Abstract
Normal peripheral blood mononuclear cells (PBMC) were cocultured with a human lung cancer cell line (LC89) transduced with the interleukin-2 (IL-2), IL-7, granulocyte-macrophage colony-stimulating factor (GM-CSF ), and tumor necrosis factor-α (TNF-α) genes to evaluate the capacity of the engineered cells to: allow survival of CD3+ and CD56+ cells, generate cytotoxic effectors with HLA class I restricted and unrestricted antitumor activity, and interfere in the molecular organization of the CD3/T-cell receptor associated signal transduction machinery. When PBMC were cultured up to 3 weeks with IL-2 releasing LC89 cells (LC89/IL-2), the number of viable CD3+ and CD56+ lymphocytes was much greater than in cultures with parental cells or with LC89 cells transduced with the other cytokine genes. After 1 week of coculture, a variable degree of restricted and unrestricted killing directed against different targets was observed. When the cultures were prolonged up to 3 weeks, LC89/IL-2 cells induced a marked increase in specific cytotoxic activity, which was coupled to a further enhancement of unrestricted lytic function. In the presence of LC89/IL-7 cells the degree of specific lysis remained unchanged, whereas unrestricted effectors were markedly decreased. No cytotoxic activity could be induced by LC89/GM-CSF and LC89/TNF-α cells in the few lymphocytes surviving after 3 weeks of culture. Coculture of parental LC89 cells with PBMC was consistently associated with a downmodulation in the expression of the CD3 ζ chain, as well as of the tyrosine kinases p56lck and ZAP-70. On the contrary, LC89/IL-2 cells, and not LC89 cells transduced with the IL-7, GM-CSF, or TNF-α gene, were capable of reverting the immunosuppressive effect exerted by the tumor cells. This protective effect could be maintained in cultures prolonged up to 4 weeks. When the same cultures were set up in Transwell, ie, with a membrane separation between cancer cells and PBMC, the expression of the CD3 ζ chain and of the p56lck and ZAP-70 tyrosine kinases remained unchanged under all culture conditions, indicating that the downmodulation of T-cell signal transduction molecules requires a direct cell to cell contact. These results show that transfer of the IL-2 gene into the DNA of human cancer cells promotes both restricted and unrestricted antitumor activity, and is capable of restoring and maintaining the expression of molecules involved in the process of T-cell mediated tumor cell recognition, thus underlining the potential role of the IL-2 gene in the design of vaccination protocols with cytokine gene transduced cancer cells.
ESCAPE FROM THE host immune system is a primary cause of tumor occurrence and progression.1,2 The reasons for cancer escape are likely to be multiple and strategies aimed at stimulating the immune compartment of the tumor bearing patient have been a long-desired goal.2-5
Over the last few years, several authors have shown the possibility of successfully transducing cytokine genes into the DNA of experimental and human cancer cell lines.5 The engineered cell lines show a stable gene insertion and constitutive cytokine release, whereas the morpho-phenotypic and proliferative features remain unchanged. When inoculated into syngeneic animals or immunosuppressed nude mice, the tumorigenic potential of the transduced neoplastic cells is often decreased or abrogated, most likely as a consequence of the activation of different cell populations — neutrophils, eosinophils, monocyte/macrophages, natural killer (NK), and T lymphocytes — capable of exerting antitumor activity. These encouraging preclinical results have prompted the design of clinical vaccination protocols using tumor cells transduced with various cytokine genes for the management of cancer patients, in an attempt to induce/amplify an antitumor response by immune competent effectors.5 6 The primary goal is to activate an adequate number of specific cytotoxic cells, with memory potential, capable of rejecting the tumor.
Recently, it has been reported that altered levels of signal transduction molecules associated with the CD3/T-cell receptor (TCR) machinery may play a role in the T-cell dysfunction present in tumor bearing hosts.7 These findings have offered a molecularly based interpretation for the tumor specific T-cell incompetence which takes place in cancer patients.
In a recent study we documented the successful and stable insertion of the genes for interleukin-2 (IL-2), IL-7, granulocyte-macrophage colony-stimulating factor (GM-CSF ) and tumor necrosis factor-α (TNF-α) into the DNA of a human lung adenocarcinoma cell line (LC89).8 Cytokine gene transduced LC89 cells maintained their morphologic and proliferative features unmodified, whereas the tumorigenic capacity in nude mice was completely abolished when transduced with the IL-2 gene and reduced following insertion of the IL-7 and TNF-α genes. Furthermore, a decrease in the mRNA expression and production of transforming growth factor β1 (TGF β1 ), a cytokine with well-documented immunosuppressive activity,9 was observed in LC89 cells transduced with the IL-7, GM-CSF, and, to a greater extent, the IL-2 gene.
In view of these results, in the present study we have set up cocultures with LC89 cells transduced with the IL-2, IL-7, GM-CSF, and TNF-α genes and normal peripheral blood mononuclear cells (PBMC) to evaluate the capacity of the engineered cells to: (1) trigger the expansion of T and NK cells; (2) generate cytotoxic effectors with HLA class I restricted and unrestricted antitumor activity; (3) preserve the T-cell signal transduction machinery, by monitoring changes in the expression of the CD3 ζ chain and of the tyrosine kinases p56lck and ZAP-70. Evidence is provided that LC89 cancer cells transduced with the IL-2 gene can fulfill these objectives.
MATERIALS AND METHODS
Tumor cell line (LC89) and cytokine gene transduction.LC89 was established from a lymph node metastasis of a male patient affected by poorly differentiated lung adenocarcinoma.10 The cell line grows in complete medium consisting of RPMI 1640 with the addition of penicillin (100 U/mL), streptomycin (100 mg/mL), 2 mmol/L glutamine and 10% fetal bovine serum (all products were purchased from Hyclone, Logan, UT).
The insertion of cytokine genes was performed by retroviral vector techniques.11 Briefly, the IL-2 and GM-CSF genes were introduced into the LXSN vector12 carrying the neomycin resistance gene; the TNF-α gene was inserted into the retroviral vector N2A,13 which also carries the neomycin resistance gene, while the IL-7 gene was introduced into the EMCV HyTK IRES vector,14 which carries the hygromycin resistance gene. All vectors were transfected into the packaging cell lines AM1215 or PA317.16 Supernatants of packaging cell lines containing the highest titer of virus were used for the infections of LC89. Infected LC89 cells were cultured in selection media and the gene insertion of cytokines was evaluated by reverse transcriptase-polymerase chain reaction.17 The IL-2 primer pairs were purchased from Clontech (Palo Alto, CA); the nucleotide sequences of the IL-7, GM-CSF, and TNF-α oligonucleotide primers were as follows: IL-7 = 5′ primer 5′-ATG-TTC-CAT-GTT-TCT-TTT-AGG-TAT-ATC-T-3′, 3′ primer 5′-GTG-TTC-TTT-AGT-GCC-CAT-CAA-3′, PCR fragment size 531 bp; GM-CSF = 5′ primer 5′-TTT-ATG-GCA-CCC-GCC-CGC-TCG-3′, 3′ primer 5′TTT-TCA-TCA-CTC-CTG-GAC-TGG-CTC-3′, PCR fragment size, 393 bp; TNF-α = 5′ primer 5′-TTT-TTC-CCC-AGG-GACCTC-TCT-3′, 3′ primer 5′-GCA-ATG-ATC-CCA-AAG-TAG-ACC-TGC-CC-3′, PCR fragment size 514 bp.
Cytokine levels were determined in the supernatants of 1 × 106 parental and gene transduced LC89 cells after 48 hours of culture using enzyme-linked immunosorbent assay (ELISA; generously provided by Medgenix Diagnostics, Fleurus, Belgium). The amounts of cytokine released by parental and gene transduced LC89 cells were as follows: IL-2 0 U/mL and 200 U/mL, IL-7 0 U/mL, and 259 U/mL, GM-CSF 5 pg/mL and 20 pg/mL, TNF-α 0 pg/mL and 16 pg/mL, respectively.
The production of cytokines by LC89 transduced cells remained stable for over 6 months.
Mixed lymphocyte tumor culture (MLTC).PBMC were obtained from healthy donors following separation on a Lymphoprep (Nycomed, Pharma AS, Oslo, Norway) gradient. 1 × 106 PBMC were mixed with 5 × 105 parental or cytokine gene transduced LC89 cells. On day 7, PBMC were collected and an aliquot tested for cytotoxicity, cytofluorimetric profile using a FACScan (Becton Dickinson, Mountain View, CA) and Western blot analysis, whereas 1 × 106 PBMC/well were restimulated with 1 × 105 parental or gene transduced LC89 cells. This restimulation procedure was repeated every 7 days up to 21 days of culture. Using the same cell proportions and culture procedures, in some experiments the MLTC were seeded into Transwell dishes (Falcon, Becton Dickinson Labware, Lincoln Park, NJ), which enabled LC89 parental and transduced cells to be cultured separate from PBMC by pored (0.45 μm pore size) membranes.
Fluorescence-activated cell sorter analysis.5 × 105 effector cells were incubated with conjugated monoclonal antibodies (MoAbs; Becton Dickinson) for 30 minutes at 4°C. After washing twice, cell preparations were analyzed on a FACScan (Becton Dickinson).
Cytotoxic assay.A standard 51Cr release assay was used. Target cells (5 × 105) were incubated with 100 μCi 51Cr for 1 hour at 37°C and then washed twice with complete medium. A total volume of 150 μL of complete medium containing 2 × 103 target cells and various numbers of effector cells at different final effector: target ratios was placed into V-bottomed microtiter plates. After incubation at 37°C for 4 hours and centrifugation at 1,200 rev/min for 10 minutes, an aliquot (100 μL) of the supernatant was collected and counted in a gamma scintillation counter. All experiments were performed in triplicate and the percent of 51Cr release calculated according to the formula: E-S/M-S × 100, where E is the mean cpm release in the presence of effector cells, S is the mean cpm spontaneously released by the target cells incubated with medium alone and M is the mean cpm release of 100 μL resuspended target cells.
Target cells were represented by the K562 cell line to study NK activity, the Raji cell line to study HLA class I unrestricted tumoral cytotoxicity and the LC89 cell line to assess specific (restricted) antitumor cytotoxicity. Inhibition of specific cytotoxicity was studied in a competition test by adding 5 × 104 cold K562 cells to every well and following incubation of LC89 target cells with the anti-HLA class I MoAb W6/32 (2 μg/well).
Immunoblotting.The expression of the TCR CD3 ζ chain and of the p56lck and ZAP-70 tyrosine kinases was studied by Western blot analysis of PBMC obtained from MLTC. Cells were lysed in buffer containing 50 mmol/L HEPES (pH 7.2) (Life Technologies Inc, Gaithersburg, MD), 150 mmol/L NaCl, 5 mmol/L Na EDTA, 1 mmol/L sodium orthovanadate (Sigma Italia, Milano, Italy), 2 mmol/L phenylmethylsulfonyl fluoride (Sigma-Aldrich srl, Milano, Italy), 100 μg/mL soybean trypsin/chymotrypsin inhibitor (Sigma), 100 μg/mL chymostatin (Sigma), and 50 mmol/L 6-O-(N-heptylcarbamoyl)-methyl-α-D-glucopyranoside (Hecameg, Vegatec, Villejuif, France). The latter is a highly purified, UV transparent, nonionic detergent derived from glucose that does not denature proteins and does not interfere with antigenicity or enzyme activity.18 Protease inhibitors were selected because of their capacity to block degradation of T-cell transducers in the presence of contaminating granulocytes.19 The cell lysates were centrifuged at 12,000 rpm for 5 minutes at 4°C, and the supernatants carefully removed. Protein quantitation was performed by reading absorbance of UV irradiation at 280 nm with a Beckman Du-70 spectrophotometer (Beckman Instruments, Palo Alto, CA). According to the cytofluorimetrically defined T-cell percent in cultures, equivalent amounts of T-cell derived proteins rather than equivalent amounts of cells were loaded per lane (15 μg/lane). Solubilized proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis under reducing conditions using an 8% Tris-glycine gel (Novex, San Diego, CA) for p56lck and ZAP-70, and a 14% Tris-glycine gel (Novex) for CD3ζ. Proteins were transferred to polyvinylidene difluoride (PVDF ) membranes (Novex), blocked with a 5% solution of nonfat dried milk in Tris-saline + 0.1% Tween 20 (Sigma), and blotted with mouse antihuman TCRζ (Coulter, Hialeah, FL), mouse anti-human p56lck (IgG2a ), or mouse antihuman ZAP-70 (IgG2a )(Transduction Laboratories, Lexington, KY). Horseradish peroxidase-labeled rabbit antimouse IgG (Amersham Italia, Milano, Italy) was used as secondary antibody and developed via an enhanced chemoluminescence (ECL) Western blotting detection kit (Amersham) on Hyperfilm-ECL (Amersham). Films were scanned using an UltroScan 2022 laser densitometer (Pharmacia Biotech, Uppsala, Sweden) and peak areas expressed in arbitrary OD units.
RESULTS
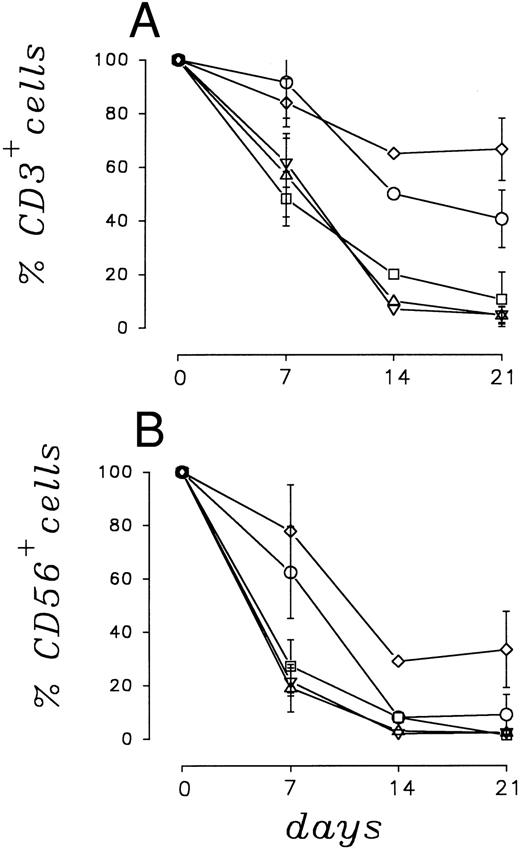
Number and survival of CD3+ and CD56+ cells.The numerical pattern of CD3+ and CD56+ PBMC cells, calculated as a percent of seeded cells, is shown in Fig 1A and B. The results represent the mean ± SE of five independent experiments. After 7 days of coculture with LC89 cells transduced with the IL-2 gene, 86.1% ± 9.1% of CD3+ and 78.1% ± 17.4% of CD56+ PBMC remained viable; after 21 days of coculture, 66.4% ± 11.6% of CD3+ and 32.8% ± 14.2% of CD56+ cells were still recovered. Cocultures for 7 days with IL-7 gene transduced LC89 cells resulted in a 91.9% ± 13.2% and 62.3% ± 17.2% viable normal CD3+ and CD56+ lymphocytes, respectively. These values decreased to 40.3% ± 10.7% and 8.9% ± 7.5% after 21 days of culture.
Survival of CD3+ (A) and CD56+ (B) cells in cocultures of PBMC with parental and cytokine gene transduced LC89 cells, evaluated as percent of the seeded cells.(□) LC89; (⋄) LC89/IL-2; (○) LC89/IL-7; (▵) LC89/GM-CSF; (▿) LC89/TNF-α.
Survival of CD3+ (A) and CD56+ (B) cells in cocultures of PBMC with parental and cytokine gene transduced LC89 cells, evaluated as percent of the seeded cells.(□) LC89; (⋄) LC89/IL-2; (○) LC89/IL-7; (▵) LC89/GM-CSF; (▿) LC89/TNF-α.
Recovery of CD3+ and CD56+ viable cells was much lower under the remaining culture conditions. In fact, CD3+ lymphocytes cocultured for 7 days with LC89 cells transduced with the GM-CSF and TNF-α genes showed a viability of 57.0% ± 15.0% and 61.7% ± 9.1%, respectively (Fig 1A). These values were superimposable to those obtained with parental LC89 cells (48.0% ± 10.1%). Similar results were obtained for CD56+ cells (Fig 1B), for which the percent of viable recovery was 19.3% ± 8.8%, 21.6% ± 5.2% and 27.3% ± 9.9% after 7 days of coculture with LC89/GM-CSF, LC89/TNF-α and parental LC89 cells, respectively. After 21 days of coculture, the recovery of viable lymphocytes was always very low. In cocultures with LC89/GM-CSF, LC89/TNF-α and parental LC89, the recovery of CD3+ cells was 4.6% ± 3.1%, 4.8% ± 3.05% and 10.6% ± 10.1%, and that of CD56+ cells 1.8% ± 1.3%, 1.5% ± 1.33% and 0.9% ± 0.8%, respectively.
Under no coculture conditions were viable monocyte/macrophages or B lymphocytes ever detected. No marked variation in the CD4/CD8 lymphocyte ratio was observed in the different cocultures between normal PBMC and parental or cytokine gene transduced LC89 cells.
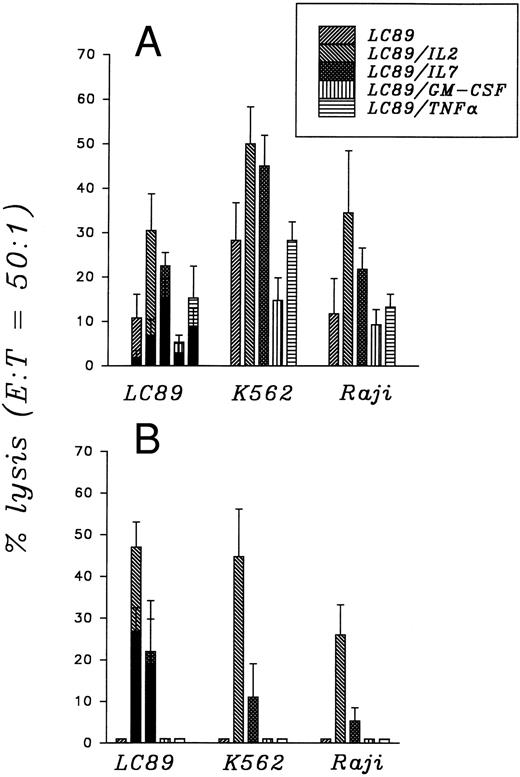
Cytotoxicity.The capacity of normal PBMC to lyse different tumor targets after 7 and 21 days of coculture with parental and cytokine gene transduced LC89 cells is illustrated in Fig 2A and B. Each result is the mean ± SE of four independent experiments.
Cytotoxic activity of PBMC cocultured for 7 (A) and 21 (B) days with parental and cytokine gene transduced LC89 cells, evaluated as percent of target lysis. (▪) in the presence of 5 × 104 cold K562 cells.
Cytotoxic activity of PBMC cocultured for 7 (A) and 21 (B) days with parental and cytokine gene transduced LC89 cells, evaluated as percent of target lysis. (▪) in the presence of 5 × 104 cold K562 cells.
PBMC cocultured for 7 days with LC89/IL-2 cells suggested the generation of both restricted and unrestricted cytotoxic effectors. In fact, when tested against LC89 a 30.5% ± 8.2% of lysis was observed; this decreased to 7.2% ± 3.4% when K562 cells were added as cold target competitor. At the same time, a high unrestricted lytic activity was recorded against both the K562 (50.1% ± 8.3%) and Raji (34.6% ± 14.0%) cell lines (Fig 2A). After 21 days of coculture, a further increase in the capacity of PBMC to lyse LC89 cells was observed (46.9% ± 6.0%), even when cold K562 cells were added as competitors (26.8% ± 5.5%) (Fig 2B). The specific activity, considered as the percent of cytotoxicity which persists in the presence of a cold competitor, induced by LC89/IL-2 cells was thus equal to 57.1%. This result was further confirmed when LC89 target cells were incubated in the presence of an anti-HLA class I MoAb which prevents recognition by specific cytotoxic effectors. Under such conditions, the capacity of PBMC to kill LC89 cells decreased to 14.2% ± 4.3%. The simultaneous presence of unrestricted cytotoxic effectors was supported by the persisting capacity of PBMC to lyse, after 21 days of coculture, both K562 (44.6% ± 11.4%) and Raji (26.0% ± 7.2%) cells.
When normal PBMC were cocultured for 7 days with LC89/IL-7 cells the degree of lysis against LC89 was 22.5% ± 3%, which decreased to 15.1% ± 4.2% in the presence of cold K562 cells. Cytotoxic activity against K562 (45.1% ± 7%) and Raji (21.4% ± 4.8%) cells was also recorded. After 21 days of coculture with LC89/IL-7 cells, the activity against LC89 remained practically unchanged (21.9% ± 12.1%), also if cold competitors were added (19.2% ± 10.4%). The high degree of restricted lysis was confirmed by the inhibitory action exerted by the W6/32 anti-HLA class I MoAb incubated with target LC89 cells which reduced the lysis to 7.3% ± 3.7%. A decrease in unrestricted cytotoxic activity was supported by the low lytic function observed when the effectors were tested against K562 (11.0% ± 8%) and Raji (5.3% ± 3.1%) cells.
PBMC cocultured for 7 days with parental LC89 and with LC89 cells transduced with the GM-CSF and TNF-α genes induced a cytotoxic function against LC89 equal to 10.7% ± 5.3%, 5.0% ± 1.6% and 15.4% ± 7.2%, respectively. These values decreased in the presence of a cold competitor (K562) to 2.1% ± 1.4%, 2.7% ± 2% and 9.3% ± 3.9%, respectively. The unrestricted activity of PBMC cocultured for 7 days with LC89, LC89/GM-CSF and LC89/TNF-α was further documented by the degree of lysis observed against K562 cells: 28.3% ± 8.5%, 14.9% ± 5% and 28.4% ± 4.2%, respectively. The lytic function against the Raji cell line was low: 4.0% ± 1.4%, 9.0% ± 3.4% and 13.3% ± 2.9%, respectively. When the cocultures were prolonged further, a progressive decrease in the number of effector cells, as reported earlier (Fig 1), was observed. At day 21, the cytotoxic activity, when assessable due to low cell recovery, was always lower than 5% against all targets.
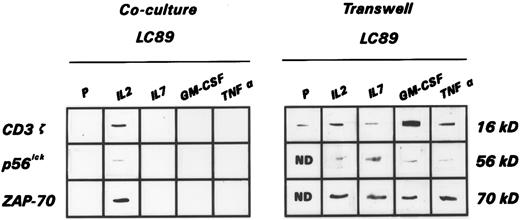
Expression of the CD3 ζ chain and of the p56lck and ZAP-70 tyrosine kinases.Coculture of parental LC89 cells with normal PBMC induced in the latter, after 7 days of incubation, a strong downmodulation in the expression of the ζ chain of the CD3, documented by Western blot analysis. This was also accompanied by a marked reduction in the expression of the tyrosine kinases p56lck, a member of the Src family, and ZAP-70, a member of the Syk family, which are known to be closely involved in the signal transduction processes. This downmodulation increased further after a more prolonged co-incubation time. Figure 3 shows the disappearance of the TCR ζ chain, as well as of p56lck and ZAP-70 after 14 days of culture from a representative experiment of the five performed with equivalent results. Findings similar to those observed with parental LC89 cells were recorded when PBMC were cocultured with LC89 cells transduced with the IL-7, GM-CSF, and TNF-α genes (Fig 3). On the contrary, the expression of the CD3 ζ chain, and of the tyrosine kinases p56lck and ZAP-70 was maintained when PBMC were cultured in the presence of LC89/IL-2 cells (Fig 3).
Expression of the CD3 ζ chain, as well as of p56lck and ZAP-70 tyrosine kinases, evaluated by immunoblotting, in a representative experiment of PBMC cocultured for 14 days with parental and cytokine gene transduced LC89 cells under cell-to-cell contact conditions (Coculture LC89) or in the presence of a pored membrane separation (Transwell LC89).
Expression of the CD3 ζ chain, as well as of p56lck and ZAP-70 tyrosine kinases, evaluated by immunoblotting, in a representative experiment of PBMC cocultured for 14 days with parental and cytokine gene transduced LC89 cells under cell-to-cell contact conditions (Coculture LC89) or in the presence of a pored membrane separation (Transwell LC89).
To assess whether the downmodulation of the intracellular signals required a direct contact between PBMC and LC89 tumor cells, Transwell culture dishes that permit soluble factors released by both effectors and stimulators to migrate, without allowing a direct cell to cell interaction, were used. Under such culture conditions, PBMC always maintained unchanged the expression of the CD3 ζ chain, as well as of the p56lck and ZAP-70 tyrosine kinases (Fig 3). These experiments were repeated four times with identical results.
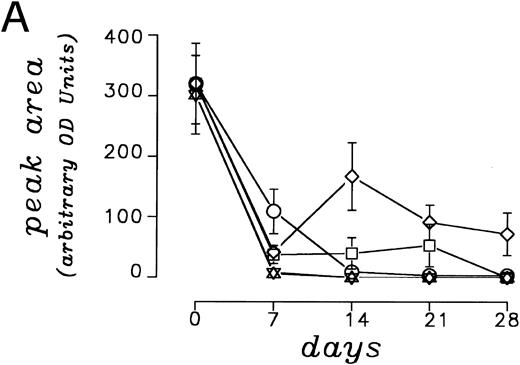
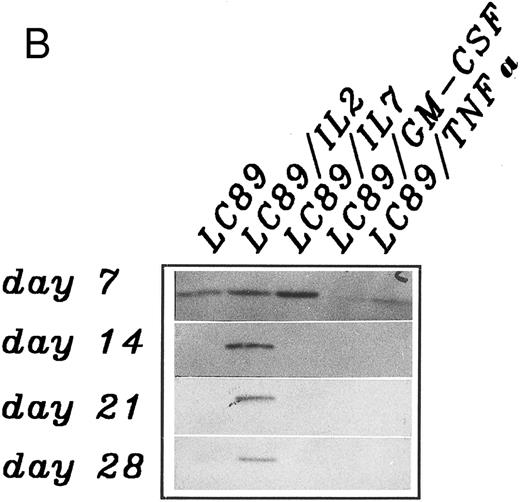
A densitometric time course monitoring of the CD3 ζ chain, as a representative indicator of the pattern of expression of CD3 associated signal transduction molecules, carried out in five independent experiments showed an initial down-modulation after 7 days of coculture between PBMC and parental and cytokine gene transduced LC89 cells (Fig 4A). By day 14, a restoration in CD3 ζ expression was documented in cocultures with LC89/IL-2 cells, which persisted for 4 weeks. No recovery of the CD3 ζ chain was observed under the other culture conditions. Panel B shows the expression of the CD3 ζ chain in a representative coculture experiment between PBMC and parental and cytokine gene transduced LC89 cells, which confirms the persistent presence of the molecule over time in the presence of LC89/IL-2 cells.
(A) Modulation of the CD3 ζ chain expression on PBMC co-cultured up to 28 days with parental or cytokine gene transduced LC89 cells, evaluated by immunoblotting and expressed as arbitrary O.D. units of peak area (mean of five independent experiments). (□) LC89; (⋄) LC89/IL-2; (○) LC89/IL-7; (▵) LC89/GM-CSF; (▿) LC89/TNF-α. (B) Expression at different time periods of the CD3 ζ chain, evaluated by immunoblotting, in a representative coculture experiment between PBMC and parental and cytokine gene transduced LC89 cells.
(A) Modulation of the CD3 ζ chain expression on PBMC co-cultured up to 28 days with parental or cytokine gene transduced LC89 cells, evaluated by immunoblotting and expressed as arbitrary O.D. units of peak area (mean of five independent experiments). (□) LC89; (⋄) LC89/IL-2; (○) LC89/IL-7; (▵) LC89/GM-CSF; (▿) LC89/TNF-α. (B) Expression at different time periods of the CD3 ζ chain, evaluated by immunoblotting, in a representative coculture experiment between PBMC and parental and cytokine gene transduced LC89 cells.
DISCUSSION
Over the last few years the possibility of using immunotherapeutic strategies in the management of neoplastic conditions has been reinforced. This is largely due to the clinical use of substances, namely cytokines, capable of stimulating the immune system of the host to both activate and resume from their anergic state effector cells physiologically devoted to exert an anticancer action.4,5 In this setting one may place gene transfer approaches aimed at using cytokine gene transduced tumor cells as vaccination therapy.5,6 Extensive data have been accumulated in experimental models which point to the effectiveness of cancer cells transduced with different cytokine genes to stimulate various effector cell populations to reject the tumor and, in particular, to induce the generation of lymphocytes with specific cytotoxic effect and with antitumor memory.20 21
In humans, it is difficult to show which cytokine gene transduced into the DNA of cancer cells may trigger more effectively an antineoplastic response. The results of the present study indicate that human lung adenocarcinoma cells transduced with the IL-2 gene cocultured with normal PBMC are capable of propagating T and NK cells, of stimulating allogeneic lymphocytes to exert HLA class I restricted and unrestricted antitumor activity, and of effectively restoring the expression of the CD3 ζ chain and of the tyrosine kinases p56lck and ZAP-70, molecules involved in the process of T-cell associated signal transduction. These effects were not obtained with cancer cells transduced with the genes for IL-7, GM-CSF, and TNF-α.
The evidence that LC89 cells transduced with the IL-2 gene are capable of maintaining viable a considerable proportion of CD3+ T lymphocytes and, to a lesser extent, also of CD56+ cells, even after 21 days of culture, is of relevance since the generation of a specific antitumor response requires a time period to become effective.22 The IL-7 gene transduced into LC89 cells also induced a certain degree of protection towards lymphocyte viability, but this was less pronounced compared to that promoted by the IL-2 gene. Transduction of the GM-CSF and TNF-α genes was ineffective and cocultured PBMC showed a survival time identical to that observed with PBMC incubated with parental LC89. These findings are in agreement with the known capacity of exogenous IL-2 to stimulate in particular T- and NK-lymphocyte subpopulations. The effects exerted by the transfer of the IL-7 gene were partly disappointing, because this cytokine has been shown to trigger a proliferative signal on both T and NK cells.23 The results observed in the gene transduced model may be caused by an insufficient level of IL-7 release. The possibility of subcloning gene transduced tumor cells in an attempt to obtain higher IL-7 secreting clones was not pursued because this would have meant carrying out a selection process, which would have restricted the immunogenic potential of the parental tumor cell line.
PBMC cocultured for 7 days with LC89 cells transduced with the IL-2 gene and, to a lesser extent, with the IL-7 and TNF-α genes displayed both unrestricted cytotoxic activity against K562 (NK target) and Raji (LAK target) cells, as well as specific activity directed against LC89 cells. When the cocultures were prolonged up to 3 weeks, the specific cytotoxic potential of lymphocytes cocultured with LC89/IL-2 against LC89 cells increased further, while lymphocytes cocultured with LC89/IL-7 showed an unchanged lytic capacity against LC89 cells and a markedly decreased activity toward unrestricted targets. Thus, lymphocytes kept in contact with a tumor that is induced to release IL-2 can be educated to extend a control of neoplastic cells both through an unrestricted activity, which plays an important role in controlling tumor expansion, and through the recruitment and expansion of specific effectors directed against the tumor itself. Data accumulated in animal models have shown that tumor cells transduced with different cytokine genes are capable of inducing an unrestricted antitumor response followed by a subsequent recruitment of specific lymphocytes.20 21 In humans, no in vivo data are at present available.
Contradicting results have been reported on the expression of the CD3 ζ chain and of tyrosine kinases in tumor bearing hosts. Although a reduction in the expression of the CD3 ζ chain and of p56lck has been described in tumor infiltrating T lymphocytes (TIL) from renal cell carcinoma patients,24 and in circulating T and NK lymphocytes from colon carcinoma patients,25 it has been reported that T lymphocytes infiltrating B-cell non-Hodgkin's lymphomas display functional defects, despite expressing normal levels of CD3 ζ, p56lck, p59fyn and ZAP-70.26 The latter proteins, however, showed alterations in their tyrosine phosphorylation pattern. Similarly, in animal models important abnormalities have been observed by some investigators,7,27,28 whereas others have described normal levels of CD3 ζ, p56lck, p59fyn and ZAP-70 in splenic lymphocytes of mice carrying different tumors, unless the tumor had reached a large volume.29 It is even more controversial whether tumor cells can directly affect the expression of T-cell signal transduction molecules. In our in vitro model, we could show that human cancer cells can diminish by day 7 and completely downmodulate by day 14 of coculture the expression in normal PBMC of the CD3 ζ chain, as well as of the tyrosine kinases p56lck and ZAP-70, all molecules involved in the T-cell mediated tumor cell recognition pathway.30 Although no difference was observed in cultures set up with cancer cells engineered to release IL-7, GM-CSF, and TNF-α compared to parental LC89 cells, IL-2 gene transduced tumor cells were capable of restoring the expression of the CD3 ζ chain, as well as of p56lck and ZAP-70, and of preserving it over time. The initial day 7 decrease of the CD3 ζ chain in the presence of LC89/IL-2 cells suggests that the small quantities of IL-2 released by the engineered cells require time to counteract the strong immunosuppressive effect exerted by the tumor cells. In vitro studies have shown that the loss of the CD3 ζ chain induced in vitro in normal T cells following stimulation with anti-CD3 can be reversed in the presence of high doses of exogenous IL-2.31 Furthermore, the defective CD3 ζ expression recorded in TIL from renal cell carcinoma and follicular lymphoma biopsies has been successfully restored with exogenous IL-2.32,33 In our model, exogenous IL-2 was also capable of preserving the expression of the CD3 ζ chain, but doses of IL-2 higher than those released by the engineered cells were required (data not shown). Although in the animal it had been previously shown that tumor cells transduced with the IL-2 gene could prevent abnormalities in the signal transduction of splenic T lymphocytes,28 such an effect had so far not been reported with human neoplastic cells.
Though an immunosuppressive effect induced by neoplastic cells on T-cell function has been previously described, the underlying mechanism(s) has still to be fully elucidated. Some authors have hypothesized a role of soluble factors, such as TGFβ1 ,8 or of other substances released by the tumor cells.27 Our data indicate that a direct contact between the neoplastic cells and PBMC is the primary event which leads to the alterations encountered within normal lymphocytes. In fact, when both parental and cytokine gene transduced LC89 cells were cocultured with normal PBMC separated by a membrane which allows soluble factor interchange, while preventing direct intercellular contact, lymphocytes presented, even after 14 days of incubation and under all culture conditions, an unchanged expression of CD3 ζ, p56lck, and ZAP-70.
Taken together, these findings show the direct downmodulation exerted by human tumor cells on the expression of T-cell associated signal molecules involved in the process of antigen recognition and document that this immunosuppressive effect can be successfully overcome by gene transfer methodologies. The potential role of the IL-2 gene for the development of immunotherapeutic vaccination strategies for the management of cancer patients gains further support by the results of this study.
ACKNOWLEDGMENT
We thank Dr D. De Groote, Medgenix Diagnostics, for the generous gift of the cytokine ELISA kits.
Supported by Associazione Italiana per la Ricerca sul Cancro (AIRC), Milan; Consiglio Nazionale delle Ricerche (CNR), Rome; Applicazioni Cliniche Della Ricerca Oncologica (ACRO) project and Istituto Superiore di Sanità, Rome. L.R. and L.M. received fellowships from the “A. Bossolasco” Foundation, University of Torino and AIRC, Milan, respectively. A.C. is a doctoral student in Oncology at the University of Torino.
Address reprint requests to Robert Foa, MD, Dipartimento di Scienze Biomediche ed Oncologia Umana, Sezione Clinica, Via Genova 3, 10126 Torino, Italy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal