Abstract
Stimulation of the erythropoietin receptor (EPO-R) or the interleukin-2 receptor (IL-2-R) by their respective ligands has been reported to activate tyrosine phosphorylation of the cytoplasmic protein, Shc. We have recently characterized a cell line, CTLL-EPO-R, that contains functional cell-surface receptors for both EPO and IL-2. Although stimulation with IL-2 or IL-15 resulted in the rapid, dose-dependent tyrosine phosphorylation of Shc, stimulation with EPO failed to activate Shc. EPO, IL-2, and IL-15 activated the tyrosine phosphorylation of the adaptor protein, Shp2, and the association of Shp2/Grb2/cytokine receptor complexes. In addition, EPO, IL-2, and IL-15 activated Raf1 and ERK2, demonstrating that the Raf1/MEK/MAP kinase pathway was activated. These results indicate that multiple biochemical pathways are capable of conferring a mitogenic signal in CTLL-EPO-R. EPO can activate the Raf1/MEK/ MAP kinase pathway via Shc-dependent or Shc-independent pathways, and Shc activation is not required for EPO-dependent cell growth in CTLL-EPO-R.
ERYTHROPOIETIN (EPO) and other hematopoietic growth factors have diverse effects on the proliferation and differentiation of blood cells. These effects are mediated through binding to specific, high-affinity cell-surface receptors. Postreceptor signal transduction pathways remain poorly understood and probably elicit both mitogenic and differentiation signals. After ligand binding, cytokine receptors activate multiple signaling cascades. For instance, a distal region (amino acids 626-763) of the interleukin-3 receptor (IL-3-R) βc chain activates Ras, resulting in c-fos and c-jun induction.1 This region is distal to a domain which associates with JAK22 and is essential for mitogenesis.3
Activation of the Ras/Raf1/MAP kinase pathway can be accomplished via the SH2 domain containing adapter proteins, Grb2 and Shc. Grb2 is a cytoplasmic adaptor protein consisting of one SH2 domain flanked by two SH3 domains.4 Grb2 binds activated growth factor receptors5-9 and other tyrosine-phosphorylated proteins such as insulin receptor substrate-1 (IRS-1)10,11 through its SH2 domain and binds the guanine nucleotide releasing factor mammalian (m)SOS1 through its SH3 domains.5-11 This leads to translocation of mSOS1 to Ras, which resides in the plasma membrane, and results in increased exchange of GDP for GTP on Ras.
Shc12 is a modular protein containing both an amino terminal phosphotyrosine binding domain (PTB)13 and a carboxy terminal SH2-domain.14 It is tyrosine phosphorylated at Y317 in response to multiple growth factors.15 Overexpression of Shc can lead to fibroblast transformation12 or neuronal differentiation of PC12 cells.16,17 The SH2 domain of Grb2 binds tyrosine-phosphorylated Shc, suggesting that a Grb2-Shc complex may be involved in receptor-mediated Ras activation. Previous studies of the EPO-R have shown that Grb2 can either bind directly or indirectly via Shc to the tyrosine-phosphorylated EPO-R.18-21
Recent data have demonstrated a third mechanism by which an activated growth factor receptor can couple Grb2 to the Ras pathway. The tyrosine-phosphatase Shp222-25 can function as an adaptor coupling the platelet-derived growth factor receptor (PDGF-R) activation to Grb2 binding.26-28 Like Shc, when Shp2 is tyrosine phosphorylated, the SH2 domain of Grb2 can associate with Shp2. Therefore, Grb2 can associate directly with activated receptors or can associate indirectly via interaction with Shc or Shp2. A physical interaction between the EPO-R and Shp2 has been observed,29 suggesting that this mechanism is important in EPO-R signal transduction. Mutation of EPO-R Y425 was found to perturb Shp2 association and expression of EPO-R Y425F in DA-3 cells resulted in decreased EPO-dependent proliferation.30
In the current study, we have compared IL-2 and EPO signal transduction mechanisms within the same cellular background (CTLL-EPO-R). The EPO-R was ectopically expressed in CTLL cells, generating a cell line that displayed IL-2-, IL-15-, and EPO-dependent proliferation.31,32 We have previously used these cells to show that IL-2 and EPO activate distinct JAK kinase family members.32 IL-2 does activate Shc as previously described,33-36 but surprisingly, EPO fails to activate Shc in these cells. We conclude that EPO activation of Shc is not required for mitogenesis and that the EPO-R can use alternate pathways to activate the Ras/Raf1/MAP kinase pathway. The EPO-R can couple to Ras in these cells either through direct Grb2 binding or via the adaptor protein, Shp2.
MATERIALS AND METHODS
Cells and cell culture.Ba/F337 and DA-3 cells38 (generously provided by J. Ihle, Memphis, TN) were maintained in RPMI 1640 medium supplemented with 10%(vol/vol) fetal calf serum (FCS) and with 5% conditioned medium from WEHI 3B cells (IL-3 medium). CTLL-2 cells39 were maintained in RPMI 1640 medium supplemented with 10% FCS and 2 U of murine recombinant IL-2 (Boehringer Mannheim, Indianapolis, IN) per milliliter (IL-2 medium). HCD-57 cells40 were cultured in IMDM supplemented with 30% FCS and 0.1 U/mL of human recombinant EPO (Kirin Brewery, Tokyo, Japan) per mL.
DNA transfection.Ba/F3-EPO-R cells and DA-3-EPO-R cells were transfected by electroporation with pLXSN-EPO-R cDNA. Selection with G418 (1.0 mg/mL) in IL-3 medium was initiated 48 hours after electroporation. Similarly, CTLL-EPO-R cells31 were generated by co-electroporation with pXM-EPO-R cDNA and pSV2-neo. Selection was performed with G418 (1.0 mg/mL) in IL-2 medium. Subclones were cultured in RPMI 1640 medium supplemented with 10% FCS and 0.5 U/mL murine recombinant EPO per milliliter.
Analysis of Shc activation and identification of associated proteins.For starvation assays, cell lines were incubated in RPMI 1640/10% FCS (no supplemental growth factor) for a 4-hour period and then stimulated for various periods with either no factor, IL-2, IL-3 (Kirin Brewery), IL-4 (Genzyme, Cambridge, MA), IL-15 (generously provided by Immunex Corp, Seattle, WA), or EPO (Kirin Brewer). Cell lysates were prepared in 50 mmol/L Tris-HCl (pH 8.0) 150 mmol/L NaCl, 1.0% Triton X-100 plus phosphatase and protease inhibitors as previously described.41 Immunoprecipitations were performed with either a Shc polyclonal antibody (Transduction Laboratories, Lexington, KY), a Grb2 polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA), an Shp2 monoclonal antibody (MoAb) (Transduction Laboratories), or an anti-N-terminal EPO-R polyclonal antibody as previously described.41 Immune complexes were isolated with Protein A-Sepharose (Sigma, St Louis, MO), washed three times with 50 mmol/L Tris HCl (pH 8.0), 150 mmol/L NaCl, 0.1% Triton X-100, 10 mmol/L Na2P2O7 , 10 mmol/L NaF, 5 mmol/L EDTA, 1 mmol/L Na3VO4 , and prepared for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as described previously.41
Glutathione-S-transferase (GST) fusion proteins expressing the SH2 domains of Grb2, Shp2, and Shc were produced in bacteria as described.42 For in vitro binding analysis, 5 μg of each fusion protein was incubated with 2 mg of lysate from CTLL-EPO-R cells stimulated with no factor, 50 U/mL IL-2, or 50 U/mL EPO. The glutathione-Sepharose beads were washed and bound proteins were resolved via SDS-PAGE as described above.
After electrophoretic transfer of proteins to nitrocellulose, the membrane was blocked and incubated with the antiphosphotyrosine MoAb 4G10, washed in 50 mmol/L Tris-HCl (pH 8.0), 150 mmol/L NaCl 0.1% Triton X-100 (TBST), followed by horseradish peroxidase (HRP)-conjugated sheep anti-mouse IgG (Amersham, Arlington Heights, IL), followed by washing in TBST. Alternatively, following blocking, the membrane was incubated with HRP-conjugated antiphosphotyrosine monoclonal antibody RC20 (Transduction Laboratories). After enhanced chemiluminescence detection, the membrane was stripped by incubation in 62.5 mmol/L Tris-HCl (pH 6.8), 2% (wt/vol) SDS, 100 mmol/L β-mercaptoethanol for 1 hour at 55°C. Membranes were blocked and reprobed with either an Shc polyclonal antibody, Grb2 MoAb (Transduction Laboratories) or an Shp2 MoAb and washed. Incubations were performed with the relevant secondary reagent, either HRP-Protein A (Amersham, Arlington Heights, IL) or HRP-sheep anti-mouse IgG and the membrane was washed before enhanced chemiluminescence detection.
Analysis of Raf1 and MAP kinase pathway activation.CTLL-EPO-R cells were depleted of factor in RPMI/1% bovine serum albumin, for an 8-hour period. Cells were stimulated with various growth factors. After lysis, an immunoprecipitation was performed with an anti-Raf1 antibody.43 The immunoprecipitations were washed with 40 mmol/L TrisHCl (pH 8.0), 150 mmol/L NaCl, 1% NP40, and then 25 mmol/L HEPES (pH 7.4), 1 mmol/L dithiothreitol, 10 mmol/L MgCl2 , 2 mmol/L MnCl2 . Forty microliters of kinase buffer was added in the presence of 15 μmol/L adenosine triphosphate (ATP), 10 μCi 32P-γ-ATP, and 0.1 μg kinase inactive purified MEK1 (kindly provided by Dr T. Sturgell, University of Virginia, Charlottesville) and reactions were allowed to proceed 30 minutes at room temperature. The reaction was stopped with SDS-PAGE loading buffer, samples were boiled, resolved by SDS-PAGE, and transferred to nitrocellulose. The phosphorylation of MEK1 was quantitated by phosphorimager analysis and the amount of Raf1 protein in each lane was quantitated by a protein immunoblot and densitometry analysis.
For analysis of ERK1/2 activation, immunoprecipitations were performed from cytokine-stimulated lysates using an anti-ERK1 antibody (Santa Cruz Biotechnology) or an anti-ERK2 antibody (Santa Cruz Biotechnology). Immunoprecipitates were washed twice in 50 mmol/L TrisHCl (pH 8.0), 150 mmol/L NaCl, 0.1% Triton X-100, 0.1 mmol/L Na3VO4 , and once in 5 mmol/L HEPES, 10 mmol/L MgCl2 , 0.1 mmol/L Na3VO4 . Kinase reactions were performed in 50 μL of 5 mmol/L HEPES, 10 mmol/L MgCl2 , 0.1 mmol/L Na3VO4 , 5 μg myelin basic protein, 1 μmol/L unlabeled ATP, 10 μCi of 32P-γ-ATP for 15 minutes at 30°C. The reaction was stopped with SDS-PAGE loading buffer, samples were boiled, resolved by SDS-PAGE, and transferred to nitrocellulose. The phosphorylation of MBP was quantitated by phosphorimager analysis and the amount of ERK2 protein in each lane was quantitated by a protein immunoblot and densitometry analysis.
RESULTS
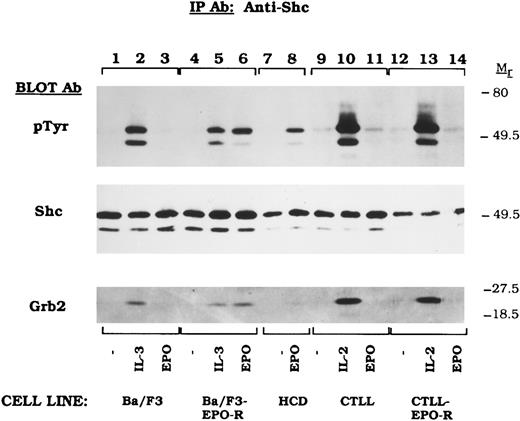
EPO-induced mitogenesis does not require Shc activation in CTLL-EPO-R cells.Previous studies showed that Shc activation was observed after stimulation of EPO-dependent hematopoietic cells.18-21,41 To further test for EPO-induced Shc tyrosine phosphorylation, we examined several EPO-dependent hematopoietic cell lines (Fig 1). The 52- and 46-kD Shc proteins were tyrosine phosphorylated by IL-3 in Ba/F3 (Fig 1, lane 2), IL-3 or EPO in BaF/3-EPO-R (Fig 1, lanes 5 and 6) and in DA-3-EPO-R (see Fig 4) transfectants as previously reported.18,19,41 HCD-57,40 a murine cell line expressing EPO-R, also activated Shc tyrosine phosphorylation upon EPO stimulation (Fig 1, lane 8). IL-2 activated Shc in CTLL (Fig 1, lane 10) CTLL-EPO-R (Fig 1, lane 13) cells. Surprisingly, EPO-dependent Shc phosphorylation was not observed after stimulation of CTLL-EPO-R-cells (Fig 1, lane 14). Similar amounts of Shc are expressed in the various cells lines as revealed by reprobing the blot for Shc (Fig 1, Shc immunoblot). Tyrosine phosphorylation of Shc at Y317 results in the binding of the adapter protein Grb2 to Shc.15 To examine this association, the blot was reprobed for Grb2 (Fig 1, Grb2 Immunoblot). Grb2 binding correlated with tyrosine phosphorylation of Shc.
EPO fails to activate Shc tyrosine phosphorylation in CTLL-EPO-R cells. Ba/F3 (lanes 1 through 3), Ba/F3-EPO-R (lanes 4 through 6), HCD-57 (lanes 7 and 8), CTLL (lanes 9 through 11), and CTLL-EPO-R (lanes 12 through 14) cells were depleted of cytokine for 4 hours and stimulated with no factor (lanes 1, 4, 7, 9, and 12), 50 U of murine IL-3 (lanes 2 and 5), 50 U of murine IL-2 per milliliter (lanes 10 and 13), or 50 U of human EPO per milliliter (lanes 3, 6, 8, 11, and 14) for 10 minutes. After cell lysis, an immunoprecipitation was performed with an Shc polyclonal antibody. Immune complexes were resolved by SDS-PAGE and blotted to nitrocellulose. The immunoblot was probed with HRP-conjugated antiphosphotyrosine (pTyr) MoAb RC20. The blot was then stripped and reprobed with an Shc polyclonal antibody (Shc immunoblot) or a Grb2 MoAb (Grb2 immunoblot).
EPO fails to activate Shc tyrosine phosphorylation in CTLL-EPO-R cells. Ba/F3 (lanes 1 through 3), Ba/F3-EPO-R (lanes 4 through 6), HCD-57 (lanes 7 and 8), CTLL (lanes 9 through 11), and CTLL-EPO-R (lanes 12 through 14) cells were depleted of cytokine for 4 hours and stimulated with no factor (lanes 1, 4, 7, 9, and 12), 50 U of murine IL-3 (lanes 2 and 5), 50 U of murine IL-2 per milliliter (lanes 10 and 13), or 50 U of human EPO per milliliter (lanes 3, 6, 8, 11, and 14) for 10 minutes. After cell lysis, an immunoprecipitation was performed with an Shc polyclonal antibody. Immune complexes were resolved by SDS-PAGE and blotted to nitrocellulose. The immunoblot was probed with HRP-conjugated antiphosphotyrosine (pTyr) MoAb RC20. The blot was then stripped and reprobed with an Shc polyclonal antibody (Shc immunoblot) or a Grb2 MoAb (Grb2 immunoblot).
EPO and IL-2 activate Grb2 association with the tyrosine phosphatase, Shp2. CTLL-EPO-R cells were depleted of cytokine for 4 hours and stimulated with no factor (lanes 1 and 6), 50 U of murine IL-2 per milliliter (lanes 2 and 7), 100 ng of murine IL-4 per milliliter (lanes 3 and 8), 100 ng of simian IL-15 per milliliter (lanes 4 and 9), or 50 U of human EPO per milliliter (lanes 5 and 10) for 10 minutes. After cell lysis, an immunoprecipitation was performed with a Grb2 polyclonal antibody (lanes 1 through 5). Immune complexes were resolved by SDS-PAGE and blotted to nitrocellulose. The immunoblot was probed with 4G10 monoclonal antiphosphotyrosine antibody followed by HRP-sheep anti-mouse IgG. The blot was then consecutively stripped and reprobed with an Shc polyclonal antibody (Shc immunoblot), an Shp2 MoAb (Shp2 immunoblot), and a Grb2 MoAb (Grb2 immunoblot). Lysate controls from CTLL-EPO-R cells are illustrated in lanes 6 through 10. Molecular mass standards are indicated. Ab, antibody.
EPO and IL-2 activate Grb2 association with the tyrosine phosphatase, Shp2. CTLL-EPO-R cells were depleted of cytokine for 4 hours and stimulated with no factor (lanes 1 and 6), 50 U of murine IL-2 per milliliter (lanes 2 and 7), 100 ng of murine IL-4 per milliliter (lanes 3 and 8), 100 ng of simian IL-15 per milliliter (lanes 4 and 9), or 50 U of human EPO per milliliter (lanes 5 and 10) for 10 minutes. After cell lysis, an immunoprecipitation was performed with a Grb2 polyclonal antibody (lanes 1 through 5). Immune complexes were resolved by SDS-PAGE and blotted to nitrocellulose. The immunoblot was probed with 4G10 monoclonal antiphosphotyrosine antibody followed by HRP-sheep anti-mouse IgG. The blot was then consecutively stripped and reprobed with an Shc polyclonal antibody (Shc immunoblot), an Shp2 MoAb (Shp2 immunoblot), and a Grb2 MoAb (Grb2 immunoblot). Lysate controls from CTLL-EPO-R cells are illustrated in lanes 6 through 10. Molecular mass standards are indicated. Ab, antibody.
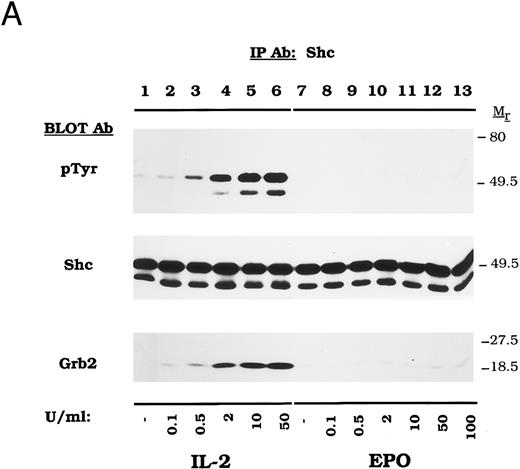
To verify the absence of EPO-dependent Shc activation in CTLL-EPO-R cells, a dose-response experiment was performed. Stimulation with increasing concentrations of IL-2 resulted in dose-dependent Shc tyrosine phosphorylation (Fig 2A, lanes 2 through 6) after a 5-minute incubation. In contrast, EPO failed to activate Shc at concentrations as high as 100 U/mL (Fig 2A, lanes 8 through 13). Equivalent amounts of Shc were immunoprecipitated with each stimulation (Fig 2A, Shc immunoblot). Grb2 was shown to bind to Shc only after stimulation in an IL-2–dependent manner (Fig 2A, Grb2 immunoblot).
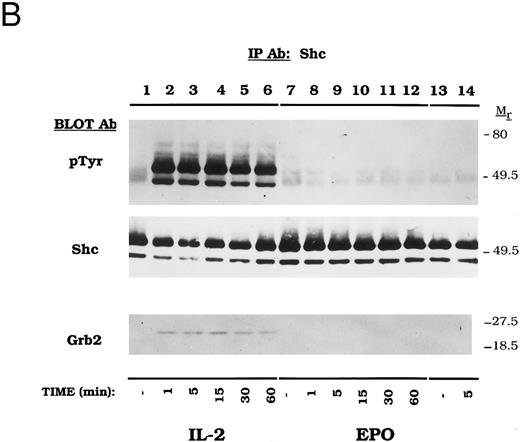
Dose-dependent activation and time course of Shc tyrosine phosphorylation in CTLL-EPO-R cells. (A) CTLL-EPO-R cells were incubated in the depleted of cytokine for 8 hours and then stimulated with no added factor (lanes 1 and 7), or various concentrations of IL-2 (lanes 2 through 6) or EPO (lanes 8 through 13) for 5 minutes as shown. After cell lysis, an immunoprecipitation was conducted with anti-Shc polyclonal antibody. Western blot analysis using the monoclonal antiphosphotyrosine 4G10 antibody was performed (pTyr immunoblot). The blot was stripped and reprobed with an anti-Shc polyclonal antibody (Shc immunoblot) or an anti-Grb2 MoAb (Grb2 immunoblot). Molecular mass standards are indicated. Ab, antibody. (B) CTLL-EPO-R subclone 22 (lanes 1 through 12) and CTLL-EPO-R subclone 5 (lanes 13 and 14) were starved for 8 hours and then stimulated with no added factor (lanes 1, 7, and 13), 50 U/mL IL-2 (lanes 2 through 6), or 50 U/mL EPO (lanes 8 through 12 and 14) for various times as shown. After cell lysis, an immunoprecipitation was conducted with anti-Shc polyclonal antibody. Western blotting using the monoclonal antiphosphotyrosine 4G10 was performed (pTyr immunoblot). The blot was stripped and reprobed with an anti-Shc polyclonal antibody (Shc immunoblot) or an anti-Grb2 MoAb (Grb2 immunoblot). Molecular mass standards are indicated. Ab, antibody.
Dose-dependent activation and time course of Shc tyrosine phosphorylation in CTLL-EPO-R cells. (A) CTLL-EPO-R cells were incubated in the depleted of cytokine for 8 hours and then stimulated with no added factor (lanes 1 and 7), or various concentrations of IL-2 (lanes 2 through 6) or EPO (lanes 8 through 13) for 5 minutes as shown. After cell lysis, an immunoprecipitation was conducted with anti-Shc polyclonal antibody. Western blot analysis using the monoclonal antiphosphotyrosine 4G10 antibody was performed (pTyr immunoblot). The blot was stripped and reprobed with an anti-Shc polyclonal antibody (Shc immunoblot) or an anti-Grb2 MoAb (Grb2 immunoblot). Molecular mass standards are indicated. Ab, antibody. (B) CTLL-EPO-R subclone 22 (lanes 1 through 12) and CTLL-EPO-R subclone 5 (lanes 13 and 14) were starved for 8 hours and then stimulated with no added factor (lanes 1, 7, and 13), 50 U/mL IL-2 (lanes 2 through 6), or 50 U/mL EPO (lanes 8 through 12 and 14) for various times as shown. After cell lysis, an immunoprecipitation was conducted with anti-Shc polyclonal antibody. Western blotting using the monoclonal antiphosphotyrosine 4G10 was performed (pTyr immunoblot). The blot was stripped and reprobed with an anti-Shc polyclonal antibody (Shc immunoblot) or an anti-Grb2 MoAb (Grb2 immunoblot). Molecular mass standards are indicated. Ab, antibody.
A time-course experiment was next performed to determine the kinetics of Shc activation in CTLL-EPO-R cells (Fig 2B). IL-2 rapidly induced Shc tyrosine phosphorylation in CTLL-EPO-R cells within 1 minute (Fig 2B, lanes 2 through 6) whereas 50 U/mL EPO failed to activate Shc phosphorylation at any time point for two independent CTLL-EPO-R subclones (Fig 2B, lanes 8 through 12 and 14). Other substrates, such as EPO-R and JAK2, demonstrated time- and concentration-dependent activation in CTLL-EPO-R cells.32 Similar amounts of Shc were immunoprecipitated in this experiment (Fig 2B, Shc Immunoblot). Again, Grb2 association with Shc was observed only upon IL-2–dependent Shc tyrosine phosphorylation (Fig 2B, Grb2 Immunoblot).
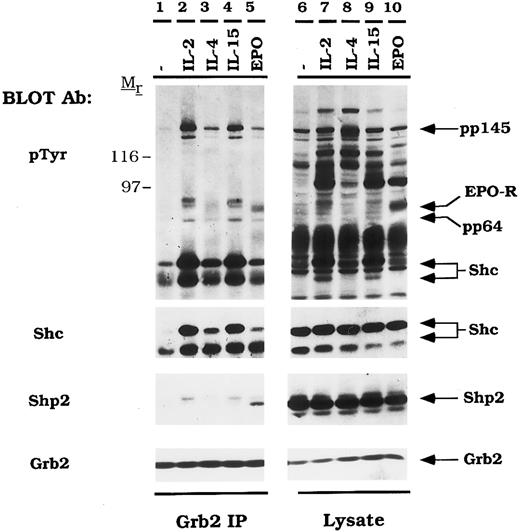
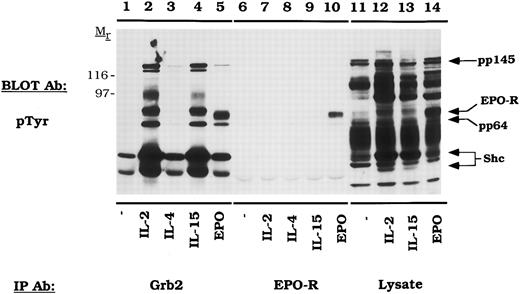
EPO activates the tyrosine phosphorylation of the EPO-R and the formation of EPO-R/ Grb2 complexes.Because EPO did not activate Shc in CTLL-EPO-R cells, we reasoned that if the Grb2/mSOS/Ras pathway was activated, it would be mediated by an Shc-independent pathway. For example, previous studies have shown that the Grb2 binds EGF-R directly and thereby activates the Ras pathway.44 CTLL-EPO-R cells were starved and stimulated with various cytokines, and cellular proteins were immunoprecipitated with either anti-Grb2 or anti-EPO-R antibodies (Fig 3). EPO activated the tyrosine phosphorylation of EPO-R (Fig 3, lane 10), which co-immunoprecipitated as a 72-kD phosphoprotein with an anti-Grb2 antibody (Fig 3, lane 5). The Shc signal (pp52 and pp46) is somewhat overexposed in this experiment, because a long exposure was required to observe other associated proteins. Multiple phosphoproteins associated with Grb2 upon IL-2 and IL-15 stimulation, including Shc, a pp80-kDa protein (possibly IL-2-R β) and pp64, pp97, and pp145. A 145-kD protein has been observed to associate with the phosphotyrosine binding domain (PTB) of Shc after fibroblast growth factor (FGF )45 stimulation and has recently been described as an SH2-domain inositol 5-phosphatase (SHIP).46-48
EPO induces tyrosine phosphorylation and the formation of Grb2/EPO-R complexes. CTLL-EPO-R cells were depleted of cytokine for 8 hours and stimulated with no factor (lanes 1 and 6), 50 U of murine IL-2 per milliliter (lanes 2 and 7), 100 ng of murine IL-4 per milliliter (lanes 3 and 8), 100 ng of simian IL-15 per milliliter (lanes 4 and 9), or 50 U of human EPO per milliliter (lanes 5 and 10) for 10 minutes. After cell lysis, an immunoprecipitation was performed with either a Grb2 polyclonal antibody (lanes 1 through 5) or an anti-amino terminal EPO-R polyclonal antibody (lanes 6 through 10). Immune complexes were resolved by SDS-PAGE and blotted to nitrocellulose. The immunoblot was probed with 4G10 monoclonal anti-phosphotyrosine antibody followed by HRP-sheep anti-mouse IgG. The blot was then stripped and reprobed with a Grb2 polyclonal antibody. Molecular mass standards are indicated. Ab, antibody.
EPO induces tyrosine phosphorylation and the formation of Grb2/EPO-R complexes. CTLL-EPO-R cells were depleted of cytokine for 8 hours and stimulated with no factor (lanes 1 and 6), 50 U of murine IL-2 per milliliter (lanes 2 and 7), 100 ng of murine IL-4 per milliliter (lanes 3 and 8), 100 ng of simian IL-15 per milliliter (lanes 4 and 9), or 50 U of human EPO per milliliter (lanes 5 and 10) for 10 minutes. After cell lysis, an immunoprecipitation was performed with either a Grb2 polyclonal antibody (lanes 1 through 5) or an anti-amino terminal EPO-R polyclonal antibody (lanes 6 through 10). Immune complexes were resolved by SDS-PAGE and blotted to nitrocellulose. The immunoblot was probed with 4G10 monoclonal anti-phosphotyrosine antibody followed by HRP-sheep anti-mouse IgG. The blot was then stripped and reprobed with a Grb2 polyclonal antibody. Molecular mass standards are indicated. Ab, antibody.
EPO and IL-2 activate tyrosine phosphorylation of Shp2 and activate formation of Shp2/Grb2 complexes.Previous studies have shown an EPO-dependent physical interaction between Shp2 and Grb2 and between Shp2 and EPO-R.29 We reasoned, therefore, that the 64-kD protein activated by IL-2, IL-15, and EPO in CTLL-EPO-R cells might be Shp2. To test this hypothesis, we performed an anti-Grb2 immunoprecipitation (Fig 4). As observed in Fig 3, multiple phosphoproteins were shown to associate with Grb2 after cytokine stimulation. A 64-kD phosphoprotein was observed after IL-2 (Fig 4, lane 2), IL-15 (Fig 4, lane 4), or EPO (Fig 4, lane 5) stimulation of CTLL-EPO-R cells. This protein was shown to be Shp2 after stripping and reprobing with a monoclonal Shp2 antibody. IL-4 failed to induce tyrosine phosphorylation and association of Shp2 with Grb2, as previously described (Fig 4, lane 8).49
Association of tyrosine phosphorylated 52- and 46-kD proteins paralleled the pattern of Shc activation shown in Fig 1 (Fig 4, pTyr immunoblot, lanes 2, 4, and 5). Subsequent stripping and reprobing the membrane showed that these proteins were in fact Shc (Fig 4, Shc immunoblot). IL-15, like its related cytokine, IL-2 also resulted in tyrosine phosphorylation and Grb2 association (Fig 4, lane 9). Tyrosine phosphorylated EPO-R was shown to co-immunoprecipitate as a 72- to 78-kD phosphoprotein (Fig 4, lane 5). A 145-kD Shc-associated protein, presumably SHIP,46-48 co-immunoprecipitated with Grb2 (Fig 4, lanes 2, 4, and 5). Equal amounts of Grb2 were immunoprecipitated in this experiment. These data show that, although EPO does not tyrosine phosphorylate Shc in CTLL-EPO-R cells, this cytokine activates Grb2/Shp2 complexes in these cells.
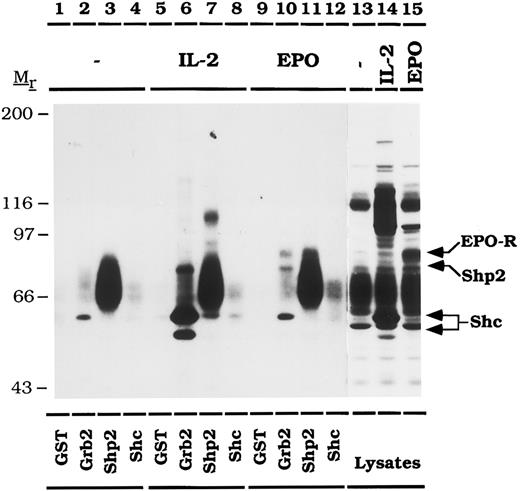
The activated EPO-R binds to the SH2 domains of Grb2 and Shp2.In CTLL-EPO-R cells, the tyrosine phosphorylated EPO-R co-immunoprecipitates with Grb2 and Shp2, but not with tyrosine phosphorylated Shc. GST fusion proteins containing the SH2 domains of Grb2, Shp2, or Shc were used to verify that the binding of Grb2 and Shp2 to the EPO-R is mediated by SH2 domain interactions (Fig 5). CTLL-EPO-R cells were stimulated in the presence of no added factor, IL-2, or EPO and incubated with various GST fusion proteins. Bound phosphoproteins were detected by antiphosphotyrosine immunoblotting. GST-SH2-Grb2 bound Shc (pp46 and pp52) only after IL-2 (Fig 5, lane 6), but not EPO (Fig 5, lane 10) stimulation, consistent with the immunoprecipitation data presented earlier. In addition, Shp2 (pp64) associated with GST-SH2-Grb2 after IL-2 (Fig 5, lane 6) or EPO (Fig 5, lane 10) stimulation. The EPO-R (pp72) was observed to associate with GST-SH2-Grb2 (Fig 5, lane 10) or GST-(N + C)-SH2-Shp2 (Fig 5, lane 11) after EPO stimulation. An additional, unidentified 60-kD phosphoprotein that is phosphorylated under conditions of cytokine deprivation is shown to associate with GST-(N + C)-SH2-Shp2 (Fig 5, lanes 3, 7, and 11). GST or GST-SH2-Shc fail to bind any phosphoproteins from CTLL-EPO-R cells.
The activated EPO-R binds directly to the SH2 domains of Grb2 and Shp2. CTLL-EPO-R cells were depleted of cytokine for 8 hours and stimulated with no factor (lanes 1 through 4, and 13), 50 U of murine IL-2 per milliliter (lanes 5 through 8, and 14) or 50 U of human EPO per milliliter (lanes 9 through 12, and 15) for 10 minutes. Lysates were incubated with 10 μg of GST (lanes 1, 5, and 9), GST-SH2-Grb2 (lanes 2, 6, and 10), GST-(N + C)-SH2-Shp2 (lanes 3, 7, and 11) or GST-SH2-Shc (lanes 4, 8, and 12). The immunoblot was probed with 4G10 monoclonal antiphosphotyrosine antibody followed by HRP-sheep anti-mouse IgG. A diffuse, constitutively phosphorylated 60-kD phosphoprotein associates with GST-(N + C)-Shp2 (lanes 3, 7, and 11). Lysate controls from CTLL-EPO-R cells are illustrated in lanes 13 through 15. The migration of EPO-R, Shp2, and Shc were determined by stripping and reprobing the membrane as shown in Fig 4 (data not shown). Molecular mass standards are indicated. Ab, antibody.
The activated EPO-R binds directly to the SH2 domains of Grb2 and Shp2. CTLL-EPO-R cells were depleted of cytokine for 8 hours and stimulated with no factor (lanes 1 through 4, and 13), 50 U of murine IL-2 per milliliter (lanes 5 through 8, and 14) or 50 U of human EPO per milliliter (lanes 9 through 12, and 15) for 10 minutes. Lysates were incubated with 10 μg of GST (lanes 1, 5, and 9), GST-SH2-Grb2 (lanes 2, 6, and 10), GST-(N + C)-SH2-Shp2 (lanes 3, 7, and 11) or GST-SH2-Shc (lanes 4, 8, and 12). The immunoblot was probed with 4G10 monoclonal antiphosphotyrosine antibody followed by HRP-sheep anti-mouse IgG. A diffuse, constitutively phosphorylated 60-kD phosphoprotein associates with GST-(N + C)-Shp2 (lanes 3, 7, and 11). Lysate controls from CTLL-EPO-R cells are illustrated in lanes 13 through 15. The migration of EPO-R, Shp2, and Shc were determined by stripping and reprobing the membrane as shown in Fig 4 (data not shown). Molecular mass standards are indicated. Ab, antibody.
EPO, IL-2, and IL-15 activate the Raf1 and MAP kinase pathway in CTLL-EPO-R cells.Other investigators have shown that effectors downstream of Ras including Raf150 and MAP kinase20 51 display EPO-dependent activation in a number of hematopoietic cell lines. To demonstrate the activation of the Ras pathway in CTLL-EPO-R cells, we analyzed Raf1 (Fig 6) and ERK2 activation (Fig 7).
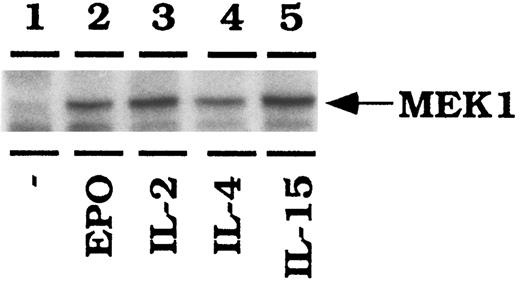
IL-2, IL-15, and EPO activate Raf1-1 in CTLL-EPO-R cells. CTLL-EPO-R cells were depleted of cytokine for 8 hours in RPMI-1 mg/mL bovine serum albumin (BSA). Stimulations were conducted with no added cytokine (lane 1), 50 U of human EPO per mL (lane 2), 50 U of murine IL-2 per milliliter (lane 3), 100 ng of murine IL-4 per milliliter (lane 4), or 100 ng of simian IL-15 per milliliter (lane 5). After cell lysis, an immunoprecipitation was performed using an anti-Raf1 antibody, followed by an in vitro kinase reaction using MEK1 as an exogenous substrate. Samples were resolved by SDS-PAGE, transferred to nitrocellulose, and then exposed to film.
IL-2, IL-15, and EPO activate Raf1-1 in CTLL-EPO-R cells. CTLL-EPO-R cells were depleted of cytokine for 8 hours in RPMI-1 mg/mL bovine serum albumin (BSA). Stimulations were conducted with no added cytokine (lane 1), 50 U of human EPO per mL (lane 2), 50 U of murine IL-2 per milliliter (lane 3), 100 ng of murine IL-4 per milliliter (lane 4), or 100 ng of simian IL-15 per milliliter (lane 5). After cell lysis, an immunoprecipitation was performed using an anti-Raf1 antibody, followed by an in vitro kinase reaction using MEK1 as an exogenous substrate. Samples were resolved by SDS-PAGE, transferred to nitrocellulose, and then exposed to film.
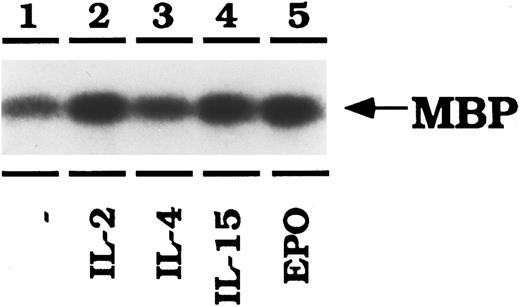
IL-2, IL-15, and EPO activate ERK2 in CTLL-EPO-R cells. CTLL-EPO-R cells were depleted of cytokine for 8 hours in RPMI-1 mg/mL BSA. Stimulations were conducted with no added cytokine (lane 1), 50 U of murine IL-2 per milliliter (lane 2), 100 ng of murine IL-4 per milliliter (lane 3), or 100 ng of simian IL-15 per milliliter (lane 4) or 50 U of human EPO per mL (lane 5). After cell lysis, an immunoprecipitation was performed using an anti-ERK2 antibody, followed by an in vitro kinase reaction using myelin basic protein (MBP) as an exogenous substrate. Samples were resolved by SDS-PAGE, transferred to nitrocellulose and then exposed to film.
IL-2, IL-15, and EPO activate ERK2 in CTLL-EPO-R cells. CTLL-EPO-R cells were depleted of cytokine for 8 hours in RPMI-1 mg/mL BSA. Stimulations were conducted with no added cytokine (lane 1), 50 U of murine IL-2 per milliliter (lane 2), 100 ng of murine IL-4 per milliliter (lane 3), or 100 ng of simian IL-15 per milliliter (lane 4) or 50 U of human EPO per mL (lane 5). After cell lysis, an immunoprecipitation was performed using an anti-ERK2 antibody, followed by an in vitro kinase reaction using myelin basic protein (MBP) as an exogenous substrate. Samples were resolved by SDS-PAGE, transferred to nitrocellulose and then exposed to film.
CTLL-EPO-R cells were depleted of cytokine for 8 hours in serum-free conditions before stimulation with the indicated cytokines. Raf1 was then immunoprecipitated from the lysates and in vitro kinase reactions were performed in the presence of kinase inactive purified MEK1 as an exogenous substrate. IL-2, IL-15, and EPO all led to an increase in MEK1 phosphorylation (Fig 6). The amount of IL-4–dependent Raf1 activation was increased over background, but less than that observed with other cytokines in this experiment. EPO stimulation resulted in a threefold stimulation of Rafl activity, similar to IL-2 and IL-15 as monitored by phosphorimager detection (data not shown). These data are representative of three independent experiments. Equal amounts of Raf1 were immunoprecipitated in this experiment as revealed by probing the membrane with a Raf1 antibody (data not shown).
Next, the activation of ERK1 and ERK2 was examined (Fig 7). Lysates from CTLL-EPO-R–stimulated cells were immunoprecipitated with anti-ERK1 or anti-ERK2 peptide-specific antibodies. An in vitro kinase reaction was then performed using myelin basic protein as an exogenous substrate. IL-2, IL-15, and EPO all activated ERK2 as detected by enhanced phosphorylation of MBP (Fig 7). The level of IL-4–dependent MBP phosphorylation was enhanced over background. EPO, like IL-2 and IL-15, activated MBP phosphorylation twofold as determined by phosphorimager detection (data not shown). These data were found to be reproducible in three independent experiments. Similarly, IL-2, IL-15, and EPO stimulations resulted in tyrosine phosphorylation of ERK2 in immunoprecipitation/Western blotting experiments (data not shown). ERK1 kinase activity was not enhanced by any of the cytokines in CTLL-EPO-R cells, although ERK1 is expressed in CTLL-EPO-R cells (data not shown).
DISCUSSION
Previous studies have shown that Shc is rapidly tyrosine phosphorylated in response to EPO,18-21,41 IL-2,33-36 IL-3,19,21,41,52-54 IL-5,53,54 granulocyte-macrophage colony-stimulating factor (GM-CSF ),52-54 growth hormone,55 and thrombopoietin.56 57 In the present study we have demonstrated that EPO activates Shc in some, but not all, EPO-dependent cell lines.
CTLL-EPO-R cells display EPO-dependent proliferation31,32 and activation of JAK232,58 and STAT558 (Barber DL, unpublished observation). Here we show that EPO stimulates the tyrosine phosphorylation of the EPO-R and Shp2 which both can bind to the SH2 adaptor protein Grb2. Stimulation with either IL-2 or IL-15 results in dose- and time-dependent Shc tyrosine phosphorylation in CTLL-EPO-R cells. However, EPO failed to stimulate Shc tyrosine phosphorylation in any of these experiments.
Why EPO fails to activate Shc tyrosine phosphorylation in CTLL-EPO-R cells is not clear. CTLL-EPO-R have similar EPO-R receptor number, affinity, and EPO dose-dependent growth characteristics when compared to other EPO-dependent cell lines.32 The EPO-R may be differentially tyrosine phosphorylated in CTLL-EPO-R cells. Therefore, Shc may be unable to dock to the EPO-R and as a result is not tyrosine phosphorylated. Conversely, Shc may associate with the tyrosine-phosphorylated EPO-R but is not itself tyrosine phosphorylated. However, EPO-R failed to associate with Shc in CTLL-EPO-R (data not shown). JAK2 can phosphorylate Shc when analyzed by in vitro kinase reactions using lysates prepared with Brij 96.21 EPO activates JAK2 in CTLL-EPO-R32; therefore, the block in Shc activation does not reside in aberrant Janus kinase activation. It has been shown that IL-2 activates the lck59-61 and Syk protein tyrosine kinases.62 However, Syk is not expressed in CTLL cells (data not shown). lck phosphorylates Shc in similar in vitro experiments.63 It is unknown which, if any, src family kinases are activated by EPO. Perhaps the failure of EPO to activate Shc in CTLL-EPO-R cells results from the inability of EPO-R to activate lck.
Several reports describe EPO-R expression in CTLL cells. One study indicated that EPO-R failed to generate an EPO-dependent response in CTLL cells.64 Overexpression of K-Ras generated an EPO-dependent growth response and was shown to be correlated with the IL-2 or EPO-dependent activation of 130- and 160-kD phosphoproteins.65 Another study demonstrated EPO-dependent growth of CTLL-EPO-R cells after a latent period of 4 to 15 days.66 We have characterized the growth and proliferative capacity of CTLL-EPO-R cells in earlier studies.31,32 Multiple reports have indicated that JAK2,32,67 STAT5,58 68 and EPO-R (this study) are all tyrosine phosphorylated, irrespective of EPO-growth dependency. The inability of certain CTLL-EPO-R subclones to proliferate in EPO therefore does not result from aberrant JAK-STAT signaling.
Despite prominent EPO-dependent Shc activation in Ba/F3-EPO-R, DA-3-EPO-R, and HCD-57, Shc failed to co-immunoprecipitate with the EPO-R from Triton X-100 lysates. Shc may bind to the EPO-R via its amino terminal phosphotyrosine binding domain (PTB),45,69 or the carboxy terminal SH2 domain. However, the EPO-R cytoplasmic tail does not contain any potential NPXY binding sites for PTB domains.13,70-72 The ability of the Shc SH2 domain to bind directly to the EPO-R under physiologically relevant conditions remains to be established. Alternatively, Shc could associate indirectly with the EPO-R through a bridging molecule such as SHIP, an inositol 5-phosphatase recently identified.46-48 The association between Shc and EPO-R in MO-7-EPO-R may result from overexpression of EPO-R in this cell line.18
Two other recent studies illustrate that cytokine-dependent Shc activation is not required for mitogenesis. Shc associates with the IL-3-R βc chain at Y577.73 Mutation of this critical tyrosine does not adversely affect GM-CSF-R–dependent proliferation, JAK-STAT activation, or Raf1 activation.73 Mutation of Y388 in the IL-2-R β-chain prevents association of Shc with the IL-2-R β.74 Furthermore, Shc was not tyrosine phosphorylated and Shc phosphorylation was not required for IL-2–dependent proliferation.74 The association of Shc with the IL-3-R βc75 or IL-2-R β-chain76 is mediated by the phosphotyrosine binding domain of Shc.
The Grb2 SH2 domain binds to tyrosine phosphorylated proteins containing the motif YXN.77 A number of the proteins examined in these experiments contain this motif. Shc phosphorylated at Y317 (YVNV) binds Grb2.15 Shp2 contains two such motifs at Y542 (YTNI) and Y580 (YENV).28 In addition, the EPO-R has a consensus Grb2 binding motif at Y463 (YENS).78 It is unknown if the EPO-R is tyrosine phosphorylated at this position. Therefore, Grb2 may bind directly to two or more substrates in these experiments.
An activated growth factor receptor may activate the Ras/Raf1/MAP kinase pathway via multiple alternative mechanisms. The tyrosine kinase receptor, PDGF-R, can activate Ras via Grb2 direct, Shc/Grb2, or SH-PTP-2/Grb2 mechanisms.26-28 Regarding EPO-dependent Ras activation, in Ba/F3-EPO-R, DA-3-EPO-R, and HCD-57 cells, all three mechanisms appear to be intact (data not shown). However, in CTLL-EPO-R, Grb2 associates with the EPO-R directly or through binding to Shp2, but without Shc activation. The level of Shp2 tyrosine phosphorylation varies among the cell lines examined in this study (data not shown). This implies that Shp2 may have a varying role in Grb2 recruitment.
EPO, IL-2, and IL-15 activated Raf1 (2.5- to 3.0-fold) and ERK2 (1.7- to 1.8-fold) kinase activity to a similar degree, as reported earlier for EPO.20,51 However, IL-4 displayed intermediate activation of Raf1 (1.8-fold) and ERK2 (1.2-fold) when compared with unstimulated controls. CTLL cells fail to proliferate in IL-4, but JAK1 and JAK3 are activated by this cytokine.32 Previous studies had failed to observe IL-4–dependent activation of the Ras signaling cascade.79 80
The biologic importance of Ras/Raf1/MAP kinase activation by the cytoplasmic tail of the EPO-R remains unknown. While the carboxy-terminus of the EPO-R activates this pathway and correlates with c-fos induction,81 this region of the carboxy terminus can be truncated without a loss of mitogenic signal in transfected Ba/F3 cells.37,82 When a truncated EPO-R lacking 108 amino acids is expressed in 32D cells, EPO-dependent Shc activation was not observed, despite normal JAK2 activation.20 However, in FDCP-1 transfectants, a truncated EPO-R lacking 154 amino acids displayed both Shc and JAK2 tyrosine phosphorylation and mitogenesis.21 The membrane proximal region of the EPO-R is required for mitogenesis and JAK2 activation.21 83 The differences observed in Shc activation in these cell lines suggest either multiple mechanisms of EPO-R–dependent Grb2 recruitment exist, or that EPO-dependent activation of additional tyrosine kinases occurs in selected hematopoietic cell lines. Although activation of the Ras/Raf1/MAP kinase may not be required for growth of immortalized cell lines, like 32D, it may be essential for growth of primary hematopoietic cells.
ACKNOWLEDGMENT
We thank David Cosman (Immunex Corp, Seattle, WA) for the gift of IL-15, Jim Griffin for GST-SH2-Shc, and Ben Neel for GST-SH2-Grb2 and GST-(N + C)-SH2-Shp2. We appreciate helpful comments on the manuscript from Martin Carroll, Cheryl Miller, Sonya Penfold, and Kodimangalam Ravichandran. We thank Anton Bennett, Ben Neel, Kodimangalam Ravichandran, Joanne Pratt, and members of the D'Andrea laboratory for helpful discussions throughout this work.
Supported by a grant from National Institutes of Health (Award RO1 DK 43889-01) (A.D.D.). D.L.B. was supported by Alberta Heritage Foundation for Medical Research and the Leukemia Society of America. A.D.D. is a Scholar of the Leukemia Society of America.
Address reprint requests to Alan D. D'Andrea, MD, Dana-Farber Cancer Institute, Pediatric Oncology, 44 Binney St, Boston, MA 02115.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal