POLYMORPHONUCLEAR leukocytes were discovered by Paul Ehrlich,1 when fixation and staining techniques made it possible to identify the lobulated nucleus and the granules that have given name to these cells and allowed for their classification as eosinophils, basophils, and neutrophils. Neutrophilic granulation refers to staining by a mixture of basic and acid, ie, neutral, dyes,1 whereas the term specific granules was used by Paul Ehrlich to distinguish true granules from artifacts with a granular appearance.2 It was later realized that two types of granules could be distinguished in neutrophils on the basis of their affinity for dye: azurophil granules, which take up the basic dye azure A at the promyelocytic stage, due to their content of acid mucopolysaccharide,3and specific granules, which do not. A clear distinction between these two types of granules was established when the peroxidase staining method for electron microscopy was adopted to identify myeloperoxidase (MPO), which is present only in azurophil granules.4,5 This distinction was corroborated by the development of subcellular fractionation techniques for separation of the granules.6-9 It has become dogma that these two types of granules are fundamentally different. Specific granules have been characterized as secretory granules that play important roles in initiating the inflammatory response,10 whereas azurophil granules are often viewed as lysosomes that are particularly active in the digestion of phagocytosed material.11-14
This simplistic view of granules has been challenged by results from subcellular fractionation experiments in which granule proteins were found in more than two peaks on density gradients.9,13,15,16 In addition, the existence of a tertiary granule type, identified by its late appearance during myeloid maturation, was indicated by electron microscopy.3 During the last 15 years, high-resolution subcellular fractionation techniques, alone or in combination with immune-electron microscopy and flow cytometry, have shown a bewildering heterogeneity of the neutrophil's granules17-22 and have furthermore identified an additional regulated exocytotic storage organelle, the secretory vesicle.23-26
A novel aspect of the physiology of granules was unraveled by the discovery that these regulated storage organelles (granules and secretory vesicles) are not just simple bags of proteolytic or bactericidal proteins that are kept in store until liberated either to the outside of the cell or to the phagocytic vacuole, but are also important reservoirs of membrane proteins that become incorporated into the surface membrane of the neutrophils when these organelles fuse with the plasma membrane and exocytose their content.17 27 In this way, granules and secretory vesicles may fundamentally change the ability of the neutrophil to interact with its environment.
Realizing this, a number of important questions immediately appear. Why this heterogeneity? Does it provide the neutrophil with the ability to differentially exocytose (or, in case of membrane proteins, translocate) proteins stored in different granules, and if so, how is this differential exocytosis controlled? Or, is the reason for the granule heterogeneity simply that some proteins must be segregated to survive inside the neutrophil, or for the neutrophil to survive, much as two-component glue has to be stored in two separate tubes, although both will be used at the same time? Whether one or the other reason applies, both raise the question of how the neutrophil can target proteins into different granule subsets.
The purpose of this review is to present the heterogeneity of granules and discuss the functional importance of this and, furthermore, to address how the neutrophil controls the structure and mobilization of these different granules.
BASIC ASPECTS OF GRANULOGENESIS
Granules start to form at the stage of neutrophil maturation marked by transition from myeloblast to promyelocyte.4,5 From here on, formation of granule proteins continues even up to the stage of segmented cells.28
In general, granules are believed to be formed by aggregation of immature transport vesicles that bud off from the trans-Golgi network (TGN), in which sorting takes place between constitutively secreted proteins and proteins that are routed into the regulated secretory pathway, ie, go to granules.29-31 The original study by Bainton et al4,5 showed that such vesicles bud off from cis-Golgi to form storage granules at the promyelocyte stage, but from the trans-Golgi at the myelocyte stage, where specific granules are formed. This implies that the sorting apparatus (if existing) is localized in the cis-Golgi in promyelocytes and moves to the TGN in more mature cells. Unlikely as this may seem, it agrees with the finding that MPO, a major protein of azurophil granules, does not contain complex carbohydrate side chains,32,33 but it is contradicted by the finding that several other azurophil granule proteins, including elastase, cathepsin G, and proteinase 3, acquire complex oligosaccharide side chains.34,35 Refining of carbohydrate side chains from simple to complex is a feature of the intermediate- and trans-Golgi stacks.36
Several regulatory steps will be required if granules are formed by fusion from smaller unit transport vesicles that bud off from the Golgi and undergo homotypic fusion until they achieve the size of a granule. It must be secured that such transport vesicles do not fuse with the plasma membrane, as do transport vesicles that mediate constitutive secretion. Furthermore, it must be secured that such transport vesicles do not fuse with already formed granules, because this would lead to mixing of granule proteins destined for different types of granules.
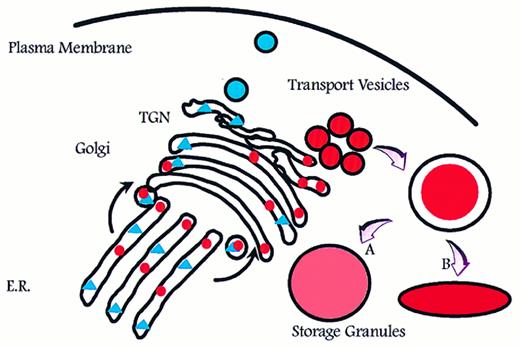
Aggregation of smaller unit vesicles to form mature granules creates a surface to volume problem because the surface area of the final granule is the sum of the surface area of the transport vesicles that fused to create the mature granule, and the volume of the mature granule is the sum of the volume of cargo carried by the transport vesicles (Fig 1). Azurophil granules are generally described as spherical or football shaped, whereas specific granules are known to adopt more irregular and elongated forms.5 This might be a reflection of volume adjustment in azurophil granules, which are known to proteolytically process a significant part of the proteins that are targeted to these.32,37,38 In this respect, azurophil granules resemble lysosomes.39 In contrast, no processing and therefore no increase in osmotic activity due to proteolysis has been observed in specific granules, with one possible exception.40
Intracellular routing of newly synthesized proteins. Newly synthesized proteins are sorted into pathways for constitutive (triangles) and regulated (circles) exocytosis. If formation of storage granules occurs by homotypic aggregation of transport vesicles, a surface to volume problem will arise that can be solved only by either adjusting (expanding) the volume to fit the surface of a spherical particle (A) or by adjusting the shape so that the surface fits the volume (B). E.R., endoplasmic reticulum; TGN, trans-Golgi network.
Intracellular routing of newly synthesized proteins. Newly synthesized proteins are sorted into pathways for constitutive (triangles) and regulated (circles) exocytosis. If formation of storage granules occurs by homotypic aggregation of transport vesicles, a surface to volume problem will arise that can be solved only by either adjusting (expanding) the volume to fit the surface of a spherical particle (A) or by adjusting the shape so that the surface fits the volume (B). E.R., endoplasmic reticulum; TGN, trans-Golgi network.
The question of whether granules of neutrophils are formed by fusion of unit vesicles has not been addressed, but patch-clamp capacitance studies that accurately quantitate the size of granules that are incorporated into the plasma membrane have been performed on horse eosinophils and rat basophils. These studies have shown that individual granules differ in size by the size of unit vesicles. This indicates that granules are formed by homotypic fusion of a finite but slightly varying number of smaller unit vesicles.41 42
CLASSIFICATION OF NEUTROPHIL GRANULES
Granules may be classified on the basis of their size, morphology, or electron density or with reference to a given protein.5 The initial classification into two major types of granules was based on the content of MPO.4 However, the granules can be further subdivided on the basis of other intragranular proteins,21,43,44 as observed in Fig 2. It should be emphasized that, according to the targeting-by-timing hypothesis that will be discussed later, classification of granules is arbitrary, because granules form a continuum from azurophil granules to gelatinase granules, sharing some proteins, eg, lysozyme,45 whereas other proteins can be chosen to serve as specific markers of one particular subset, eg, MPO, lactoferrin, and gelatinase. A primary question is whether classification of granules is physiologically meaningful. A secondary question is whether it is of use in clinical hematology, eg, for classification of myeloproliferative or myelodysplastic disorders.
Classification of granules in neutrophils. Peroxidase-positive (azurophilic or primary) granules are characterized by the content of MPO and may be further divided based on their content of defensins into large, defensin-rich granules and the smaller defensin-poor granules. The peroxidase-negative granules may be divided into specific (secondary) granules and gelatinase (tertiary) granules on the basis of their relative content of lactoferrin and gelatinase. All granules contain lysozyme. Secretory vesicles share some of their membrane proteins with peroxidase-negative granules, whereas others are unique to secretory vesicles. Def., defensins; Gela., gelatinase; Tert., tertiary.
Classification of granules in neutrophils. Peroxidase-positive (azurophilic or primary) granules are characterized by the content of MPO and may be further divided based on their content of defensins into large, defensin-rich granules and the smaller defensin-poor granules. The peroxidase-negative granules may be divided into specific (secondary) granules and gelatinase (tertiary) granules on the basis of their relative content of lactoferrin and gelatinase. All granules contain lysozyme. Secretory vesicles share some of their membrane proteins with peroxidase-negative granules, whereas others are unique to secretory vesicles. Def., defensins; Gela., gelatinase; Tert., tertiary.
It is well established that major differences exist between the different granule subsets regarding the extent to which these are mobilized both in vitro and in vivo.46-50 Gelatinase granules (identified by gelatinase) are mobilized more readily than specific granules (identified by lactoferrin),16,51,52 which again are exocytosed more readily than azurophil granules (identified by MPO). This hierarchy applies both when neutrophils isolated from peripheral blood are stimulated with various secretagogues and when exudate neutrophils collected in a skin window chamber are analyzed and further stimulated.48,50 It therefore makes sense from a functional point of view to classify the neutrophil granules into peroxidase-positive (or azurophilic or primary) granules, defined by their content of MPO, and to further subdivide the peroxidase-negative granules into specific (or secondary) granules, defined by their content of lactoferrin, and gelatinase (or tertiary) granules, defined by their high concentration of gelatinase20 21 (Fig 2). A number of proteins have been identified in these granules. Table 1 gives the content of the different types of granules. The localization of some of these proteins has been determined by electron microscopy, some by subcellular fractionation, and some by mobilization, assuming that proteins that are mobilized together also localize together.
Content of Human Neutrophil Granules and Secretory Vesicles
| Azurophil Granules . | Specific Granules . | Gelatinase Granules . | Secretory Vesicles . |
|---|---|---|---|
| Membrane | |||
| CD6359 289 | |||
| CD68290 | |||
| V-type H+-ATPase291 | Membrane | ||
| CD11b64,104 | |||
| CD15 antigens305 | |||
| CD66306 | |||
| CD67306 | |||
| Cytochrome b55817,52,105 | |||
| fMLP-R131,307 308 | |||
| Fibronectin-R309 | |||
| G-proteinα-subunit310 311 | |||
| Laminin-R309 | |||
| NB 1 antigen312 | |||
| 19-kD protein313 | |||
| 155-kD protein314 | |||
| Rap1, Rap2315,316 | |||
| SCAMP170 | |||
| Thrombospondin-R317 | |||
| TNF-R318 | |||
| Urokinase-type plasminogen activator-R319 | |||
| VAMP-2170 | |||
| Vitronectin-R309 | Membrane | ||
| CD11b64,109,327-330 | |||
| Cytochrome b55820 | |||
| Diacylglycerol-deacylating enzyme331 | |||
| fMLP-R109 131 | |||
| SCAMP170 | |||
| Urokinase-type plasminogen activator-R319 | |||
| VAMP-2170 | |||
| V-type H+-ATPase291 | Membrane | ||
| Alkaline phosphatase23-26 66 | |||
| CR165 | |||
| Cytochrome b55862,333 | |||
| CD11b62,64 | |||
| CD14334 | |||
| CD1663 334 * | |||
| fMLP-R131 | |||
| SCAMP170 | |||
| Urokinase-type plasminogen activator-R319 | |||
| V-type H+-ATPase291 | |||
| VAMP-2170 | |||
| CD10, CD13, CD45335 * | |||
| C1q-receptor336 * | |||
| DAF60 * | |||
| Matrix | |||
| Acid β-glycerophosphatase11 | |||
| Acid mucopolysaccharide292 | |||
| α1-Antitrypsin293 | |||
| α-Mannosidase11 | |||
| Azurocidin/CAP37/heparin binding protein294-296 | |||
| Bactericidal permeability increasing protein297 | |||
| β-Glycerophosphatase7 | |||
| β-Glucuronidase7 298 | |||
| Cathepsins11 | |||
| Defensins43 299 | |||
| Elastase300 | |||
| Lysozyme7,8 298 | |||
| Myeloperoxidase301 | |||
| N-Acetyl-β-glucosaminidase7 | |||
| Proteinase-3302 | |||
| Sialidase303 | |||
| Ubiquitin-protein304 | Matrix | ||
| β2-Microglobulin320 | |||
| Collagenase321 | |||
| Gelatinase21 322 | |||
| hCAP-18117 | |||
| Histaminase323 | |||
| Heparanase324 | |||
| Lactoferrin301 | |||
| Lysozyme7,8 298 | |||
| NGAL126 | |||
| Urokinase-type plasminogen activator319 325 | |||
| Sialidase303 | |||
| SGP2840 | |||
| Vitamin B12-binding protein326 | Matrix | ||
| Acetyltransferase332 | |||
| β2-Microglobulin20 | |||
| Gelatinase16,21 107 | |||
| Lysozyme45 | Matrix | ||
| Plasma proteins24 25 (including tetranectin) |
| Azurophil Granules . | Specific Granules . | Gelatinase Granules . | Secretory Vesicles . |
|---|---|---|---|
| Membrane | |||
| CD6359 289 | |||
| CD68290 | |||
| V-type H+-ATPase291 | Membrane | ||
| CD11b64,104 | |||
| CD15 antigens305 | |||
| CD66306 | |||
| CD67306 | |||
| Cytochrome b55817,52,105 | |||
| fMLP-R131,307 308 | |||
| Fibronectin-R309 | |||
| G-proteinα-subunit310 311 | |||
| Laminin-R309 | |||
| NB 1 antigen312 | |||
| 19-kD protein313 | |||
| 155-kD protein314 | |||
| Rap1, Rap2315,316 | |||
| SCAMP170 | |||
| Thrombospondin-R317 | |||
| TNF-R318 | |||
| Urokinase-type plasminogen activator-R319 | |||
| VAMP-2170 | |||
| Vitronectin-R309 | Membrane | ||
| CD11b64,109,327-330 | |||
| Cytochrome b55820 | |||
| Diacylglycerol-deacylating enzyme331 | |||
| fMLP-R109 131 | |||
| SCAMP170 | |||
| Urokinase-type plasminogen activator-R319 | |||
| VAMP-2170 | |||
| V-type H+-ATPase291 | Membrane | ||
| Alkaline phosphatase23-26 66 | |||
| CR165 | |||
| Cytochrome b55862,333 | |||
| CD11b62,64 | |||
| CD14334 | |||
| CD1663 334 * | |||
| fMLP-R131 | |||
| SCAMP170 | |||
| Urokinase-type plasminogen activator-R319 | |||
| V-type H+-ATPase291 | |||
| VAMP-2170 | |||
| CD10, CD13, CD45335 * | |||
| C1q-receptor336 * | |||
| DAF60 * | |||
| Matrix | |||
| Acid β-glycerophosphatase11 | |||
| Acid mucopolysaccharide292 | |||
| α1-Antitrypsin293 | |||
| α-Mannosidase11 | |||
| Azurocidin/CAP37/heparin binding protein294-296 | |||
| Bactericidal permeability increasing protein297 | |||
| β-Glycerophosphatase7 | |||
| β-Glucuronidase7 298 | |||
| Cathepsins11 | |||
| Defensins43 299 | |||
| Elastase300 | |||
| Lysozyme7,8 298 | |||
| Myeloperoxidase301 | |||
| N-Acetyl-β-glucosaminidase7 | |||
| Proteinase-3302 | |||
| Sialidase303 | |||
| Ubiquitin-protein304 | Matrix | ||
| β2-Microglobulin320 | |||
| Collagenase321 | |||
| Gelatinase21 322 | |||
| hCAP-18117 | |||
| Histaminase323 | |||
| Heparanase324 | |||
| Lactoferrin301 | |||
| Lysozyme7,8 298 | |||
| NGAL126 | |||
| Urokinase-type plasminogen activator319 325 | |||
| Sialidase303 | |||
| SGP2840 | |||
| Vitamin B12-binding protein326 | Matrix | ||
| Acetyltransferase332 | |||
| β2-Microglobulin20 | |||
| Gelatinase16,21 107 | |||
| Lysozyme45 | Matrix | ||
| Plasma proteins24 25 (including tetranectin) |
This localization is based on kinetics of upregulation in response to stimulation with inflammatory mediators, but has not yet been demonstrated by subcellular localization by immunocytochemistry.
SECRETORY VESICLES
When considering exocytosis of granules, it should be kept in mind that exocytosis of granule content is a consequence of fusion of granule membrane with the plasma membrane and incorporation of granule membrane into the plasma membrane. In many cells, this membrane is rapidly retrieved for re-use (adipocytes, neurons),53-57 but in neutrophils, the membrane of mobilized granules largely remains part of the plasma membrane.58-61 In this way, membrane proteins located to the membrane of granules translocate to the surface membrane and furnish the cell with new receptors and other functional proteins.17
The observation that the β2 -integrin Mac-1 (αmβ2 , CD11b/CD18) became incorporated into the plasma membrane without corresponding exocytosis of granule content led to the discovery of the most rapidly mobilizable intracellular structure in neutrophils, the secretory vesicle.23,24,26,58 Secretory vesicles are important because of their membrane, which is particularly rich in receptors.62-65 The only known intravesicular content of secretory vesicles is plasma. Albumin therefore currently serves as marker of these vesicles.25 This indicates that secretory vesicles are endocytic in origin. It should be noted that secretory vesicles are not part of a constitutive endocytosis/exocytosis organelle. Once mobilized, secretory vesicles are not reformed, either in vitro or in vivo.66-70
SPECIFIC FUNCTIONS OF GRANULES AND SECRETORY VESICLES
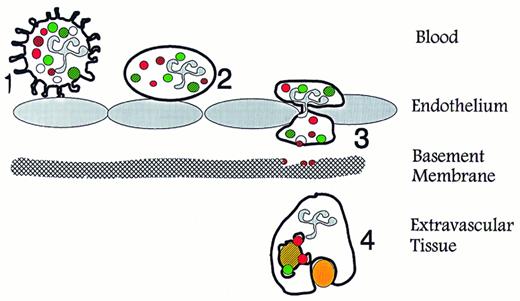
When combining the known content of the different granules and secretory vesicles with their order of mobilization, it becomes clear that these serve different and significant functions (Fig 3). Our view on secretory vesicles is that these are mobilized when the neutrophil establishes the primary rolling contact with activated endothelium, which is mediated primarily by selectins and their ligands, eg, PSGL-1.71-74 The mobilization of secretory vesicles may be mediated by signaling through the selectins75,76 or by inflammatory mediators liberated from the endothelium.77,78 This view is in agreement with the observation that secretory vesicles have been completely mobilized in neutrophils that are collected in plasma in a skin window chamber.50 Thus, mobilization of secretory vesicles transforms the neutrophil to a β2 -integrin presenting cell79,80 and in this way changes the cell from a generally passive cell, well suited for circulation, to a highly responsive cell, primed for migration into tissues.81-93
Specific functions of neutrophil granules and secretory vesicles in relation to diapedesis and phagocytosis. (1) Primary contact between endothelium and circulating neutrophils is established via selectins and their ligands, which causes the neutrophils to roll along the activated endothelium. This contact may transduce signals in the neutrophils that mobilize secretory vesicles. (2) Integration of the membrane of secretory vesicles and its associated CD11b/CD18 enhances the potential of the neutrophil for firm adhesion to endothelium. (3) Exocytosis of gelatinase from gelatinase granules may help degradation of type IV collagen in basement membranes. (4) Mobilization of specific granules to the surface membrane may enhances the phagocytic potential of the neutrophils by providing CR3 (CD11b/CD18). Fusion of azurophil and specific granules with the phagosome creates conditions for oxygen-dependent and -independent bactericidal activity. (Modified and reprinted with permission.200 ).
Specific functions of neutrophil granules and secretory vesicles in relation to diapedesis and phagocytosis. (1) Primary contact between endothelium and circulating neutrophils is established via selectins and their ligands, which causes the neutrophils to roll along the activated endothelium. This contact may transduce signals in the neutrophils that mobilize secretory vesicles. (2) Integration of the membrane of secretory vesicles and its associated CD11b/CD18 enhances the potential of the neutrophil for firm adhesion to endothelium. (3) Exocytosis of gelatinase from gelatinase granules may help degradation of type IV collagen in basement membranes. (4) Mobilization of specific granules to the surface membrane may enhances the phagocytic potential of the neutrophils by providing CR3 (CD11b/CD18). Fusion of azurophil and specific granules with the phagosome creates conditions for oxygen-dependent and -independent bactericidal activity. (Modified and reprinted with permission.200 ).
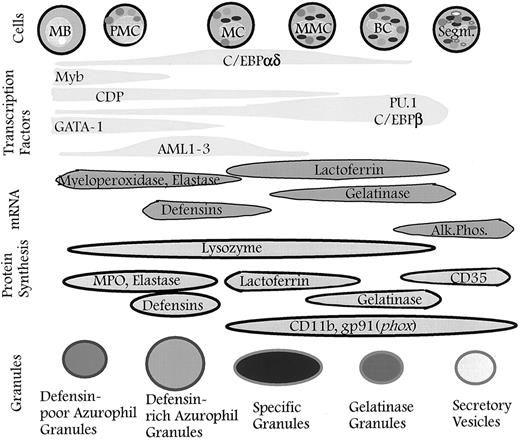
Granules defined by timing of biosynthesis of their characteristic proteins. The granules formed at any given stage of maturation of neutrophil precursors will be composed of the granule proteins synthesized at that time. The different subsets of granules identified are the result of differences in the biosynthetic windows of the various granule proteins during maturation and not the result of specific sorting between individual granule subsets. When the formation of granules ceases, secretory vesicles will form337 (this point has not yet been proven). The control of biosynthesis is exerted by transcription factors that control the expression of genes for the various granule proteins. It cannot be ruled out that posttranscriptional control occurs so that biosynthesis of proteins is not a precise reflection of the corresponding mRNA levels. MB, myeloblast; PMC, promyelocyte; MC, myelocyte; MMC, metamyelocyte; BC, band cell; Segm., segmented cell.
Granules defined by timing of biosynthesis of their characteristic proteins. The granules formed at any given stage of maturation of neutrophil precursors will be composed of the granule proteins synthesized at that time. The different subsets of granules identified are the result of differences in the biosynthetic windows of the various granule proteins during maturation and not the result of specific sorting between individual granule subsets. When the formation of granules ceases, secretory vesicles will form337 (this point has not yet been proven). The control of biosynthesis is exerted by transcription factors that control the expression of genes for the various granule proteins. It cannot be ruled out that posttranscriptional control occurs so that biosynthesis of proteins is not a precise reflection of the corresponding mRNA levels. MB, myeloblast; PMC, promyelocyte; MC, myelocyte; MMC, metamyelocyte; BC, band cell; Segm., segmented cell.
Type IV collagen, a major constituent of basement membranes, and type V collagen of interstitial tissues, are substrates of gelatinase.94-96 It is likely that exocytosis of gelatinase from gelatinase granules is essential for migration of neutrophils through basement membranes.97,98 Although evidence for disruption of the basement membrane has not been obtained by in vivo experiment,99 in vitro experiments support the need for gelatinase activity in migration of neutrophils through Matrigel and amnion membranes,100 but the appropriate gene knock-out experiment has not been performed to address this. A human gene knock-out is provided by the rare condition, specific granule deficiency,101,102 although the functional defects in this condition, which include inability of neutrophils to infiltrate into tissues, may be caused by the lack of expression of other granule proteins than gelatinase.103
Although subject to much previous debate,104-109 the membrane of specific granules has not definitively been shown to be different from that of gelatinase granules, except by quantitative measures (total area of specific granules is larger than the total area of gelatinase granules).20,21 The membranes of specific-and gelatinase granules relate to phagocytosis and intracellular killing, because these are the main stores of Mac-1 (αmβ2 , CD11b/CD18) and of the flavocytochrome b558 (gp91phox/p22phox ), an essential component of the NADPH oxidase.20,104 105
With respect to intragranular proteins, specific granules are dominated by lactoferrin,8,110 the function of which is still unknown. Of the constituents listed in Table 1, the zymogen collagenase should be mentioned because this, like gelatinase of gelatinase granules, most likely is important for the ability of the neutrophil to make its way through tissues.111-113 Azurophil granules are characterized by their content of hydrolytic and bactericidal proteins such as elastase, bactericidal permeability-increasing protein, defensins, and MPO.5,7,13 Azurophil granules have been further subdivided on the basis of several proteins.114 Defensins are the dominating protein in a major subset of azurophil granules.43 Three novel proteins of specific granules that may turn out to contribute significantly to the function of neutrophils have recently been identified. These are NGAL,115,116 hCAP-18,117-119 and SGP28.40
NGAL.NGAL is a member of the lipocalin family of proteins, which were recently reviewed by Flower.120 Lipocalins, the archetype of which is retinol-binding protein, are 25- to 30-kD proteins that have a three-dimensional structure121 that has been likened to a coffee filter.122 Lipocalins are known to bind small lipophilic substances (eg, retinol). NGAL, so named because of its ability to complex with gelatinase (neutrophil gelatinase associated lipocalin),115,116 is the human homologue of the mouse protein 24p3 that was identified in SV40 transformed kidney cells.123,124 A closely homologous protein was recently identified as the protein whose expression is increased the most in neu-transformed rat mammary carcinomas. This protein was termed neu-related lipocalin.125 NGAL is normally found only in the specific granules of human neutrophils,126,127 but its production is significantly induced in colon epithelial cells during inflammation (Crohn's disease, ulcerative colitis, appendicitis, and diverticulitis), as evidenced both by immune-histochemistry and by in situ hybridization.128 The synthesis of NGAL has been shown to be induced in peripheral blood neutrophils treated with granulocyte-macrophage colony-stimulating factor (GM-CSF ).129 Two studies on the subcellular localization of the fMLP receptor by photo-affinity labeling identified NGAL as a 25-kD fMLP-binding protein of specific granules.130 131 Our hypothesis is that NGAL may participate in regulating the inflammatory response by binding small lipophilic inflammatory mediators such as fMLP, platelet activating factor (PAF ), leukotriene B4 (LTB4 ), and lipopolysaccharide (LPS).
hCAP-18.hCAP-18, also named FALL-39, was recently identified independently by three groups.117-119 It is the only human member of the cathelicidin family of bactericidal peptides132 (see Zanetti et al133 and Levy134 for recent reviews). These peptides were first discovered in neutrophils of ruminants.135 Cathelicidins are proteins that share a high level of homology in the N-terminal 14-kD part of the protein that is homologous to cathelin, a protein purified from porcine neutrophils,136 whereas the C-terminal parts of cathelicidins are highly diverse, ranging from 12135 to 100 amino acids.137 The C-termini are highly cationic. Some are extremely rich in proline and arginine,135,138,139 whereas others, including the C-terminal 39 aminoacids of hCAP-18, form an amphipatic α-helix.119 Indeed, we discovered hCAP-18 as a protein highly enriched in the Triton-phase during Triton X-114 phase separation of proteins from specific granules.117
Cathelicidins are stored as intact proteins in specific granules (or large granules of ruminant neutrophils), but the C-termini may be cleaved off when the proteins are exposed to elastase140,141 during concomitant degranulation of specific and azurophil granules, and the bactericidal activity of the C-terminal peptides is unleashed.118,141 The C-terminal peptides are not only microbicidal, but may also be toxic to eucaryotic cells.142
SGP28.SGP28 is a glycoprotein of specific granules with a molecular weight of 28 kD, hence its name.40 It is homologous to two other proteins: Tpx-1, which is a human testis specific protein,143 and sperm-coating glycoproteins of rat epididymis.144 These proteins constitute a family of cystein-rich proteins termed CRISPs (cysteine-rich secretory proteins).145 SGP28 was independently cloned from a salivary gland cDNA library and termed CRISP-3 and was found expressed (Northern blotting) also in pancreas and epithelial cells of the large bowel.145 Part of SGP28 shows high amino acid sequence homology to plant proteins known as pathogenesis-related proteins. There are proteins that are believed to be important for resistance to infections caused by both virus, bacteria, and fungi.146
FUNCTIONAL INTERPLAY OF GRANULES
The generation of reactive oxygen metabolites that is essential for proper microbicidal activity of neutrophils is dependent on components of both peroxidase-negative granules (which harbor the flavocytochrome b558 , an essential component of the NADPH oxidase) and of azurophil granules (which contain MPO that transforms the relatively innocuous product of the NADPH oxidase, H2O2 , to hypochlorous acid147 ). As mentioned above, proteases from azurophil granules may activate the cathelicidin present in specific granules by proteolytically removing the inhibitory (and protective) N-terminal cathelin-like part of the protein. Likewise, gelatinase and collagenases may be converted from their proform to active proteases by elastase liberated from azurophil granules97 or by reactive oxygen metabolites generated by the NADPH oxidase.112 148
CONTROL OF GRANULE EXOCYTOSIS
As alluded to above, secretory vesicles and the different kinds of granules are mobilized in a hierarchy, which seems adjusted to the different roles these organelles play during the journey of the neutrophil from the circulation to the inflammatory focus. Because all granules and secretory vesicles appear randomly distributed throughout the cytosol in the circulating cell21,25,62,63 and localized towards the lamellipod in the (fMLP-) activated cell,70 this hierarchy must rely on mechanisms that discriminate between the different granule subsets. However, hierarchic mobilization excludes independent mobilization, ie, mobilization of specific granules without concomitant and more extensive mobilization of gelatinase granules. This indicates that differences in the mobilization of the granule subsets are due to quantitative differences and not to qualitative differences in the machinery that controls exocytosis of the individual granule subsets.
Elevation of intracellular Ca2+ is known to elicit exocytosis of storage granules in a variety of cells,47,149-152 but the molecular mechanism by which this occurs is unknown. Differences in sensitivity toward intracellular Ca2+ as a signal to elicit mobilization were observed among the different granule subsets49,51,153 and secretory vesicles, in correspondence with the hierarchy of their mobilization. It has been shown that a few cytosolic proteins translocate to granules in a Ca2+-dependent way (annexins I, II, III, IV, VI, and XI).154-160 No clear-cut qualitative differences were observed between binding of different annexins to the different granule subsets, although annexin III was suggested to bind preferentially to specific granules.156 Furthermore, the Ca2+ concentrations that elicited binding of annexins were found to differ among the different granule subsets.155 A non-annexin Ca2+-binding protein, later identified as grancalcin,161 was found to be translocated selectively to secretory vesicles.154 However, the role, if any, of these proteins in regulation of exocytosis is unclear.
Recently, the studies of intracellular transport (from ER to Golgi and within Golgi) have been extended to exocytosis, and a unifying hypothesis called the SNAP/SNARE-hypothesis has been forwarded according to which specific targeting of granules and vesicles is provided by specific interaction of v-SNAREs (present on the membrane of donor organelles) with their cognate t-SNAREs (present on the target membrane). According to this hypothesis, fusion is mediated by the combined action of the cytosolic factors NSF (N-ethyl maleimide sensitive factor) and the attachment proteins for NSF, the SNAPs (soluble NSF-attachment proteins), in further combination with Ca2+, synaptotagmin, and other membrane proteins.30,31,162,163 The experimental evidence for the relevance of this mechanism to control exocytosis is derived mainly from studies on neuronal tissue.164-166 One of the strongest indications to support this hypothesis is the observation that the neurotoxins botulinus and tetanus toxins specifically cleave SNARE proteins.167-169
In neutrophils, the t-SNARE protein, syntaxin-4, is found exclusively in the plasma membrane, whereas the cognate v-SNARE, VAMP-2, is present in the membrane of the mobilizable granules and secretory vesicles, with the highest density on the membrane of secretory vesicles and gelatinase granules followed by specific granules. VAMP-2 could not be detected on azurophil granules.170 This could indicate that exocytosis of the neutrophil granules and of secretory vesicles is stochastic, ie, that the likelihood that any given granule will be exocytosed, is determined entirely by the density of fusion-proteins in the membrane of that granule (as recently observed in sea urchin eggs171 ) and that no qualitative differences exist among fusion proteins associated with the different types of granules.
The signal transduction pathways that lead to granule exocytosis are not completely known. Involvement of G-proteins is indicated by the ability of the nonhydrolyzable GTP-γ-S to induce exocytosis in permeabilized neutrophils172,173 and by localization of distinct G-proteins to granules.174 Patch clamp capacitance measurements provide a powerful tool to analyze granule exocytosis. Using this technique, incorporation of granule membrane into the plasma membrane is quantitated as increase in the electrical capacitance of the plasma membrane.175 This technique has documented the role of GTP and Ca2+ in control of granule exocytosis and holds promise for further delineation of the molecular mechanism of exocytosis.176
The differences among different granule subsets in their ability to become exocytosed have been shown to extent also to orientation of exocytosis. Whereas peroxidase-negative granule are often characterized as secretory granules that mainly mediate extracellular release of their proteins,177,178 peroxidase-positive granules have been characterized as specialized lysosomal structures4,11 that do not participate significantly in extracellular release, but form a digestive organelle for phagocytosed particles.178 179
The notion that azurophil granules are specialized lysosomes has recently been challenged by the observation that the membrane of azurophil granules is devoid of the characteristic lysosomal membrane proteins (LAMPs),180,181 which instead are found in multilamellar and multivesicular bodies in the neutrophil, probably defining these organelles as the true lysosomal structures of neutrophils.181 182
TARGETING OF PROTEINS TO INDIVIDUAL GRANULE SUBSETS
The mechanism for controlling the protein profile of the individual granule subsets, including the proteins that determine their later exocytosis, must be highly efficient to account for the differences in granule composition and for the hierarchy in exocytosis presented above. Two essentially unrelated steps are involved in targeting of proteins to granules. The first step is sorting between transport vesicles that mediate constitutive exocytosis of secretory proteins and insertion of membrane proteins into the plasma membrane and vesicles that will form storage granules capable of undergoing regulated exocytosis. The second step is sorting between different subsets of storage granules.
Sorting between regulated and constitutive exocytotic pathways.Very little is known with respect to mechanisms that sort proteins into the constitutive or the regulated exocytotic pathways in general, let alone in myeloid cells. Sorting of insulin and neuropeptides is (partly) controlled by pH and Ca2+-mediated aggregation,183-185 without involvement of carrier proteins or membrane attachments.186 The well-known targeting of mannose 6-phosphate containing glycoproteins to lysosomes via the cation-dependent and cation-independent mannose 6-phosphate receptors187 has not been shown to be important for targeting of proteins to granules in neutrophils. Although MPO is decorated with mannose 6-phosphate that is recognized by the cation-independent mannose 6-phosphate receptor,33,181 this is not important for sorting to azurophil granules.33,188 In addition, azurophil granules contain proteins that do not have the mannose 6-phosphate label (eg, lysozyme189 and defensins190 ). Recently, MPO was found associated with calreticulin as a chaperone,191 but the significance of this for sorting is unknown. No common primary protein structure has been identified in proteins that are retained versus those that are constitutively secreted. N-terminal hydrophobic domains have been suggested as a sorting signal in some neuropeptides.192
An essential question is whether retention signals are common for all cells, ie, is a protein that in neutrophils is retained in granules also retained if transfected to other cell types? If so, is this dependent on whether the cells have the capacity for formation of granules? This has been studied with defensins, the constituents of azurophil granules, as probe. It has been observed that defensins can localize to granules in nonmyeloid cells, indicating that sorting to storage granules occurs by mechanisms common to all cells.193 This is also supported by transfection of other azurophil granule proteins to other myeloid cells.194,195 Yet, to prove the point, it is necessary to show active sorting between two proteins synthesized in the same cell, but handled differently by the sorting machinery. Because granules are so abundant in granulocytes and appear to be formed rapidly, at least as evaluated in eosinophils,196 there may be little need for a sorting mechanism if the bulk of cargo will go to granules anyway. This question must be addressed by examining whether all proteins that are routed through the Golgi go to granules with the same efficiency or whether some are more efficiently retained than others.
No studies have been presented to address the route of transport of membrane proteins that eventually localize to granules in granulocytes. In neuroendocrine cells, the targeting of peptidylglycyl α-amidating monooxygenase seems to be mediated by two targeting signals: a carboxyterminal cytoplasmic domain that retains the protein in the TGN (as opposed to being routed to the plasma membrane) and a lumenal domain that is essential for routing to secretory granules.197 P-selectin, as opposed to E-selectin, is targeted to its intracellular localization in endothelial cells by specific signals present in the cytosolic carboxy-terminal tail.198 199 Such have not been reported regarding the localization of flavocytochrome b558 and CD11b/CD18, proteins that are localized to the membrane of peroxidase-negative granules. Thus, it has not been addressed whether there is need for specialized targeting or whether sufficient amounts of the membrane proteins going through the Golgi will go passively to the granules at the time of their formation.
Sorting between individual granule subsets.In view of the considerable heterogeneity of neutrophil granules, sorting of proteins into different granules would require a very complex sorting mechanism if it was dependent on sorting information present in the individual proteins. We have forwarded the hypothesis that there is no specific sorting of proteins to individual granule subsets and that all granule proteins that are synthesized at the same time will localize to the same granules.28,200,201 This implies that the differences in protein content that define the different subsets of granules result from differences in the biosynthetic window of the various granule proteins in relation to maturation, as depicted in Fig 4. This extends the original observation of Dorothy F. Bainton that peroxidase-positive granules are formed at the promyelocyte stage, whereas peroxidase-negative granules are formed at the myelocyte stage.5 We were able to show that the differences among peroxidase-negative granules with regard to their content of gelatinase and lactoferrin could be explained by differences in the time of biosynthesis of these proteins, ie, lactoferrin synthesis occurred in cells at the myelocyte/metamyelocyte stage, whereas maximal gelatinase synthesis occurred at the metamyelocyte/band cell stage.28
Although the available information regarding the localization of proteins and their time of biosynthesis is in agreement with this hypothesis,37,202-204 formal proof that no targeting is required for sorting into individual granule subsets was achieved by our analysis of the granule protein NGAL. NGAL is synthesized at the same stage as lactoferrin in normal neutrophil precursors28 and colocalizes with lactoferrin in specific granules.126 When NGAL was transfected to HL-60 cells under control of a constitutively active cytomegalovirus promoter, NGAL became synthesized at the same time as the endogenous granule protein MPO. NGAL was efficiently retained in granules of these HL-60 cells and colocalized with MPO in azurophil granules.201 However, NGAL, so targeted to azurophil granules by changing its timing of expression, was slowly degraded in the proteolytic milieu of the azurophil granules or their precursors.201 This illustrates that, in addition to the need for sequential mobilization of different granule proteins, the need for segregating proteins that cannot coexist may be another reason for the development of neutrophil granule heterogeneity.
The degradation of NGAL in azurophil granules or their precursors is in line with a number of observations that point to the azurophil granule as the place of terminal processing of many of its proteins. A variety of proteins localized to azurophil granules (MPO,32 defensins,37 cathepsin G, and elastase38 ) are proteolytically modified to their mature form after exit from the TGN. This is supported by the observation that defensins and MPO are not proteolytically modified when targeted to storage granules of cells of nonmyeloid origin.193,205 Signals present in the N-terminus of defensins have been shown to be important for correct targeting to granules.206 This most likely illustrates that the N-terminal pro-piece neutralizes the highly cationic C-terminus190 and prevents the protein from becoming stuck to membranes en route through the ER and Golgi and underscores the importance of final processing occurring in azurophil granules and not before. The fact that pro-segments are described for a variety of azurophil granule proteins, but not for granule proteins localized to peroxidase-negative granules, may be a matter of semantics. A variety of the specific granule proteins are only active after N-terminal trimming of a pro-piece has occurred (cathelicidin, gelatinase, and collagenase, as previously discussed), but this only takes place after exposure to azurophil granule proteins or to external proteases.97,113,140,141,207 Thus, it is possible that pro-segments play a role for correct transport through the biosynthetic machinery, but maybe not for specific sorting, as was recently suggested.208 As mentioned in the legend and text to Fig 1, the proteolytic processing in granules may even affect the shape of the granules by generating osmotically active substances.
GRANULE-DEFICIENT GRANULOCYTES
HL-60 cells share with other human promyelocytic cell lines, eg, NB4 cells, the inability to express endogenous proteins normally localized to specific granules, despite the fact that the gene structure, including the 5′-untranslated regions assumed to control transcription of these genes, are intact, where investigated.209-211 Yet, these cells retain the ability to express proteins normally localized in the membrane of specific granules when driven into maturation by retinoic acid or dimethyl sulfoxide (DMSO).204,212 We therefore investigated the fate of the transfected specific granule protein NGAL in HL-60 cells when these were forced to maturation by retinoic acid and DMSO and had stopped the synthesis of endogenous azurophil granule proteins.213 In these cells, newly synthesized NGAL was not retained, but was routed to the extracellular medium, and the endogenous specific granule membrane protein, the flavocytochrome b558 was localized to the plasma membrane and not to granules. This is in contrast to the undifferentiated transfected cells, in which NGAL was localized to azurophil granules, as previously discussed. This illustrates that a shift of routing from the regulated secretory pathway to the constitutive secretory pathway takes place, when HL-60 cells mature. This indicates that the ability to maintain a regulated secretory pathway is dependent on continued synthesis of proteins that are needed for formation of regulated storage granules. This is consistent with the observation that formation of transport vesicles that mediate constitutive secretion continues after cycloheximide treatment, whereas formation of transport vesicles that mediate regulated secretion is blocked.214 It has recently been observed that transfection of fibroblasts with synaptotagmin is able to transform constitutive secretion to regulated secretion.215 It appears, therefore, that the difference between regulated and constitutive secretion may rely on a few proteins and is not due to fundamental differences in the budding and targeting mechanisms of the TGN. The proteins that are needed for formation of regulated storage granules have not been identified in myeloid cells.
The rare condition termed specific granule deficiency is characterized by defects in expression of defensins and of matrix proteins of specific granules, but preservation of the ability to form some membrane proteins that are normally localized to specific granules.103 These membrane proteins colocalize with the plasma membrane in specific granule deficient neutrophils216 and, in this respect, these cells resemble differentiated HL-60 cells.
If, as we believe, targeting of proteins to granules is entirely dependent on the time of biosynthesis, then control mechanisms must exist to secure that vesicles, budding off the TGN and carrying newly synthesized proteins, do not fuse with already formed granules. One such mechanism could be that granules bud off from Golgi at their final size with no further homotypic aggregation, although the observations that have been made on the size of granules do not support this, as previously mentioned. On the other hand, all cells that contain regulated storage granules must have a mechanism to control the size of the granules, otherwise the cells would continue to put all proteins into an ever-growing granule. The targeting-by-timing hypothesis therefore does not invoke further control mechanisms than are already needed in all cells that form granules. An indication that factors exist that limit the size of storage vesicles is given by the observation that such factors seem to be absent from cells of the promyelocytic mouse cell line 32D cl3, which accumulate granule proteins into large intracellular sacs,206 and by the large granules of neutrophils, eosinophils, and other cells from patients with the Chédiak-Higashi syndrome,22,217,218 now shown to be defective in Lyst protein.219
CONTROL OF GRANULE PROTEIN BIOSYNTHESIS
The control of granule protein biosynthesis can be on either the transcriptional or the translational level. Whereas the transcriptional control is exerted by transcription factors, translational control is more elusive.220 221 Although growth factors may exert translational control, translational control has not been shown to be relevant for expression of myeloid granule proteins.
The stage of maturation of neutrophil precursors at which biosynthesis of granule proteins starts is in agreement with the start of transcription of the relevant genes in the cases in which this has been investigated (MPO,202,211,222-224 defensins,211,224-228 lactoferrin,211,224,229,230 vitamin B12 -binding protein,211,224 CD11b,204 and alkaline phosphatase202 ), although no distinct temporal difference between gelatinase and lactoferrin mRNA expression was observed during maturation in a mouse myeloid cell.231 It is not quite clear whether there is a complete correlation between stop of protein biosynthesis and downregulation of mRNA. This indicates that start of protein synthesis is controlled by transcription, whereas the termination of protein synthesis, at least for some granule proteins, may be under translational control.
TRANSCRIPTION FACTORS INVOLVED IN CONTROL OF GRANULE PROTEIN EXPRESSION
Of the largely myeloid-specific granule proteins listed in Table 1, the following have been cloned at the genomic level and their 5′-upstream or flanking sequences have been characterized: MPO,232 elastase,233,234 cathepsin G,233,235,236 proteinase 3,237 lysozyme,238 defensin,239 lactoferrin,210 gp91phox,240-243 p22phox,244 CD11b,245-247 CD18,248-250 vitamin B12 -binding protein,251 hCAP-18/FALL-39,132 NGAL (Cowland and Borregaard, EMBL database accession no. X99133), gelatinase,252,253 and alkaline phosphatase.254 Putative binding sites for a variety of transcription factors have been identified in these promoter regions, but only a few promoter regions have been characterized by functional analysis (expression and gel shift analysis, and experiments of nature [CGD states241,255]). These include MPO,256,257 cathepsin G,236 lysozyme,238 elastase,234,258 CD11b,246,247,259,260 CD18,248-250 gp91242,243,261 and gelatinase.253
GATA-1.The transcription factor GATA-1 is upregulated in the very early stages of commitment of the hematopoietic progenitor cells as a result of induction of cell division.262 This results in a gradual increase in the concentration of GATA-1 over a period of 1 to 2 days,263 followed by an abrupt downregulation and the disappearance of GATA-1 within 4 to 5 days from cells committed to the myelomonocytic lineage.262 A GATA-1 site capable of binding the GATA-1 factor is found in the genes for lactoferrin and for the Mac-1 subunit CD11b, but it has not been shown that these sites are of any importance for the control of expression of these genes.210 247
AML (PEBP2/CBP).The AML (PEBP2/CBP) transcription factors are a family of heterodimeric proteins consisting of a common β-subunit and a specific DNA-binding α-subunit, of which three different types are known in humans.264 This is further varied by different splice variants of the individual α-unit transcripts.264,265 A functionally important PEBP2/CBP site has been identified in the promoters of the human GM-CSF266 and in the mouse MPO and elastase genes.267,268 PEBP2/CBP is upregulated in the murine 32D cl3 cells upon induction of differentiation (days 1 to 3) and downregulated upon exit from the promyelocytic stage (days 5 to 6).267 Besides the regulatory effects of upregulation and downregulation of the AML (PEBP2/CBP) factors and the need for co-operativity with other transcription factors for optimal function, a positive and negative regulatory effect of two differently spliced AML-1 transcripts has been observed on differentiation of murine 32D cl3 cells.265
c-Myb.c-Myb is found to be highly expressed in immature hematopoietic cells and completely absent from mature granulocytes.269-271 Overexpression of c-myb causes continued proliferation of progenitor cells and blocks differentiation of the cells.272 In humans, only genes expressed at early stages of granulocyte differentiation, such as elastase213,267 and MPO,268 have been shown to carry a functional important Myb site. Potential c-Myb sites have also been identified in the early expressed proteinase-3 and azurocidin genes. Co-operativity with C/EBP has been shown for the min-1 gene273,274 and with both C/EBP and PU.1 for the elastase gene.213
PU.1.PU.1 is specific for the hematopoietic system and highly expressed in B-lymphocytic, granulocytic, and monocytic cells.275,276 PU.1 is present at all stages of granulocyte differentiation.276,277 Its expression increases gradually up to the myelocytic stage of differentiation and then remains constant.276,277 This is reflected by the observation that many genes contain a functionally important PU.1 site, both some with an early onset of transcription (elastase,234 PU.1,278 GM-CSF receptor,279 and G-CSF receptor280 ) and some with a later onset of transcription during granulocytic differentiation (CD11b,247,281 CD18,249,282 and FcγRI283,284). Based on its pattern of expression, it can be concluded that PU.1 is likely to confer hematopoietic specificity to gene expression, but by itself is unable to exert a granulocytic- or stage-specific expression of the target genes. The latter has in some instances been shown to be achieved by co-operativety with other lineage- and/or stage-restricted transcription factors, as in the case of the elastase213 and GM-CSF receptor promoters.279
C/EBPα.The C/EBP family of transcription factors contains at least 6 members, of which three (C/EBPα, -β, and -δ) are restricted to the myeloid cells within the hematopoietic lineages.285 C/EBPα and C/EBPδ expression is intense in immature myeloid cells and increases until the onset of the promyelocytic stage of differentiation and then declines rapidly, whereas the expression of C/EBPβ is low in immature cells, but increases steadily during the course of granulocytic maturation to reach a maximum in the mature granulocytic cell.285 A number of genes have been shown to carry a functionally important C/EBP site such as the elastase,213min-1,273 274 G-CSF receptor,286 and GM-CSF receptor genes.279 Co-operativity with c-Myb has been shown for the elastase,213 GM-CSF,279 and min-1 promoters.273,274 For the elastase promoter, additional co-operativity has also been observed with PU.1.213
CCAAT displacement protein (CDP).CDP is a repressor of the ubiquitous CCAAT-binding transcription factor CP1. CDP has been shown to be involved in the regulation of the gp91phox gene240,242 and to play a regulatory role in the transcriptional regulation of proteins localized to the matrix of specific granules.287 These granule proteins are all expressed at the myelocytic stage of neutrophil development. This may indicate that de-repression of gene expression, due to downregulation of CDP over the course of granulocytic differentiation, is important for the stage-specific expression of some granule proteins.
It is likely that it is the appearance (activation) or disappearance (suppression) of one or more lineage- and developmental-specific transcription factors that, alone or in combination, determines the developmental expression pattern of neutrophil-specific genes in concert with ubiquitous transcription factors (Fig 4). No single transcription factor has been shown to confer myeloid-specific expression of genes, but PU.1, which is specific for hematopoietic cells, should be able to determine myeloid-specific gene expression in combination with the C/EBPs, which are found in many different cell types but, within the hematopoietic lineages, only in myeloid cells. Early myeloid expression can then be achieved by the requirement of c-Myb and/or a PEBP2/CBP binding for gene expression. Late granulocytic gene expression can be achieved by the disappearance of one or more repressors, such as CDP, or the appearance of a yet unidentified positive regulatory protein during differentiation. Another possibility is that the C/EBP sites of some of the genes expressed at the early stages of granulocytic differentiation have a high affinity for C/EBPα and a low affinity for C/EBPβ and that the opposite is the case for genes expressed at later stages. The expression patterns of these granulocyte-specific genes may then simply reflect the expression patterns of C/EBPα and C/EBPβ. Although no difference in specificity towards the recognized DNA core sequence has been ascribed for the two C/EBP transcription factors,288 a difference in binding affinity and specificity might be achieved by subtle differences in the surrounding sequences. Activation of transcription factors as a result of ligand binding to the GM-CSF and G-CSF receptors may also contribute to the regulation of late gene expression.
CONCLUSION
The granules of human neutrophils should be viewed as a continuum that reflects the differences in the time of biosynthesis of the various proteins that characterize the granules. With the growing knowledge about the stages of neutrophil maturation at which the different granule proteins are synthesized and about transcription factors involved in controlling this process, the subsets of neutrophil granules that may now be identified not only provide the basis for understanding essential parts of neutrophil physiology and pathophysiology, but also give a more detailed framework on which to put the defects in neutrophil maturation that are characteristic of myeloproliferative and myelodysplastic disorders.
ACKNOWLEDGMENT
The authors express their sincere appreciation of the collaboration with the previous and present staff at the Granulocyte Research Laboratory who contributed with much of the work that forms the basis of this review (Charlotte Horn, Ole Bjerrum, Lars Kjeldsen, Henrik Sengeløv, Karsten Lollike, Ole Sørensen, Sanne Christiansen, Veronique Le Cabec, Tomas Bratt, and Kristina Arnljots). We also thank Dr John K. Spitznagel for helpful information.
Address reprint requests to Niels Borregaard, MD, PhD, Rigshospitalet L-4042, 9 Blegdamsvej, DK-2100 Copenhagen, Denmark.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal