9 | 17.2 ± 2.49 | 3.2 |
| 14 d | 8.42 ± 3.52 | 55.5 ± 11.7 | 40.1 ± 8.71 | 231 ± 7.20 | 11.8 | 2,197 ± 249 | 7.2 ± 0.9 | 32.7 ± 1.52 | 6.2 |
| Duration PEG-rHu MGDF Therapy (2.5 μg/kg/d) . | Megakaryocytes . | Platelets . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| . | Ratio to NE (×103)* . | No. . | Volume (×103 fL) . | Mass . | Increase (×N) . | Concentration (×109/L) . | Volume (fL) . | Mass turnover . | Increase (×N) . |
| . | . | (×106 megas/kg) . | . | (×1010 fL/kg) . | . | . | . | (×105 fL plats/μL/d) . | . |
| Baseline | 1.20 ± 0.26 | 6.36 ± 1.41 | 30.1 ± 3.60 | 19.5 ± 6.62 | 1.0 | 416 ± 88 | 8.3 ± 1.0 | 5.17 ± 1.27 | 1.0 |
| 3 d | 2.21 ± 0.84 | 11.7 ± 4.45 | 57.2 ± 3.39 | 67.4 ± 29.5 | 3.4 | 474 ± 108 | 8.0 ± 0.5 | 7.95 ± 1.64 | 1.5 |
| 7 d | 6.25 ± 2.73 | 33.2 ± 14.6 | 48.6 ± 1.44 | 160 ± 68.6 | 8.2 | 1,084 ± 153 | 7.6 ± 0.9 | 17.2 ± 2.49 | 3.2 |
| 14 d | 8.42 ± 3.52 | 55.5 ± 11.7 | 40.1 ± 8.71 | 231 ± 7.20 | 11.8 | 2,197 ± 249 | 7.2 ± 0.9 | 32.7 ± 1.52 | 6.2 |
Ratio of marrow megakaryocytes to nucleated erythroid cells determined by comparing nucleated GPIIb/IIIa-positive cells to nucleated glycophorin A-positive cells using flow cytometry (see Materials and Methods).
PEG-rHuMGDF (2.5 μg/kg/d) expanded megakaryocyte volume to 57.2 ± 3.39 ×103 f L by the third day of injections (P < 10−4v the baseline of 30.1 ± 3.60 × 103 f L), and increased megakaryocyte number to 55.5 ± 11.7 × 106/kg by day 14 (P < 10−4v baseline of 6.36 ± 1.41 × 106/kg). Megakaryocyte modal ploidy was increased from 16 to 32, with increases in 64N and the appearance of a 128N cohort (P = .01). Megakaryocytes mass, the product of the total number of megakaryocytes and their mean volume,6 18 increased greater than 10-fold to 231 ± 7.20 × 1010 f L/kg by day 14 (P < .0001 compared with basal values).
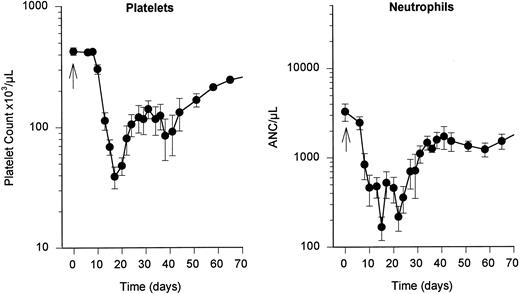
Effects of hepsulfam on hematopoiesis in untreated controls.The hematologic effects of hepsulfam (1.5 g/m2 intravenous bolus) was determined in six control rhesus macaques that received daily subcutaneous injections of saline for 21 days (Fig 2). Thrombocytopenia of less than 100 × 109 platelets/L was observed between day 12 and day 25 following hepsulfam treatment, with a nadir of 39 ± 20 × 109 platelets/L on day 17 (Fig 2, left). Thereafter, peripheral platelet counts slowly returned to normal values, ie, platelet counts remained significantly reduced at 3 months, as previously reported.22
Effects of hepsulfam on hematopoiesis. Hepsulfam (1.5 g/m2 intravenous bolus) produced thrombocytopenia (<100 × 109platelets/L) between day 12 and 25, with a nadir of 39 ± 20 × 109 platelets/L on day 17 (left). Neutropenia of less than 1 × 109 neutrophils/L occurred between day 8 and 30 following hepsulfam, with a nadir of 0.167 ± 0.120 × 109 neutrophils/L on day 15 (right).
Effects of hepsulfam on hematopoiesis. Hepsulfam (1.5 g/m2 intravenous bolus) produced thrombocytopenia (<100 × 109platelets/L) between day 12 and 25, with a nadir of 39 ± 20 × 109 platelets/L on day 17 (left). Neutropenia of less than 1 × 109 neutrophils/L occurred between day 8 and 30 following hepsulfam, with a nadir of 0.167 ± 0.120 × 109 neutrophils/L on day 15 (right).
Neutropenia of less than 1 × 109 neutrophils/L occurred between day 8 and day 30 following hepsulfam administration, with a nadir of 0.167 ± 0.120 × 109 neutrophils/L on day 15 (Fig 2, right). After hepsulfam treatment and in association with repeated blood sampling, anemia developed progressively, reaching a nadir hemaglobin of 8 g/dL on day 24, with gradual normalization over the subsequent month. Reticulocytes disappeared transiently from the circulation between days 5 and 12 following hepsulfam dosing.
Effects of PEG-rHuMGDF on posthepsulfam hematopoietic regeneration.Daily subcutaneous injections of PEG-rHuMGDF (2.5 μg/kg/d) beginning 1 day following hepsulfam injections and continuing for 21 days produced 7-day serum trough levels of 1.49 ± 0.29 ng/mL and 14-day trough levels of 1.91 ± 0.16 ng/mL (P > .2). The peripheral platelet counts exhibited a biphasic response, increasing progressively over prechemotherapy values for 9 days, then declining transiently to near baseline on day 15 (P = .01 comparing peak at 9 days and valley at 15 days using paired analysis), and subsequently climbing twofold over baseline by day 24 (P = .05 comparing valley at day 15 and peak at day 24 using paired analysis; peak platelet count of 856 ± 594 × 109/L v basal level of 416 ± 88 × 109/L; P = .01), thereafter falling to control levels over 10 days (Fig 3, left). PEG-rHuMGDF increased the proportion of megakaryocytes attaining the 64N and 128N ploidy classes (P < .005 in both cases compared with normal controls), with a reciprocal reduction in the 16N ploidy class (P < .005 compared with normal controls).
Effects of PEG-rHuMGDF on posthepsulfam hematopoietic regeneration. Daily subcutaneous injections of PEG-rHuMGDF (2.5 μg/kg/d), beginning 1 day after hepsulfam administration and continuing for 21 days, produced a biphasic increase in the peripheral platelet counts at or above prechemotherapy values (left). PEG-rHuMGDF dosing after hepsulfam chemotherapy shortened the duration of neutropenia from 22 to 12 days (P = .01), although the neutropenic nadir was not significantly changed (right). The open symbols depict the counts for untreated hepsulfam controls, and the closed symbols designate counts in PEG-rHuMGDF-treated hepsulfam animals.
Effects of PEG-rHuMGDF on posthepsulfam hematopoietic regeneration. Daily subcutaneous injections of PEG-rHuMGDF (2.5 μg/kg/d), beginning 1 day after hepsulfam administration and continuing for 21 days, produced a biphasic increase in the peripheral platelet counts at or above prechemotherapy values (left). PEG-rHuMGDF dosing after hepsulfam chemotherapy shortened the duration of neutropenia from 22 to 12 days (P = .01), although the neutropenic nadir was not significantly changed (right). The open symbols depict the counts for untreated hepsulfam controls, and the closed symbols designate counts in PEG-rHuMGDF-treated hepsulfam animals.
PEG-rHuMGDF dosing had no significant effect on the nadir of neutropenia produced by hepsulfam chemotherapy, ie, 0.224 ± 0.112 × 109 neutrophils/L, compared with 0.167 ± 0.120 × 109/L for hepsulfam alone (P > .3). However, the duration of neutropenia was significantly shortened from 22 days to 12 days (P < .01), and reached basal values after 30 days (Fig 3, right). Endogenous levels of G-CSF were not detectably increased. PEG-rHuMGDF did not affect the suppressive effect of hepsulfam on the reticulocyte count (P > .4; data not shown).
Effects of rHu-GCSF on posthepsulfam hematopoietic regeneration.Daily subcutaneous injections of rHu-GCSF (10 μg/kg/d) beginning 1 day after hepsulfam treatment and continuing for 21 days produced 7-day serum trough levels of 0.43 ± 0.27 ng/mL and 14-day trough levels of 1.42 ± 1.09 ng/mL (P > .3). rHu-GCSF alone had no effect on the nadir or duration of postchemotherapy thrombocytopenia, ie, the platelet count remained <100 × 109 platelets/L between day 12 and day 25, with a nadir of 56 ± 32 × 109/L on day 14 (Fig 4, left; P = .7). The endogenous Mpl-ligand levels increased to 0.24 ± 0.07 ng/mL at 7 days and 0.36 ± 0.08 ng/mL at 14 days (P > .5). Although hepsulfam markedly reduced hematopoietic progenitors, identifiable megakaryocytes shifted ploidy to higher classes compared with normal controls, similar to the effects produced by elevated levels of exogenous Mpl-ligand.
Effects of rHu-GCSF on posthepsulfam hematopoietic regeneration. rHu-GCSF (10 μg/kg/d) beginning 1 day after hepsulfam treatment and continuing for 21 days did not significantly improve the nadir or duration of postchemotherapy thrombocytopenia (left). Twentyone days of rHu-GCSF (10 μg/kg/d) injections shortened the period of neutropenia (<1 × 109 neutrophils/L) from 22 to 4 days (right); the intensity of the neutropenia was not significantly affected (right). The open symbols depict the counts for untreated hepsulfam controls, and the closed symbols designate counts in rHu-GCSF–treated hepsulfam animals.
Effects of rHu-GCSF on posthepsulfam hematopoietic regeneration. rHu-GCSF (10 μg/kg/d) beginning 1 day after hepsulfam treatment and continuing for 21 days did not significantly improve the nadir or duration of postchemotherapy thrombocytopenia (left). Twentyone days of rHu-GCSF (10 μg/kg/d) injections shortened the period of neutropenia (<1 × 109 neutrophils/L) from 22 to 4 days (right); the intensity of the neutropenia was not significantly affected (right). The open symbols depict the counts for untreated hepsulfam controls, and the closed symbols designate counts in rHu-GCSF–treated hepsulfam animals.
Posthepsulfam injections of rHu-GCSF (10 μg/kg/d) shortened the period of neutropenia (<1 × 109 neutrophils/L) from 22 days to 4 days (Fig 4, right; P = .005), although the nadir was not significantly affected (0.554 ± 0.490 × 109 neutrophils/L v hepsulfam control of 0.167 ± 0.120 × 109 neutrophils/L; P = .3). rHu-GCSF did not affect reticulocyte counts significantly (P = .5).
Effects of combining PEG-rHuMGDF and rHu-GCSF on hematopoietic regeneration.Administering daily subcutaneous injections of PEG-rHuMGDF (2.5 μg/kg/d) concurrently with rHu-GCSF (10 μg/kg/d) produced platelet counts of 127 ± 85 × 109/L (Fig 5, left), compared with 39 ± 20 × 109/L for hepsulfam alone controls (P = .03), and 856 ± 594 × 109/L for posthepsulfam PEG-rHuMGDF alone (P = .02). This combination of PEG-rHuMGDF and rHu-GCSF produced a neutropenic nadir of 1.18 ± 0.99 × 109/L (P = .01 compared with hepsulfam only controls). The 7-day serum trough levels of PEG-rHuMGDF averaged 1.06 ± 0.11 ng/mL, and the 14-day mean trough level was 2.40 ± 0.92 (P > .1). The rHu-GCSF 14-day mean trough level was 2.02 ± 1.92 ng/mL (P = 0.6 v 1.42 ± 1.09 ng/mL for rHu-GCSF alone).
Effects of combining PEG-rHuMGDF and rHu-GCSF on hematopoietic regeneration. Administering PEG-rHuMGDF (2.5 μg/kg/d, or 10 μg/kg/d) concurrently with rHu-GCSF (10 μg/kg/d) increased platelet counts in a dose-dependent fashion (left) compared with hepsulfam alone controls (P < .03 in both cases), but significantly less than the peak platelet count of 856 ± 594 × 109/L for posthepsulfam PEG-rHuMGDF (2.5 μg/kg/d) alone (P < .02 in both cases). Combination PEG-rHuMGDF and rHu-GCSF shortened the period of neutropenia (<1 × 109 neutrophils/L) to 4 days (right); (P = .001 v hepsulfam alone controls) without significantly increasing the nadir (P < .2 for hepsulfam only controls). The (○) depict the counts for untreated hepsulfam controls. The (▴) represent counts in hepsulfam animals concurrently receiving 2.5 μg/kg PEG-rHuMGDF and 10 μg/kg rHu-GCSF. The (•) designate counts in hepsulfam animals concurrently receiving 10 μg/kg PEG-rHuMGDF and 10 μg/kg rHu-GCSF.
Effects of combining PEG-rHuMGDF and rHu-GCSF on hematopoietic regeneration. Administering PEG-rHuMGDF (2.5 μg/kg/d, or 10 μg/kg/d) concurrently with rHu-GCSF (10 μg/kg/d) increased platelet counts in a dose-dependent fashion (left) compared with hepsulfam alone controls (P < .03 in both cases), but significantly less than the peak platelet count of 856 ± 594 × 109/L for posthepsulfam PEG-rHuMGDF (2.5 μg/kg/d) alone (P < .02 in both cases). Combination PEG-rHuMGDF and rHu-GCSF shortened the period of neutropenia (<1 × 109 neutrophils/L) to 4 days (right); (P = .001 v hepsulfam alone controls) without significantly increasing the nadir (P < .2 for hepsulfam only controls). The (○) depict the counts for untreated hepsulfam controls. The (▴) represent counts in hepsulfam animals concurrently receiving 2.5 μg/kg PEG-rHuMGDF and 10 μg/kg rHu-GCSF. The (•) designate counts in hepsulfam animals concurrently receiving 10 μg/kg PEG-rHuMGDF and 10 μg/kg rHu-GCSF.
Increasing PEG-rHuMGDF to 10 μg/kg/d in combination with rHu-GCSF (10 μg/kg/d) produced an exaggerated biphasic platelet response with a transient thrombocytopenic nadir on day 15 and subsequent peak platelet response of 509 ± 459 × 109 platelets/L (P < 10−4v hepsulfam only controls, and P = .01 v 127 ± 85 × 109 platelets/L for 2.5 μg/kg/d PEG-rHuMGDF combination dosing, and P = .03 v 856 ± 594 × 109 platelets/L for 2.5 μg/kg/d PEG-rHuMGDF only dosing; Fig 5).
Posthepsulfam injections of PEG-rHuMGDF (2.5 μg/kg/d) on days 1 to 22, with the addition of rHu-GCSF (10 μg/kg/d) from days 8 to 22, reproduced the biphasic increase in posthepsulfam platelet counts, peaking at 747 ± 317 × 109/L (P = .7 v 856 ± 594 × 109/L produced by 2.5 μg/kg/d PEG-rHuMGDF alone, P < 10−4v 56 ± 32 posthepsulfam rHu-GCSF alone, and P = .001 v prehepsulfam pretreatment baseline of 416 ± 88 × 109/L; Fig 6, left). Neutropenia was completely abolished by this dosing regimen (Fig 6, right), ie, neutrophil count >3.5 × 109/L throughout the postchemotherapy interval (P = .01 v the nadir neutrophil count for rHu-GCSF therapy alone). The serum trough concentrations of PEG-rHuMGDF for 7 days (1.34 ± 0.54 ng/mL) and 14 days (1.71 ± 0.45 ng/mL) were similar (P = .3). The 14-day trough levels of rHu-GCSF were 1.34 ± 1.16 ng/mL, similar to the 1.79 ± 1.25 ng/mL in the cohort receiving concurrent combined dosing (P = .5).
Prevention of both thrombocytopenia and neutropenia. PEG-rHuMGDF (2.5 μg/kg/d) singly for 7 days after hepsulfam, and adding daily rHu-GCSF (10 μg/kg/d) subsequently for 2 weeks restored the posthepsulfam platelet response produced by 2.5 μg/kg/d PEG-rHuMGDF alone (left) and abolished the period of neutropenia (right). The (○) depict the counts for untreated hepsulfam controls. The (•) represent counts in hepsulfam animals receiving 2.5 μg/kg PEG-rHuMGDF from day 1 through day 22, and 10 μg/kg rHu-GCSF from day 8 through day 22.
Prevention of both thrombocytopenia and neutropenia. PEG-rHuMGDF (2.5 μg/kg/d) singly for 7 days after hepsulfam, and adding daily rHu-GCSF (10 μg/kg/d) subsequently for 2 weeks restored the posthepsulfam platelet response produced by 2.5 μg/kg/d PEG-rHuMGDF alone (left) and abolished the period of neutropenia (right). The (○) depict the counts for untreated hepsulfam controls. The (•) represent counts in hepsulfam animals receiving 2.5 μg/kg PEG-rHuMGDF from day 1 through day 22, and 10 μg/kg rHu-GCSF from day 8 through day 22.
Coadministration of rHu-GCSF did not appear to significantly affect the levels of PEG-rHuMGDF, ie, 14-day levels of PEG-rHuMGDF were similar at 1.91 ± 0.16, 2.40 ± 0.92, and 1.71 ± 0.45 ng/mL for PEG-rHuMGDF (2.5 μg/kg/d only), PEG-rHuMGDF (2.5 μg/kg/d) and rHu-GCSF (10 μg/kg/d), and PEG-rHuMGDF (2.5 μg/kg/d) followed by rHu-GCSF (10 μg/kg/d), respectively (P = .219 by ANOVA). The 14-day serum trough concentrations of rHu-GCSF were similar for dosing rHu-GCSF (10 μg/kg/d) alone, concurrent administration with PEG-rHuMGDF (2.5 μg/kg/d), and delayed co-administration beginning 1 week after initiating PEG-rHuMGDF (2.5 μg/kg/d), ie, 1.42 ± 1.09, 2.02 ± 1.92, and 1.61 ± 1.34 ng/mL (P = .48 by ANOVA).
DISCUSSION
This study demonstrates in a rhesus model of hepsulfam-induced marrow suppression that stimulating megakaryocytopoiesis with PEG-rHuMGDF (2.5 μg/kg/d) abolishes postchemotherapy thrombocytopenia and attenuates neutropenia, while stimulating neutrophil production by rHu-GCSF (10 μg/kg/d) markedly truncates the period of postchemotherapy neutropenia without increasing platelet production, and coadministration of PEG-rHuMGDF and rHu-GCSF eliminates both thrombocytopenia and neutropenia when rHu-GCSF therapy is added after initiating postchemotherapy PEG-rHuMGDF treatment.
The present rhesus hepsulfam myelosuppressive model is useful for the preclinical evaluation of hematopoietic cytokines because it reproduces chemotherapeutic myelosuppression in patients, ie, predictable and severe thrombocytopenia lasting 13 days and neutropenia lasting 22 days, with gradual return to baseline values.22 Hepsulfam is an alkylating agent structurally related to busulfan (1,4 butanediol dimethanesulfonate), that is toxic to early hematopoietic progenitor cells,23,24,37 with little associated damage to non-hematologic tissues. The rhesus hepsulfam model has been used previously to characterize the effects of other recombinant human cytokines (GM-CSF, interleukin-3 [IL-3], IL-6, IL-11) on early hematopoietic progenitor cells and hematopoietic recovery.22 38
PEG-rHuMGDF stimulates megakaryocyte-lineage growth and development from hematopoietic progenitors (Fig 1 and Table 1),16,28,39,40 clears slowly from circulating blood as a result of pegylation,16 and is well tolerated in preclinical16,28,41,42 and initial clinical studies.43 Thus, PEG-rHuMGDF is a potent megakaryocyte lineage-dominant growth factor, analogous to the other late-acting hematopoietic cytokines, recombinant human erythropoietin (rHu-EPO) and rHu-GCSF. Under physiologic conditions the peripheral concentrations of erythrocytes, neutrophils, and platelets are maintained constant by independently regulating the production of each specific hematopoietic lineage via the corresponding hematopoietic cytokine to compensate for changing peripheral demands.2,13,14,16,18,44-48 Mpl ligands, similar to other cytokines regulating hematopoiesis, trigger intracellular signaling pathways in hematopoietic progenitors that mediate megakaryocyte development, proliferation, and differentiation, including tyrosine phosphorylation of membrane-associated kinases and adaptor molecules, ie, the Shc-Ras-raf1-MEK-ERK pathway, the Jak2-STAT3 and 5 pathway, PLC-γ, and Mpl itself.14 49-54 The differential between the increase in megakaryocyte mass and platelet turnover probably represents an unsteady state, although ineffective production cannot be ruled out.
After initiating PEG-rHu-MGDF therapy following hepsulfam-induced myelosuppression, there is a 3-day delay before the peripheral concentration of platelets rises (Fig 3), demonstrating that the processes of final megakaryocyte cytoplasmic maturation and platelet delivery are largely independent of PEG-rHuMGDF, consistent with studies of platelet release from megakaryocytes in vitro.17 Thereafter, the peripheral platelet counts increase progressively over prechemotherapy values for 9 days, then decline transiently to near baseline values on day 15, subsequently climb twofold over baseline by day 24, and fall to baseline levels over the ensuing 10 days (Fig 3, left). Because hepsulfam has destroyed the majority of hematopoietic progenitor cells, the initial platelet peak represents PEG-rHuMGDF stimulation of marrow megakaryocytes that survived hepsulfam toxicity, and responded to PEG-rHuMGDF-induced maturation mitosis (endoreduplication) and delivery of the corresponding expanded cytoplasm as additional circulating platelets (Table 1). The approximate doubling of the platelet count over baseline in the initial peak supports the interpretation that PEG-rHuMGDF induces on average one additional endomitotic event, thereby doubling substrate cytoplasm dedicated to platelet production. Furthermore, this result implies that most morphologically identifiable marrow megakaryocytes resist the lethal effects of hepsulfam. The subsequent valley in the platelet counts is attributable to hepsulfam's lethal contraction of the early hematopoietic progenitor cell reservoir that has not yet been replaced by PEG-rHuMGDF–induced proliferation. The second platelet peak reflects compensatory proliferative amplification of megakaryocyte progenitors and their subsequent maturation mediated by PEG-rHuMGDF after myelosuppressive chemotherapy. The steady fall in the concentration of peripheral platelets after discontinuing PEG-rHuMGDF therapy (Figs 1 and 4) reflects the 5 to 6 day survival time of circulating platelets together with continuing platelet production from already mature marrow megakaryocytes. As the concentration of circulating platelets increases over baseline in response to PEG-rHuMGDF postchemotherapy, the mean platelet volume decreases by approximately 15%, returning to normal only after PEG-rHuMGDF injections are discontinued, similar to the reduction in platelet volume observed without associated hepsulfam chemotherapy.16 28
The effects of PEG-rHuMGDF on postchemotherapy platelet production in the present study are in accord with reports that recombinant Mpl-ligands accelerate platelet recovery in several different murine chemotherapy models, including myelosuppressive chemotherapy,55,56 combination of carboplatin and irradiation,41,57,58 and bone marrow59-61 or peripheral blood stem cell transplantation.62 PEG-rHuMGDF specifically produces log-linear dose-dependent responses in platelet recovery and prevents thrombocytopenia in mice receiving carboplatin chemotherapy,55 and significantly reduces the severity and duration of thrombocytopenia, and improves mortality in the more severe model using irradiation plus carboplatin.41 Transplantation of PEG-rHuMGDF-mobilized peripheral blood progenitor cells results in accelerated platelet recovery, especially if PEG-rHuMGDF is administered following transplantation.62 In addition, bone marrow transplants from donor mice pretreated with rHu-TPO exhibit accelerated platelet recovery.60 In nonhuman primates, rHu-MGDF or PEG-rHuMGDF accelerates platelet recovery following sublethal total body irradiation (TBI)63; control animals exhibit more than 12 days of severe thrombocytopenia (<20,000 μL), while animals receiving either rHu-MGDF or PEG-rHuMGDF for 18 days average ≤0.5 days of severe thrombocytopenia. In this model, the concurrent administration of PEG-rHuMGDF and rHu-GCSF, both beginning 1 day after TBI, accelerated multilineage hematopoietic recovery without evidence of lineage competition.63 Additional studies in this model indicate that PEG-rHuMGDF is effective even when administered three times per week, but that there is significant decrease in efficacy if therapy is initiated on day 5, as opposed to day 1.42 Recently, rHu-TPO has been reported not to improve platelet recovery following myeloablative TBI and infusion of small numbers of highly purified CD34 positive DR dull cells.19
The present study shows that PEG-rHuMGDF alone facilitates postchemotherapy neutrophil recovery, as evidenced by its capacity to shorten the period of neutropenia following severe myelosuppressive chemotherapy from 22 to 12 days (Fig 3, right; <0.01 compared with untreated hepsulfam controls). Although the mechanism(s) whereby PEG-rHuMGDF improve neutrophil recovery are not presently understood, recent evidence suggests that megakaryocytes may produce cytokines exhibiting hematopoietic activities, including IL-1, IL-3, IL-6, and GM-CSF.64-66 Alternatively, there is considerable cytokine pleiotropy evident for lineage-dominant cytokines in vitro.13,49,67-70 The possibility that PEG-rHuMGDF might increase the production of endogenous G-CSF sufficiently to mediate the improved neutrophil response is not excluded, since the present assay lacks the sensitivity to rule out a biologically significant increase in endogenous G-CSF levels, and mRNA levels for G-CSF in megakaryocytes were not measured. The present observations are in agreement with the reported improvement in neutrophil counts produced by rHu-MGDF in nonhuman primates following radiation-induced myelosuppression.42 Myelosuppressed mice receiving PEG-rHuMGDF alone, also show improved leukocyte and red blood cell recoveries, and markedly reduced mortality (94 % v 15% with PEG-rHuMGDF ). However, rHu-GCSF therapy alone does not improve platelet recovery (Fig 4, left), despite measurable increases in serum endogenous Mpl-ligand.
In the present study rHu-GCSF (10 μg/kg/d) alone reduces the time for postchemotherapy neutropenia from 22 days to 4 days, although the intensity of the neutropenic nadir is not significantly attenuated (Fig 4, right), and neither the duration nor intensity of thrombocytopenia is improved (Fig 4, left). The present data showing that rHu-GCSF does not induce megakaryocyte growth and development, has been observed by others previously.14 71 Presumably, the explanation for the inability of rHu-GCSF to prevent transient neutropenia, as opposed to the elimination of thrombocytopenia by high-dose PEG-rHuMGDF, is related in part to the removal of neutrophils from the peripheral blood within hours after neutrophils enter the blood stream, contrasted to the platelets' 5- to 6-day lifespan after entry into the circulation.
Coadministration of rHu-GCSF with PEG-rHuMGDF reproduces the postchemotherapy neutrophil responses induced by rHu-GCSF alone, and produces dose-dependent increases in postchemotherapy peripheral platelet counts compared with untreated hepsulfam controls, ie, 39 ± 20 × 109/L for untreated hepsulfam, 127 ± 85 × 109/L and 509 ± 459 × 109/L for 2.5 μg/kg/d and 10 μg/kg/d PEG-rHuMGDF, respectively. However, the platelet responses following concurrent therapy with rHu-GCSF and PEG-rHuMGDF are significantly less than the posthepsulfam response produced by PEG-rHuMGDF treatment alone, ie, 856 ± 594 × 109/L (P < .02 in both cases). The hypothetical mechanism(s) whereby rHu-GCSF may blunt PEG-rHuMGDF–induced platelet responses include lineage competition for early pluripotent progenitors, relative ligand-receptor specificities, binding affinities, signal transduction, receptor redundancy/pleiotropy, and altered pharmacokinetics, impacting on a markedly depleted reservoir of early hematopoietic progenitors surviving hepsulfam chemotherapy. The present study does not confirm or exclude any of these possibilities. However, changes in pharmacokinetics do not appear to be the explanation, as the pharmacokinetics of rHu-GCSF and PEG-rHuMGDF in the present study are not significantly affected by either of these cytokines. It should also be noted that coadministration of rHu-GCSF and PEG-rHuMGDF in a rhesus model of TBI-myeloablation, did not show diminution in megakarycoyte responsiveness.63
Because PEG-rHuMGDF induces both platelet and neutrophil responses after marrow suppressive chemotherapy (Fig 4), we studied the effects on hematopoietic recovery of adding rHu-GCSF after instituting PEG-rHuMGDF therapy. By administering PEG-rHuMGDF for 1 week before adding rHu-GCSF for 2 subsequent weeks, both the platelet and neutrophil counts are maintained at or above baseline values, which was not achievable by concurrently administering the two cytokines.
Thus, in this rhesus model of hepsulfam marrow suppression, initiating PEG-rHuMGDF therapy for 7 days and adding rHu-GCSF thereafter completely eliminates postchemotherapy thrombocytopenia and neutropenia. The combination of lineage-dominant PEG-rHuMGDF and rHu-GCSF following potentially curative myelosuppressive chemotherapy may be a useful strategy for minimizing hematopoietic toxicity in patients receiving high-dose marrow-lethal chemotherapy with marrow or peripheral blood progenitor cell transplantation. The present study demonstrates the importance of optimizing the dose and schedule of cytokine combinations following severe myelosuppressive chemotherapy.
ACKNOWLEDGMENT
We thank Deborah White and Evan Dessasau for expert technical assistance. We also thank Dr William P. Sheridan for useful suggestions during this study and for reviewing the manuscript.
Supported by National Institutes of Health Grants No. HL31950 and RR00165 and by a research grant from Amgen, Inc.
Address reprint requests to Laurence A. Harker, MD, Division of Hematology and Oncology, Emory University School of Medicine, PO Drawer AR, Atlanta, GA 30322.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal