Abstract
Graft failure is a mortal complication in allogeneic bone marrow transplantation (BMT); T cells and natural killer cells are responsible for graft rejection. However, we have recently demonstrated that the recruitment of donor-derived stromal cells prevents graft failure in allogeneic BMT. This finding prompted us to examine whether a major histocompatibility complex (MHC) restriction exists between hematopoietic stem cells (HSCs) and stromal cells. We transplanted bone marrow cells (BMCs) and bones obtained from various mouse strains and analyzed the cells that accumulated in the engrafted bones. Statistically significant cell accumulation was found in the engrafted bone, which had the same H-2 phenotype as that of the BMCs, whereas only few cells were detected in the engrafted bones of the third-party H-2 phenotypes during the 4 to 6 weeks after BMT. Moreover, the BMCs obtained from the MHC-compatible bone showed significant numbers of both colony-forming units in culture (CFU-C) and spleen colony-forming units (CFU-S). These findings strongly suggest that an MHC restriction exists between HSCs and stromal cells.
WE HAVE demonstrated that allogeneic bone marrow transplation (BMT) can be used to prevent or treat autoimmune disease in non-obese diabetic (NOD),1,2 (NZB × NZW)F1,3 BXSB,4 (NZW × BXSB)F1,5 KK-Ay,6 FGS,7 and MRL/lpr mice.8 MRL/lpr mice, which possess radioresistant abnormal hematopoietic stem cells (HSCs), show a recurrence of autoimmune diseases within 5 months of conventional allogeneic BMT.3 However, we have recently found that the recurrence can be prevented by the transplantation of both bone marrow cells (BMCs) and bones.8 The aim of bone grafts was to recruit donor-derived stromal cells that can effectively support the donor-derived HSCs. Using another chimeric resistant combination (DBA2 → C57BL/6), we have also found that chimerism resistance can be overcome by BMT plus bone grafts.9 These findings suggest that donor-derived HSCs migrate into major histocompatibility complex (MHC)-matched engrafted bones, where they proliferate under the influence of MHC-matched stromal cells. We therefore examined whether an MHC restriction exists between HSCs and stromal cells in vivo. In the present study, we show that an MHC restriction does, indeed, exist.
MATERIALS AND METHODS
Mice.Female C57BL/6N(B6), BALB/c, C3H/HeN(C3H), C57BL/ 10, B.10D2, and B.10BR mice were purchased from Japan SLC (Hamamatsu, Japan) and DBA/1 mice from Charles River Japan (Yokohama, Japan). All mice were used at the age of 8 to 9 weeks.
BMT and bone grafts.Recipient mice, having received lethal doses of irradiation 1 day before, were subcutaneously engrafted with femurs from which the BMCs had been flushed out with a 23-gauge needle, as previously described.10 The radiation doses used are listed in Table 1. The BMCs were collected by flushing them out from the femurs and the tibias of the donor mice using 23-gauge needles. The cell suspensions were prepared by repeated aspiration through the needle. We usually obtained 2 × 107 cells from one femur of DBA/1, B6, or BALB/c mice, although a smaller number (≃1.8 × 107 cells) was obtained from the C3H mice. These BMCs were treated with anti-Thy1.2 monoclonal antibody (MoAb) (clone 30H-12; American Type Culture Collection [ATCC], Rockville, MD) plus rabbit complement (Pel-Freez, Brown Deer, WI) to deplete the T cells. The resultant cells were passed through Sephadex G10 (Pharmacia, Upssala, Sweden) columns twice to remove the macrophages and stromal cells. After the femurs had been engrafted, the recipient mice were injected intravenously with 2 × 107 BMCs. All mice were maintained under specific pathogen-free conditions.
Mouse Combinations for BMT With Bone Grafts
| Experiment No. . | Recipients (radiation) . | Donors . | No. of Mice . | Grafted Bones . | |||
|---|---|---|---|---|---|---|---|
| . | . | . | . | H-2b . | H-2d . | H-2k . | H-2q . |
| 1 | DBA/1 (8.5 Gy) | B6 | 7 | B6 | BALB/c | C3H | DBA/1 |
| 2 | DBA/1 | BALB/c | 11 | B6 | BALB/c | C3H | DBA/1 |
| 3 | DBA/1 | C3H | 4 | B6 | BALB/c | C3H | DBA/1 |
| 4 | DBA/1 | B10 | 7 | B10 | B10D2 | B10BR | DBA/1 |
| 5 | DBA/1 | B10BR | 11 | B10 | B10D2 | B10BR | DBA/1 |
| 6 | C3H (9.5 Gy) | B6 | 3 | B6 | BALB/c | C3H | — |
| 7 | C3H | BALB/c | 9 | B6 | BALB/c | C3H | — |
| 8 | B6 (9.5 Gy) | C3H | 3 | B6 | BALB/c | C3H | |
| 9 | B6 | BALB/c | 16 | B6 | BALB/c | C3H | — |
| 10 | DBA/1 | B10D2 | 2 | B10 | B10D2 | — | — |
| BALB/c | |||||||
| DBA/2 | |||||||
| 11 | DBA/1* | B6 | 3 | B6 | BALB/c | C3H | DBA/1 |
| 12 | DBA/1* | BALB/c | 3 | B6 | BALB/c | C3H | DBA/1 |
| 13 | DBA/1* | C3H | 4 | B6 | BALB/c | C3H | DBA/1 |
| 14 | DBA/1 | B6 | 4 | B6† | BALB/c† | C3H† | DBA/1† |
| 15 | DBA/1 | BALB/c | 4 | B6† | BALB/c† | C3H† | DBA/1† |
| Experiment No. . | Recipients (radiation) . | Donors . | No. of Mice . | Grafted Bones . | |||
|---|---|---|---|---|---|---|---|
| . | . | . | . | H-2b . | H-2d . | H-2k . | H-2q . |
| 1 | DBA/1 (8.5 Gy) | B6 | 7 | B6 | BALB/c | C3H | DBA/1 |
| 2 | DBA/1 | BALB/c | 11 | B6 | BALB/c | C3H | DBA/1 |
| 3 | DBA/1 | C3H | 4 | B6 | BALB/c | C3H | DBA/1 |
| 4 | DBA/1 | B10 | 7 | B10 | B10D2 | B10BR | DBA/1 |
| 5 | DBA/1 | B10BR | 11 | B10 | B10D2 | B10BR | DBA/1 |
| 6 | C3H (9.5 Gy) | B6 | 3 | B6 | BALB/c | C3H | — |
| 7 | C3H | BALB/c | 9 | B6 | BALB/c | C3H | — |
| 8 | B6 (9.5 Gy) | C3H | 3 | B6 | BALB/c | C3H | |
| 9 | B6 | BALB/c | 16 | B6 | BALB/c | C3H | — |
| 10 | DBA/1 | B10D2 | 2 | B10 | B10D2 | — | — |
| BALB/c | |||||||
| DBA/2 | |||||||
| 11 | DBA/1* | B6 | 3 | B6 | BALB/c | C3H | DBA/1 |
| 12 | DBA/1* | BALB/c | 3 | B6 | BALB/c | C3H | DBA/1 |
| 13 | DBA/1* | C3H | 4 | B6 | BALB/c | C3H | DBA/1 |
| 14 | DBA/1 | B6 | 4 | B6† | BALB/c† | C3H† | DBA/1† |
| 15 | DBA/1 | BALB/c | 4 | B6† | BALB/c† | C3H† | DBA/1† |
Recipient mice were injected with MoAbs (ascites) against CD4, CD8, and NK1.1.
Bones were irradiated (8Gy) (Table 4).
Preparation of BMCs in engrafted bones.The mice were killed 4 to 6 weeks after receiving BMT plus bone grafts. The BMCs in each engrafted femur were flushed out with RPMI-1640 medium containing 2% fetal bovine serum (FBS; Hyclone Laboratories, Logan, UT) using a 1-mL syringe with a 26-gauge needle. The BMCs in the engrafted femurs were suspended in 1 mL RPMI-1640 medium containing 2% FBS. The cells were counted separately.
Colony-forming unit assays.Colony-forming unit (CFU) assays were performed using cells in grafted and recipient bones as described in Table 1. In vitro hematopoietic colony-forming assays of the BMCs in each engrafted femur were performed as previously described.11 Duplicate methylcellulose cultures were set up using 35-mm petri dishes (FALCON 1008; Becton Dickinson Labware, Lincoln Park, NJ). One milliliter of culture in each 35-mm dish contained an appropriate concentration of BMCs in engrafted femurs, 0.8% methylcellulose (4,000 cps; Nacalai Tesque, Kyoto, Japan), 30% FBS, 1% deionized bovine serum albumin (BSA; Sigma Chemical, St Louis, MO), 5 × 10−5 mol/L 2-mercaptoethanol, 2 U erythropoietin (Connaught Lab, Willowdale, Canada), and 10% WEHI-3 culture supernatant (WEHI-3–conditioned medium [CM]: Collaborative Research, Bedford, MA) in Iscove's modified Dulbecco's medium (IMDM; GIBCO-BRL, Grand Island, NY); the erythropoietin was excluded for the CFU-granulocyte-macrophage (GM) assay. The dishes were incubated at 37°C in a humidified atmosphere with 5% CO2 in air. After 14 days in culture, the mixed hematopoietic colonies (CFU-GEMM), erythroid burst (burst-forming unit [BFU]E), and granulocyte-macrophage colonies (CFU-GM) were counted. To identify the CFU-GEMM, we used the criteria described by Powell et al.12
CFU-spleen (S) assays were performed as previously described.13 Briefly, 24 hours before cell transfer, the recipient mice (H-2, identical to bone marrow donor) were irradiated from a 60Co source at a rate of 1 Gy/min. Appropriate numbers of BMCs collected from the engrafted femurs were injected into the recipient mice to determine the CFU-S counts. The mice were killed 14 days later, and their spleens were removed and fixed in Bouin's solution. Visible surface colonies were counted. Irradiated mice that were not given cell preparations had fewer than one colony per spleen. For CFU-S assays, radiation doses used were less than those for BMT (as shown in Table 1); 9.5 Gy → 8.5 Gy in recipients (C3H, B6, B10, B10BR or B10D2) and 8.5 Gy → 7.5 Gy in the DBA/1 recipient; at these doses, no endogenous CFU-S count was observed.
Reproducible results were obtained, and representative data are therefore shown in the figures and tables.
Treatment of recipient mice.To deplete T cells and natural killer (NK) cells from the recipient mice, the mice that received BMT plus bone grafts were injected intraperitonealy with 0.5 mL of GK1.5 (rat anti-mouse CD4) ascites (cytotoxic titer 1:10,000), 2.43 (rat anti-mouse CD8) ascites (cytotoxic titer 1:10,000), and PK136 (rat anti-mouse NK1.1) ascites, as previously reported (experiments 11, 12, and 13).11 To examine the effects of irradiation on the bone marrow stromal cells, we first transplanted the bones, then irradiated (8 Gy) the mice and reconstituted them with BMCs (experiments 14 and 15).
RESULTS
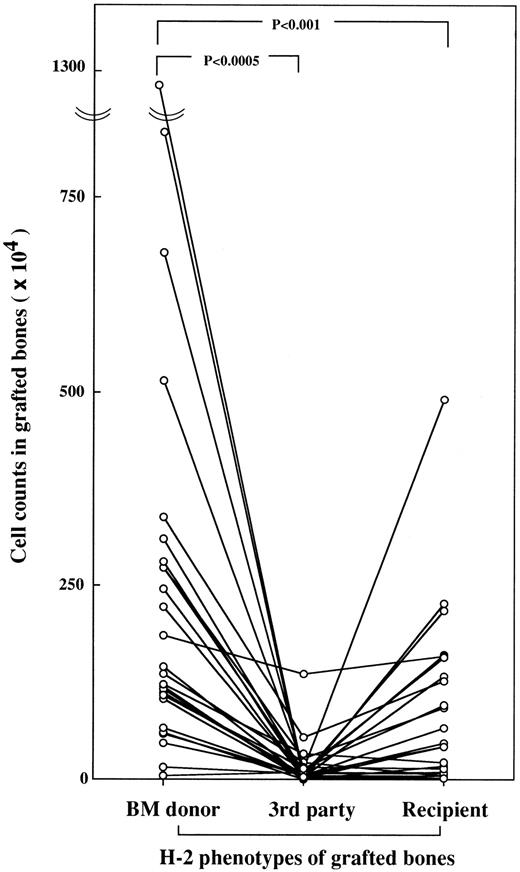
Accumulation of hematopoietic cells in MHC-compatible bones.We engrafted B10D2(H-2d) and B10BR(H-2k) bones under the skin of DBA/1(H-2q) mice that had been lethally irradiated and transplanted with B10BR BMCs. The hematopoietic reconstitution in the engrafted bones was sequentially examined from 2 to 16 weeks after transplantation. Until 2 weeks after transplantation, the cell counts in all engrafted bones were the same. However, the cell count in the engrafted B10BR bone, which is the same H-2 as the transplanted BMCs, increased to 179 × 104 cells at 4 weeks and to 750 × 104 cells at 8 weeks, then reached a plateau (755 × 104 cells) at 16 weeks after transplantation, whereas the cell count in the H-2–mismatched B10D2 bone increased slightly (4.0 ± 5.2 × 104 cells at 4 weeks, 0.7 ± 1.2 × 104 cells at 8 weeks, and 65.0 ± 72.1 × 104 cells at 16 weeks after transplantation). As the next step, we examined the cell accumulation in the engrafted bones in various combinations of recipients, bone marrow donors, and engrafted bones, as shown in Table 1. Since we found differences in the accumulated cell counts between H-2–matched bones and H-2–mismatched bones at 4 weeks after transplantation in the preliminary experiments, all recipient mice were killed and examined at 4 to 6 weeks after transplantation. We statistically analyzed the cell counts in the engrafted bones of each mouse using a paired t-test. As shown in Fig 1, statistically significant cell accumulation was found in the engrafted bones with the same H-2 phenotype as that of the BMC donor, whereas few cells had accumulated in the engrafted bones of the third-party H-2 phenotypes. Cell accumulation was also found in the engrafted bones of recipient H-2 phenotypes, but significantly less than in the bones of the BM donor phenotypes.
Accumulation of BMCs in bone marrow donor bones. All combinations of mice and engrafted bones are shown in Table 1. Cell counts in each engrafted bone were made separately at 4 to 6 weeks after BMT. Cell counts in bones from third-party mice are expressed as the mean number of two engrafted bones. Individual mice are shown by a line graph.
Accumulation of BMCs in bone marrow donor bones. All combinations of mice and engrafted bones are shown in Table 1. Cell counts in each engrafted bone were made separately at 4 to 6 weeks after BMT. Cell counts in bones from third-party mice are expressed as the mean number of two engrafted bones. Individual mice are shown by a line graph.
To examine whether minor histocompatibility complexes (minor HCs) such as Mls also have an effect on cell accumulation, B10D2(Mlsb, H-2d) BMCs plus bones from DBA/2 (Mlsa, H-2d), BALB/c (Mlsb, H-2d), and B10 (Mlsb, H-2b) mice were transplanted into the DBA/1 (Mlsa, H-2q) mice (experiment 10 in Table 1). Significant numbers of cells had accumulated in the DBA/2 bones (315 ± 78 × 104 cells) that were MHC-matched but Mls-mismatched, whereas only a few were found in the B10 bones (17 ± 10 × 104 cells) that were MHC-mismatched but had the same phenotype in minor HCs.
Accumulation of CFU-GM in H-2–matched grafted bones. Formation of CFU-GM colonies in BMCs (per bone) from each grafted bone was evaluated 4 to 6 weeks after allogeneic BMT [B6 → DBA/1] (), [BALB/c → DBA/1] (), and [C3H → DBA/1] () with bone grafts (B6, BALB/c, C3H, and DBA/1). Data are expressed as the mean ± SD of three to five mice. *P < .05.
Accumulation of CFU-GM in H-2–matched grafted bones. Formation of CFU-GM colonies in BMCs (per bone) from each grafted bone was evaluated 4 to 6 weeks after allogeneic BMT [B6 → DBA/1] (), [BALB/c → DBA/1] (), and [C3H → DBA/1] () with bone grafts (B6, BALB/c, C3H, and DBA/1). Data are expressed as the mean ± SD of three to five mice. *P < .05.
We next examined the H-2 phenotypes of the cells that had accumulated in the engrafted bones. These cells showed the MHC phenotypes of the transplanted BMCs; more than 99% of the cells in the engrafted bones were derived from transplanted BMCs (data not shown).
Accumulation of progenitor cells in MHC-compatible bones.To examine whether the cells that had accumulated in the engrafted bones were progenitor cells, they were cultured in methylcellulose. Since we obtained reproducible results in experiments 1 to 9 (Table 1), the results of experiment 1 ([B6 → DBA/1]), experiment 2 ([BALB/c → DBA/1]), and Experiment 3 ([C3H → DBA/1]) are shown in Fig 2 as representative data. Significant numbers of cells with CFU-GM activity were observed in the bones that were MHC-matched, whereas only a few with CFU activity were found in the MHC-incompatible third-party bones. In some experiments (nos. 1, 2, and 4), we performed assays for CFU-GEMM, CFU-GM, CFU-G, CFU-M, and BFU-E. Results from experiment 4 ([B10 → DBA/1]) are shown in Table 2 as representative data. In all kinds of colony formation, the BM donor bones showed significant numbers of CFUs (BFUs), in contrast to those of the third-party bones. A more significant difference was recorded in numbers of BFU-E and CFU-GEMM between the BM donor and third-party bones.
Accumulation of Hematopoietic Progenitor Cells in MHC-Matched Grafted Bones
| CFU-C . | CFU-C/Grafted Bone . | |||
|---|---|---|---|---|
| [B10 → DBA/1] . | B10 . | B10D2 . | B10BR . | DBA/1 . |
| CFU-M | 1208.8 ± 947.5 | 1.3 ± 3.5 | 66.3 ± 49.8 | 411.3 ± 85.8 |
| CFU-G | 96.3 ± 74.2 | 0.0 ± 0.0 | 5.0 ± 7.6 | 38.8 ± 13.6 |
| CFU-GM | 243.8 ± 126.8 | 1.3 ± 3.5 | 3.8 ± 7.4 | 81.3 ± 40.2 |
| BFU-E | 62.5 ± 45.6 | 0.0 ± 0.0 | 0.0 ± 0.0 | 25.0 ± 17.7 |
| CFU-GEMM | 51.3 ± 23.6 | 0.0 ± 0.0 | 0.0 ± 0.0 | 10.0 ± 9.3 |
| CFU-C . | CFU-C/Grafted Bone . | |||
|---|---|---|---|---|
| [B10 → DBA/1] . | B10 . | B10D2 . | B10BR . | DBA/1 . |
| CFU-M | 1208.8 ± 947.5 | 1.3 ± 3.5 | 66.3 ± 49.8 | 411.3 ± 85.8 |
| CFU-G | 96.3 ± 74.2 | 0.0 ± 0.0 | 5.0 ± 7.6 | 38.8 ± 13.6 |
| CFU-GM | 243.8 ± 126.8 | 1.3 ± 3.5 | 3.8 ± 7.4 | 81.3 ± 40.2 |
| BFU-E | 62.5 ± 45.6 | 0.0 ± 0.0 | 0.0 ± 0.0 | 25.0 ± 17.7 |
| CFU-GEMM | 51.3 ± 23.6 | 0.0 ± 0.0 | 0.0 ± 0.0 | 10.0 ± 9.3 |
Furthermore, to examine whether HSCs accumulate in MHC-compatible engrafted bones, the accumulated BMCs were again transferred into H-2–identical recipients. The assay for day 14 CFU-S, which are thought to be derived from pluripotent HSCs, was performed (experiments 1, 2, 3, 4, 6, and 7). The data in experiment 4 are shown in Fig 3 as representative data. The MHC-compatible bone contained a significant number of cells with CFU-S day 14 activity, whereas only few cells with CFU-S day 14 activity were found in the bones of third-party origin.
Accumulation of CFU-S in H-2–matched grafted bones. BMCs obtained from each grafted bone of [B10 → DBA/1] mice were injected into irradiated (8.5 Gy) B10 mice. Mice were killed on day 12 and the colonies counted. Each bar represents mean ± SD of three mice. Representative data of four experiments are shown.
Accumulation of CFU-S in H-2–matched grafted bones. BMCs obtained from each grafted bone of [B10 → DBA/1] mice were injected into irradiated (8.5 Gy) B10 mice. Mice were killed on day 12 and the colonies counted. Each bar represents mean ± SD of three mice. Representative data of four experiments are shown.
Accumulation of Hematopoietic Cells in MHC-Compatible Grafted Bones Even After Depletion of Host T Cells and NK Cells
| BM Donor . | Recipient . | Treatment . | No. of Cells in Grafted Bone . | |||
|---|---|---|---|---|---|---|
| . | . | . | B6 . | BALB/c . | C3H . | DBA/1 . |
| B6 | DBA/1 | (+) | 240.7 ± 157.1*,3-151 | 54.2 ± 63.3 | 14.6 ± 19.2 | 83.1 ± 67.6 |
| B6 | DBA/1 | (−) | 306.9 ± 281.0 | 11.8 ± 10.8 | 17.8 ± 33.1 | 46.5 ± 61.1 |
| BALB/c | DBA/1 | (+) | 53.0 ± 51.7 | 219.5 ± 115.4 | 61.2 ± 45.0 | 38.3 ± 35.3 |
| BALB/c | DBA/1 | (−) | 12.3 ± 16.7 | 53.0 ± 88.4 | 5.2 ± 4.4 | 46.9 ± 37.4 |
| C3H | DBA/1 | (+) | 107.7 ± 54.3 | 47.3 ± 49.0 | 139.0 ± 23.3 | 27.0 ± 2.8 |
| C3H | DBA/1 | (−) | 31.0 ± 5.7 | 2.0 ± 1.4 | 152.5 ± 99.7 | 28.5 ± 6.4 |
| BM Donor . | Recipient . | Treatment . | No. of Cells in Grafted Bone . | |||
|---|---|---|---|---|---|---|
| . | . | . | B6 . | BALB/c . | C3H . | DBA/1 . |
| B6 | DBA/1 | (+) | 240.7 ± 157.1*,3-151 | 54.2 ± 63.3 | 14.6 ± 19.2 | 83.1 ± 67.6 |
| B6 | DBA/1 | (−) | 306.9 ± 281.0 | 11.8 ± 10.8 | 17.8 ± 33.1 | 46.5 ± 61.1 |
| BALB/c | DBA/1 | (+) | 53.0 ± 51.7 | 219.5 ± 115.4 | 61.2 ± 45.0 | 38.3 ± 35.3 |
| BALB/c | DBA/1 | (−) | 12.3 ± 16.7 | 53.0 ± 88.4 | 5.2 ± 4.4 | 46.9 ± 37.4 |
| C3H | DBA/1 | (+) | 107.7 ± 54.3 | 47.3 ± 49.0 | 139.0 ± 23.3 | 27.0 ± 2.8 |
| C3H | DBA/1 | (−) | 31.0 ± 5.7 | 2.0 ± 1.4 | 152.5 ± 99.7 | 28.5 ± 6.4 |
Data are expressed as the mean ± SE.
Underlined values are the number of hematopoietic cells in grafted bones with the same MHC phenotype as the BMC.
Effects of T-cell and NK-cell depletion on MHC restriction. There is a possibility that the lack of cell accumulation in the third-party bones was due to graft rejection by radioresistant host T cells and NK cells. To examine this possibility, we depleted the NK cells and T cells from the recipients by injecting them with anti-NK 1.1, anti-CD8, and anti-CD4 MoAbs (experiments 11, 12, and 13 in Table 1). We confirmed the complete depletion of CD4+ and CD8+ from the peripheral blood by anti-CD8 and anti-CD4 MoAb treatment (data not shown), as previously described.11 After the anti-NK1.1 MoAb treatment, NK activity (the specific lysis to YAC-1 cells) in the normal B6 spleen decreased from 21% to 4% at a target/effector ratio of 1:100. As shown in Table 3, the number of cells that had accumulated in the third-party bones increased more after the MoAbs treatment than in the untreated recipients, suggesting that some immune responses to the third-party MHC determinants had been generated. Nevertheless, there was a significant difference in the accumulation of cells with CFU-S between the MHC-compatible bone and the third-party bones (CFU-S data not shown) even after treatment with MoAbs.
In some experiments, to rule out the possibility of graft rejection by T cells and NK cells remaining in the bones, the bones were treated with MoAbs against CD4, CD8, and NK1.1 plus complement before being grafted into irradiated recipients. However, the MHC restriction was not affected (data not shown).
Effects of bone radiation on MHC restriction.The radiosensitivity of stromal cells in the bone marrow has long been the subject of animated controversy.14-22 We have recently found that stromal cells are quite radioresistant8; they can survive even after 40 Gy of irradiation, but cannot proliferate. Since it is well known that engrafted bones supply a stromal microenvironment for hematopoiesis8-10 and that cell-to-cell interaction between stromal cells and pluripotent HSCs is necessary for proliferation and differentiation of pluripotent HSCs,23 we attempted to determine whether radiation injury of stromal cells exerts effects on the MHC restriction between HSCs and engrafted bones. As shown in Table 4, the MHC restriction was abrogated by irradiation, which hindered stromal cell generation. Since the bones of BALB/c mice are generally thinner and softer than those of the three other strains examined, the stromal cells of BALB/c bones are more sensitive to radiation than those of other mouse strains. The lower level of cell (CFU) accumulation in the engrafted BALB/c bones in Table 4 seems to reflect this. These findings strongly suggest that stromal cells are injured by as little as 8 Gy of irradiation functionally (from the aspect of MHC restriction).
Abrogation of MHC Restriction After Radiation
| BM Donor . | Recipient . | Radiation . | . | Grafted Bones From4-150 . | |||
|---|---|---|---|---|---|---|---|
| . | . | . | . | B6 . | BALB/c . | C3H . | DBA/1 . |
| B6 | DBA/1 | (−) | Cell counts (× 104) | 248.0 ± 231.3 | 28.3 ± 29.1 | 13.0 ± 15.4 | 43.6 ± 45.6 |
| CFU-GM | 708 ± 650 | 75 ± 95 | 23 ± 39 | 200 ± 154 | |||
| B6 | DBA/1 | (+) | Cell counts (× 104) | 16.0 ± 16.24-150 | 1.0 ± 1.4 | 19.5 ± 26.1 | 13.8 ± 21.2 |
| CFU-GM | 108 ± 85 | 2 ± 3 | 123 ± 119 | 170 ± 214 | |||
| BM Donor . | Recipient . | Radiation . | . | Grafted Bones From4-150 . | |||
|---|---|---|---|---|---|---|---|
| . | . | . | . | B6 . | BALB/c . | C3H . | DBA/1 . |
| B6 | DBA/1 | (−) | Cell counts (× 104) | 248.0 ± 231.3 | 28.3 ± 29.1 | 13.0 ± 15.4 | 43.6 ± 45.6 |
| CFU-GM | 708 ± 650 | 75 ± 95 | 23 ± 39 | 200 ± 154 | |||
| B6 | DBA/1 | (+) | Cell counts (× 104) | 16.0 ± 16.24-150 | 1.0 ± 1.4 | 19.5 ± 26.1 | 13.8 ± 21.2 |
| CFU-GM | 108 ± 85 | 2 ± 3 | 123 ± 119 | 170 ± 214 | |||
Grafted bones were irradiated (8Gy) together with recipient mice before BMT.
Data are expressed as the mean ± SD.
DISCUSSION
There are several reports discussing the disadvantages of BMT across MHC barriers.24 Graft failure is one of the problems in allogeneic BMT. Recently, we have demonstrated that the recruitment of donor-derived stomal cells by bone grafts effectively prevents graft failure.7-9 This finding suggests that graft failure may be due to an MHC restriction between the HSCs and the stromal microenvironment independent of immunologic rejection. We have previously reported that the bone from which the BMCs are flushed out does not contain HSCs, but retains stromal cells.10 Based on these findings, we attempted to examine by bone grafting whether an MHC restriction exists between HSCs and stromal cells in vivo. In this study, we have obtained the following results: (1) donor-derived BMCs (particularly HSCs) accumulate in bones of the same H-2 type; (2) comparing numerous histocompatibility complexes, differences in the MHCs (but not minor HCs) are responsible for the restriction; (3) this restriction does not appear to be the result of immunologic reactions such as host-versus graft reaction or graft-versus-host reaction; and (4) radiation injury of stromal cells abrogates the restriction. Based on these findings, it is strongly suggested that HSCs require the MHC-matched microenvironment for their proliferation and differentiation. The bone marrow hematopoietic-stromal microenvironment is thus an integral and prerequisite component of stem cell support both in vivo25 and in vitro.26
It has generally been thought that the transplantation of highly purified HSCs safely extends the clinical application of BMT to MHC-incompatible situations. However, highly purified HSCs have recently been shown to reliably engraft in MHC-matched genetic combinations, but not in allogeneic recipients.27-31 Kaufman et al described how the addition of “facilitating cells (CD8+/CD45R+/TCR−)” permitted the engraftment of purified HSCs in MHC-disparate recipients.31 They argued that donor facilitating cells would somehow interact directly with the host stroma to block engraftment or interfere with the survival of the host HSCs. Furthermore, we think the facilitating cells may suppress the host stromal cells and support their replacement by donor-derived stromal cells.
We have very recently established a new method for purifying pluripotent HSCs (P-HSCs) in mice23 and have found that P-HSCs are Lin−/class Ihigh/CD71−. Using these purified P-HSCs, we have also found that an MHC restriction does, indeed, exist even in vitro between P-HSCs and stromal cells (manuscript in preparation); P-HSCs show marked proliferation when cocultured with MHC-compatible stromal cells, but not with MHC-incompatible stromal cells. We are in the process of clarifying the MHC restriction using this in vitro assay system.
Recently, Heike et al32 have established a SCID-hu model by coimplantation of a bone fragment and BMCs from an adult human, which permits long-term human hematopoiesis. This supports our findings; the bone grafts replace the host stroma with donor-derived stromal cells, which in turn support the engraftment and repopulation of the donor HSCs.
In conclusion, we have demonstrated using a new in vivo method that an MHC restriction exists between HSCs and stromal cells. We are in the process of clarifying the mechanism underlying the “recognition” between HSCs and stromal cells.
ACKNOWLEDGMENT
The authors thank Yuki Matsui and Yoshiko Shinno for expert technical assistance, and Keiko Ando for help in the preparation of the manuscript.
Supported in part by a grant for Experimental Models for Intractable Diseases from the Ministry of Health and Welfare of Japan, by grants-in-aid for Scientific Research (02152117, 02670162, 03454177) from the Japanese Ministry of Education, Science and Culture, a grant-in-aid from the Osaka Cancer Research Association, and the Research Aid of Inoue Foundation for Science.
Address reprint requests to Susumu Ikehara, MD, PhD, First Department of Pathology, Kansai Medical University, 10-15 Fumizono-cho, Moriguchi-city, Osaka 570, Japan.

![Fig. 2. Accumulation of CFU-GM in H-2–matched grafted bones. Formation of CFU-GM colonies in BMCs (per bone) from each grafted bone was evaluated 4 to 6 weeks after allogeneic BMT [B6 → DBA/1] (), [BALB/c → DBA/1] (), and [C3H → DBA/1] () with bone grafts (B6, BALB/c, C3H, and DBA/1). Data are expressed as the mean ± SD of three to five mice. *P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/1/10.1182_blood.v89.1.49/2/m_bl_0009f2.jpeg?Expires=1769117112&Signature=j2Ct6m2ry~oB3R7MEtSm1j5WjvZb4oAtUaoi70UfbJ3psTk5KH8nBy5mt0PbuKhPlQybtjxQii4bkJLLnGtDoCb4O6avZjkLbjz9OrdpFWrA8VZQvWj5Bxzc8Th1yLC2Qe9TIGas6aREwiMpxHMVo~IFQ1xLZXJavyNf-l1T3dhoC7sY65rgGvReaXfv50knqG-X6plj5T2LGqNJJl7wvMLEfOwycK8F~Wv1Y32GEMSCmOOh2tb6QpmXpFOyQotaSJ2Iy2ab02JNBgM8Qfw6aFmWeqmM~bHWT50gMntBG5cTrtaPrIyCEZ5ATzTCOyzBFjjNSyAB6lAOpdJUxsQktg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Accumulation of CFU-S in H-2–matched grafted bones. BMCs obtained from each grafted bone of [B10 → DBA/1] mice were injected into irradiated (8.5 Gy) B10 mice. Mice were killed on day 12 and the colonies counted. Each bar represents mean ± SD of three mice. Representative data of four experiments are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/1/10.1182_blood.v89.1.49/2/m_bl_0009f3.jpeg?Expires=1769117112&Signature=QVtwc6kBnoDJ~AUsEHOre8Td3t-Tg9e-l1wFJJ3XUv4Zo9aiR7YlZBVZx1ZOZUjERP8T3LSWJ1Fee2yP6hMshe~rS0A13vSJi18bYxD1SfNAgEAk37QSlmrlQe5g735~h5Tlpo8gornD1axN911xxMqzHS703jzeJi2asRfeb9qFQtFAv~u8-qaWwkspLQhSN2LR66~Vhkr27iOIV3oA9rcZAbxOKewKsQaz3GsQEVjvZ6uEJrkoZj-hdPTzEAuy2u2lIGyqdU~KXQXXVdLWpD1CFVbOjmOEvFRG3lqN0eHRmcfZzJYFGoWmypJhCiTzAq3YNvLB7Sl82e6mFtDaOg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal