Abstract
Identification of human hematopoietic stem cells and analysis of molecular mechanisms regulating their function require biological assays that permit differentiation in all hematopoietic lineages simultaneously. In this study, we established conditions that permit the joint expression of the B-lymphoid and myeloid potential from cord blood-derived CD34+CD38lowCD19−/CD10− primitive progenitors that lack B-specific markers and transcripts. When cocultured during 6 weeks with the murine stromal cells MS-5 in the absence of exogenous human cytokines, CD34+CD38lowCD19−CD10− cells generated a high number of CD19+ B cells. Virtually all of these cells expressed a CD34−CD10+CD19+cIgM− phenotype of late pro-B cells and transcripts of Pax-5, λ-like, and μ chain were detected. We further show that 7% of CD34+CD38lowCD19− cells from cord blood, when grown individually with MS-5 cells, generated both CD19+ and CD11b+ cells after 6 weeks. Efficient B-cell differentiation was also observed in vivo after transplantation of human cord blood-derived unfractionated mononuclear cells or CD34+CD19+CD10− cells into immune-deficient mice. In contrast to the in vitro situation, all stages of B-cell differentiation were observed in vivo, including pro-B, pre-B, and sIgM+ B cells. Interestingly, human progenitors with the ability to differentiate along both B-lymphoid and granulocytic pathways were also detected among human CD34+CD38low cells in the marrow of chimeric mice 6 to 7 weeks after transplantation. Both in vitro and in vivo systems will offer an invaluable tool to further identify the lymphoid and myeloid potentialities of primitive progenitor cells isolated from fetal as well as adult human hematopoietic tissues and characterize stromal-derived signals that regulate their function.
TRANSPLANTATION experiments with donor stem cells genetically marked by retroviral insertion have unambiguously shown the coexistence in adult murine marrow of cells with multilineage potential as well as with a more lymphoid- or myeloid-restricted potential1 and similar approaches are attempted in humans through the reconstitution of immune-deficient mice with genetically marked human cells.2 However, molecular mechanisms associated with the commitment of these stem cells and their potential regulation by exogenous molecules will be best dissected in vitro, which requires appropriate biological assays that support the full differentiative potential of these early stem cells. The main difficulty comes from the fact that conditions designed for an optimal differentiation of primitive progenitor cells such as long-term culture-initiating cells (LTC-IC) towards the myeloid pathways3,4 are not permissive for B-cell5 and T-cell differentiation. However, in Dexter-type murine cultures, even though they do not terminally differentiate, B-cell progenitors persisted that reconstitute the lymphoid lineages when transplanted into irradiated recipients.5-7 Subsequently, it has been shown that B-lymphoid development can be easily obtained by switching myeloid conditions to lymphoid conditions, as originally described by Whitlock and Witte.8 Using this approach, the joint expression of both myeloid and lymphoid potentials of a bipotent progenitor closely related to totipotent murine long-term reconstituting cells can be obtained.9
A human equivalent of the Whitlock-Witte system has not yet been reported for postnatal human hematopoietic cells and, until recently, only CD34+CD19+ or CD34+CD10+ pro-B cells isolated from fetal marrow could be grown efficiently in the presence of stromal cells and cytokines,10-15 whereas methods to obtain B cells from more primitive progenitor cells were not available.16 However, very recently, CD19+ cells have been generated from cord blood-derived CD34+ cells17,18 or from more primitive Thy1+19-21 or CD38lowHLADr+22 subsets isolated from fetal or adult marrow23 by growing these primitive cells with stromal cells of murine origin with or without human cytokines. Efficient support of human B lymphopoiesis by a murine environment was further documented by the preferential development of CD19+ B cells in the marrow of immune-deficient SCID and NOD-SCID mice transplanted with human cells.24-27 CD19+ cells might represent up to 50% to 60% of the human cells 5 to 9 weeks after transplantation independently of the route of injection, intravenously24,26 or into human fetal bone implants.19 28
We now confirm and extend these results and show for the first time that human CD34+CD38lowCD19− cord blood-derived primitive cells, which lack B-specific transcripts, generated high numbers of late pro-B cells after 6 weeks when cultured in Whitlock-Witte conditions on a murine stromal cell line in the absence of additional human cytokines. Furthermore, single-cell experiments showed that 7% of CD34+CD38lowCD19− cord blood cells generated both myeloid and B-lymphoid progeny, thus identifying a progenitor that is at least bipotent. These CD34+CD38low early progenitors were also detected in the marrow of immune-deficient SCID and NOD-SCID mice transplanted with cord blood cells. Interestingly, the whole hierarchy of B-cell precursors was identified in vivo from CD34+CD19+ pro-B cells to surface IgM-expressing B cells, which was in contrast with the block in differentiation at a late CD34−CD19+cIgM− pro-B stage observed in vitro.
MATERIALS AND METHODS
Purification of CD34+ and CD34+CD38lowCD19− Cells From Human Cord Blood
Cord blood cells were collected after informed consent was obtained following procedures used for banking of cord blood cells for transplantation. Cord blood was diluted with phosphate-buffered saline (PBS) without magnesium (−Mg) and calcium (PBS −Ca−Mg) (1/1, vol/vol) and light-density (<1.077 g/mL) mononuclear cells (CB-MNC) were obtained after centrifugation over Lymphoprep (Nyegaard, Oslo, Norway). CD34+ cells were first purified with a Celprate LC34 Biotin cell separation column (CellPro, Bothell, WA) or the MiniMACS device following the procedures recommended by the manufacturer. The CD34+ population was further fractionated by cell sorting immediately or after an overnight incubation in 30% fetal calf serum (FCS; GIBCO-BRL, Cergy Pontoise, France) at 4°C.
CD34+ cells were incubated for 45 minutes at 4°C in α-minimum essential medium (α-MEM) supplemented with 5% FCS and 100 μg/mL DNase (labeling medium) with a 1/5 dilution of phycoerythrin (PE)-labeled anti-CD34 monoclonal antibody (MoAb) HPCA2 (Becton Dickinson, San Jose, CA) and fluorescein isothyocyanate (FITC)-labeled anti-CD38. MoAb (Immunotech, Marseille, France). In some experiments, three-color labeling was performed using Cy5-PE–labeled anti-CD34 (Immunotech), PE-labeled anti-CD19 (Becton Dickinson), and FITC-labeled anti-CD38 MoAbs. In experiments in which CD34+ cells lacking CD19 and CD10 were sorted, both PE-labeled anti-CD19 and PE-labeled anti-CD10 (Becton Dickinson) MoAbs were mixed. Cells were washed once after incubation with the antibodies and were suspended in labeling medium at a concentration of 4 to 5 × 106 cells/mL for separation by cell sorting. Cells were analyzed and sorted on a FACSvantage (Becton Dickinson) equiped with an INNOVA70-4 Argon ion laser (Coherent Radiation, Palo Alto, CA) tunned at 488 nm and operating at 500 mW. Cells expressing high levels of CD34 with no or low expression of CD38 and negative for CD19 (and in some experiments CD10) were sorted. Highly diffusive cells and/or too large objects were rejected. Positivity or negativity for the CD38 among the CD34+ cells was determined using control cells labeled with the PE-HPCA2 and an irrelevant FITC-IgG1 MoAb. Compensation was set up with single-stained samples. In cord blood samples, in contrast with bone marrow, very few CD34+ cells can be defined as CD38− based on comparison with the isotype-matched control antibody. Therefore, we selected a population (CD38low) that included the 20% to 25% CD34+ cells that expressed the lowest amounts of CD38 antigen (see Fig 1C). To initiate long-term cultures at limiting dilutions, cells from the CD34+ fractions were directly sorted into 96-well tissue culture plates precoated with the appropriate feeder cells using the automatic cloning design unit of the FACSvantage (Becton Dickinson).
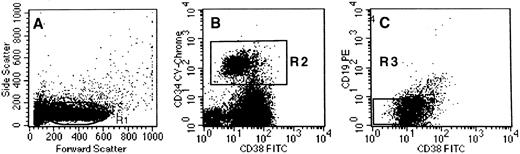
Selection of CD34+CD38lowCD19− cells sorted from cord blood. CD34+ cells were first purified from cord blood and then labeled with PE-cy5-CD34, PE-CD19, and FITC-CD38. (A) defines the morphologic gate. Analysis was performed in the CD34+ gate (R2, B). The R3 gate in (C) indicates the limits defined for the sorting gate (the CD38 limit is set at 101).
Selection of CD34+CD38lowCD19− cells sorted from cord blood. CD34+ cells were first purified from cord blood and then labeled with PE-cy5-CD34, PE-CD19, and FITC-CD38. (A) defines the morphologic gate. Analysis was performed in the CD34+ gate (R2, B). The R3 gate in (C) indicates the limits defined for the sorting gate (the CD38 limit is set at 101).
Establishment of Conditions Permissive for B-Lymphoid Differentiation
The potential of CD34+CD38lowCD19− cord blood cells to differentiate into B cells was tested in the presence of murine stromal cells MS-529 (kindly provided by K. Mori, Niagata University, Niagata, Japan). One or 2 days before their use in coculture experiments, MS-5 cells were plated in 24-well plates (6 × 104 cells/well in 1 mL) or in flat-bottomed 96-well plates (6,000 cells per well in 100 μL). Cocultures were initiated by seeding 1,000 to 40,000 cells from selected CD34+ fractions in 24-well plates precoated with MS-5 cells. Limiting dilution cocultures were also initiated in 96-well plates (1 to 100 cells per well) using the automated cell device unit from the FACSvantage.
Cultures were maintained in three different conditions referred to as lymphoid, switch, and myeloid. Cells grown in lymphoid conditions were incubated from the start in α-MEM (GIBCO) supplemented with 3% (vol/vol) of a preselected batch of FCS (Techgen, Les Ulis, France), 10−4 mol/L 2β-mercaptoethanol (ME), 200 mmol/L glutamine, 1,000 U/mL penicillin, and 100 U/mL streptomycin, and the cultures were kept continuously at 37°C in a humidified atmosphere with 5% CO2 with a twice weekly change of half the medium. Switch conditions reproduced the two-stage culture system initially described by Whitlock-Witte.8 During a first 4-week period, cells were kept in standard long-term culture medium, ie, α-MEM supplemented with 12.5% FCS, 12.5% horse serum (GIBCO-BRL), and 2β-ME, and subsequently switched for a further 2-week period in lymphoid conditions (see above). Hydrocortisone was omitted from standard long-term culture medium because its addition drastically reduced nucleated and myeloid progenitor cells output at week 5 in long-term cultures established on MS-5.30 Cocultures were also maintained continuously in standard long-term culture medium without hydrocortisone (myeloid conditions). Initial experiments were performed with standard α-MEM containing phenol red, whereas, later on during the study, α-MEM without phenol red was used.31
At the end of the 6-week culture period, nonadherent and adherent cells from each well were harvested and labeled with a panel of lineage-specific antibodies and analyzed by flow cytometry (see below). In experiments initiated with individual cells, only those wells in which proliferation had occurred were analyzed by flow cytometry. In two experiments initiated with 5 and 10 CD34+CD38lowCD19−CD10− cells, each well was divided in two; half of the cells were labeled and the other half were plated in standard methylcellulose colony assays to quantitate colony-forming units–granulocyte-macrophage (CFU-GM).
Analysis by Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) of B-Specific Transcripts in Cultured Cells
cDNA from 2 × 105 cells was first prepared from total RNA, using 150 ng of oligo dN6 as primer and the reverse transcriptase (BRL, Cergy-Pontoise, France) according to the manufacturer's instructions. PCR was performed in a Cetus apparatus using 1/20 of the cDNA, 1.5 U of Taq polymerase (Promega, Charbonnières, France), 2.5 mg of T4 gene 32 protein (Boehringer Mannheim, Meylan, France), and 50 pmol of each oligonucleotide as primer: human β2-microglobulin: sense, 5′ACCCCCACTGAAAAAGATGA3′, and antisense, 5′ATCTTCAAACCTCCATGATG3′, at 54°C; CD34: sense, 5′CTCTTCTGTCCAGTCACAGACC3′, and antisense, 5′GAATAGCTCTGGTGGCTTGCAA3′, at 64°C; CD10: sense, 5′CTGTGACAATGATCGCACTCTATG3′, and antisense, 5′GATTCCAGTGCATTCATAGTAATCTC3′, at 65°C; λ-like: sense, 5′ATGCATGCGGCCGCGGCATGTGTTTGGCAGC3′, and antisense, 5′ATCCGCGGCCGCATCGATAGGTCACCGTCAAGATT3′, at 60°C; Pax-5: sense, 5′AGCAGGACAGGACATGGAGGA, and antisense, 5′ATCCTGTTGATGGAACTGACGC3′, at 64°C; CD19: sense, 5′TCACCGTGGCAACCTGACCATG3′, and antisense, 5′GAGACAGCACGTTCCCGTTACTG3′, at 67°C; VHconsensus-Cμ: sense, 5′GACACGGCCGTGTATTACTG3′, and antisense, 5′ATCCGCGGCCGCGGAATTCTCACACAGGAGACGA3′, at 60°C; Vkconsensus-Ck: sense, 5′ATGACCCAGTCTCCATCCTCCCTG3′, and antisense, 5′ATGCGGCCGCGGGAAGATGAAGACAGATG3′, at 65°C.
To insure that PCR products were not derived from DNA, primers were defined from discrete exons for each transcript. Quantification was performed by comparison to human β2-microglobulin PCR products. The first cycle was run as follows: denaturation at 94°C for 3 minutes, annealing at temperatures indicated above for 1 minute, and synthesis at 72°C for 2 minutes. The next 34 cycles were run similarly, except that denaturation lasted only 1 minute. For the last cycle, the synthesis time was 7 minutes. One fourth of the amplified products was run in agarose gel made 1% or 2% in 1× TBE.
Assessment of B-Lymphoid Potential in Immune-Deficient Mice Reconstituted With Human Cord Blood Cells
Mice.Homozygous CB17-scid/scid (hereafter called CB17-SCID) and NOD-LtSz-scid/scid (hereafter called NOD-SCID) were originally obtained from Dr John Dick (Hospital for Sick Children, Toronto, Ontario, Canada). Mouse breeding pairs were housed in the animal facilities of the Institut Gustave Roussy under sterile conditions in air-filtered containers. NOD-SCID mice received a sublethal dose of irradiation (3.5 Gy, cobalt-60 Eldorado S irradiator; AECL Medical, Ontario, Canada) before human cell transplantation. We did not observe radiation-induced death of immunodeficient mice at this dosage. Mice were anesthesized shortly with ether and were injected intravenously with either 20 × 106 light-density CB-MNC (4 experiments), 5 × 105 CD34+ cells (1 experiment), or 60,000 CD34+CD19−CD10− cells (2 experiments), all derived from cord blood.
Assessment of the phenotype and B-lymphoid potential of human cells from transplanted NOD-SCID mice.At indicated time points, transplanted mice were euthanazied and marrow cells were flushed from the femurs and tibias with α-MEM/10% FCS. Nucleated cells were counted and used either for phenotypic analysis and/or assessment of hematopoietic progenitor cells. Human cell engraftment was evaluated by labeling cells with FITC-conjugated antihuman CD45 MoAb (Immunotech) or FITC-conjugated MoAb recognizing a polymorphic determinant on HLA-ABC (W6/32; Serotec-Innotest, Besançon, France). Detailed phenotype was performed as described below for the in vitro studies. The species specificity of mouse antihuman antibodies was tested by labeling marrow cells from untreated mice with the MoAbs used for phenotying. Background levels of immunofluorescence were measured using isotypic controls. In some experiments, human CD34+ or CD34+CD38low populations were sorted from murine marrow cell suspensions using the FACSvantage and used to initiate long-term cocultures on MS-5 in switch or lymphoid conditions (see above).
Phenotypic Analysis of Cells Generated in MS-5 Cell Cocultures or In Vivo After Engraftment in NOD-SCID Mice
Detection of cell surface antigens by immunofluorescence was performed using human-specific MoAbs. PE-labeled antibodies recognizing the CD34 (HPCA2) and CD19 antigens were from Becton Dickinson. FITC-labeled antibodies recognizing CD34 (HPCA-2), CD20, CD22, and CD10 were purchased from Becton Dickinson. FITC-labeled anti-CD38 and anti-CD11b MoAbs were from Immunotech. PE- or FITC-labeled isotype-matched nonimmune mouse Ig were used as negative controls. Analysis was performed on a FACSort (Becton Dickinson). When two-color labeling was performed, compensation was set up with single-stained samples. Low forward scatter elements (red blood cells or debris) were excluded from the analysis and 10,000 events were collected and analyzed using the Cellquest software (Becton Dickinson). For detection of intracytoplasmic μ chains, cells were permeabilized with the Fix and Perm kit from Caltag (Burlingame, CA). Surface and intracytoplasmic μ chains were identified with an FITC-goat F(ab′)2 anti-IgM (μ) (Caltag) and compared with results obtained with the FITC-goat F(ab′)2 control.
RESULTS
CD34+CD38lowCD19− Primitive Cord Blood Progenitors Cultured With Murine Stromal Cells MS-5 Generate CD19+ B Cells
We first designed in vitro conditions permissive for the simultaneous differentiation of human primitive progenitor cells in both the B and myeloid lineages. CD34+CD38low cells from cord blood were used as a source of primitive cells. Because most CD34+ cord blood cells stained positively for CD38, we deliberately selected the 20% to 25% of CD34+ cells that expressed the lowest amount of CD38 (referred to as CD38low, Fig 1C). These CD34+CD38low cells did not coexpress CD19 and the few CD34+CD19+ cells were CD38bright (Fig 1C). However, to completely exclude contamination by CD19+ or CD10+ pro-B cells, CD34+CD38low cells were further depleted of CD19+ and in some experiments of both CD19+ and CD10+ cells. CD34+CD38lowCD19− cells were cultured in the presence of murine stromal cell MS-5, which supports the myeloid differentiation of CD34+CD38low LTC-IC.25,30 To permit B-cell differentiation, the standard myeloid conditions were changed to either lymphoid or switch conditions (see the Materials and Methods for details).8,17 32
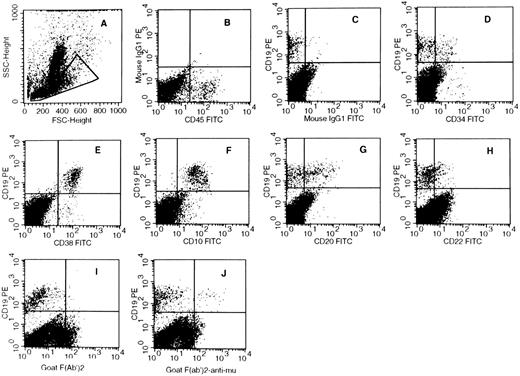
In each of 10 separate experiments in which CD34+CD38lowCD19− cells were grown in lymphoid conditions, a high number of CD19+ B cells was reproducibly observed. Thus, as shown in Table 1, 43% ± 5.4% (n = 10) (mean ± SEM) of nucleated cells produced in the culture after 6 weeks expressed the CD19 antigen. These small cells were also easily identified by visual inspection and by flow cytometry by their scatter properties (small FSC and SSC) characteristic of lymphoid cells (Fig 2C). After 1 or 2 weeks in culture, CD19+ cells were already detectable and represented 4% and 17%, respectively, of nucleated cells (2 experiments). A similarly high proportion (30%) of CD19+ cells was observed in 4 separate experiments initiated with CD34+CD38lowCD19− cells further depleted of CD10+ cells, thus excluding the possibility that CD10+CD19− lymphoid precursors may contribute to the output of B cells (Table 1). In these wells maintained in lymphoid conditions, myeloid cells represented only 12% ± 3.5% (mean ± SEM, n = 10) of nucleated cells. In contrast, in cultures established in switch conditions, even though CD19+ cells were present and represented 8.4% ± 3.6% (n = 8) of nucleated cells, myeloid cells recognized by their typical FSC and SSC (Fig 2A) and by the expression of the myeloid marker CD11b were predominant (39% ± 6% of nucleated cells in 8 experiments). Cell amplification was equivalent in cultures maintained in both lymphoid and switch conditions and represented 42- ± 5.4-fold (n = 10) and 54- ± 8.4-fold (n = 8) increases in the input number of nucleated cells, respectively.
Phenotypic Analysis of CD19+ Cells Produced by Human CD34+CD38lowCD19− and CD34+CD38lowCD19−CD10− Cord Blood Cells Cultured for 6 Weeks on the Murine Stromal Cells MS-5
| Cell Fraction . | No. of Experiments . | Conditions . | % CD19+ . | % of CD19+ Cells Expressing . | ||||
|---|---|---|---|---|---|---|---|---|
| . | . | . | . | CD34 . | CD38 . | CD10 . | CD20 . | CD22 . |
| CD34+38low19− | 10 | Lymphoid | 43 ± 5.4 | <1 | 100 | 100 | 4 ± 1 | <1 |
| 8 | Switch | 8.4 ± 3.6 | <1 | 100 | 100 | 1.3 ± 0.3 | <1 | |
| CD34+38low19−10− | 4 | Lymphoid | 30 ± 11 | 0 | 100 | 100 | 6.3 ± 3 | 0 |
| Cell Fraction . | No. of Experiments . | Conditions . | % CD19+ . | % of CD19+ Cells Expressing . | ||||
|---|---|---|---|---|---|---|---|---|
| . | . | . | . | CD34 . | CD38 . | CD10 . | CD20 . | CD22 . |
| CD34+38low19− | 10 | Lymphoid | 43 ± 5.4 | <1 | 100 | 100 | 4 ± 1 | <1 |
| 8 | Switch | 8.4 ± 3.6 | <1 | 100 | 100 | 1.3 ± 0.3 | <1 | |
| CD34+38low19−10− | 4 | Lymphoid | 30 ± 11 | 0 | 100 | 100 | 6.3 ± 3 | 0 |
One thousand sorted cells of the indicated phenotype were cultured in 24 wells precoated with confluent MS-5 cells in either lymphoid or switch conditions (see the Materials and Methods for details). At the end of the 6-week culture period, cell phenotype was analyzed by flow cytometry. The proportion of CD19+ cells was calculated in a morphologic gate including 80% of nucleated cells. The proportion of CD19+ cells coexpressing another B-lineage–specific marker was calculated in each experiment and the mean ± SEM of these calculations is shown.
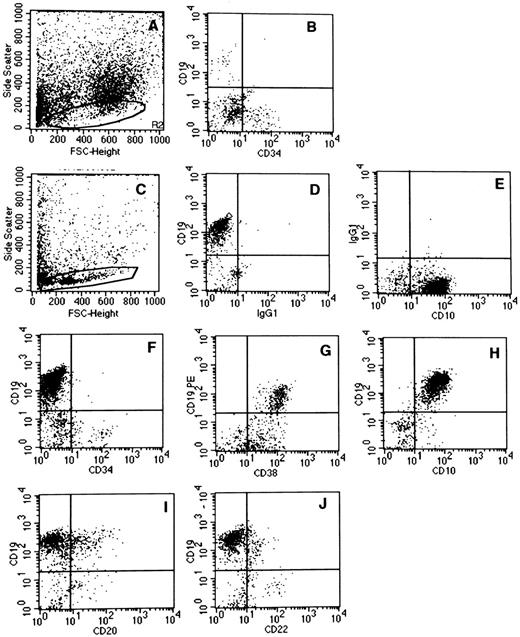
Phenotype of CD19+ B cells produced in cocultures of CD34+CD338lowCD19− cord blood cells with MS-5 murine stromal cells. One thousand CD34+CD38lowCD19− cells were seeded in 24-well plates on preestablished MS-5 cell underlayers. Cells were grown in either switch conditions (A and B) or lymphoid conditions (C through J) for 6 weeks. At the end of the culture period, the total content of the well (adherent and nonadherent cells) was analyzed by flow cytometry after labeling with human MoAbs recognizing human B-cell antigens. Quadrants limits defining positivity and negativity were set up using cells labeled with isotype-matched FITC- (D) and PE-labeled (E) control antibodies. Analysis is shown in the lymphoid gate circled in (A) and (C). (A) and (B) are from one experiment and (C) through (J) are from a separate experiment.
Phenotype of CD19+ B cells produced in cocultures of CD34+CD338lowCD19− cord blood cells with MS-5 murine stromal cells. One thousand CD34+CD38lowCD19− cells were seeded in 24-well plates on preestablished MS-5 cell underlayers. Cells were grown in either switch conditions (A and B) or lymphoid conditions (C through J) for 6 weeks. At the end of the culture period, the total content of the well (adherent and nonadherent cells) was analyzed by flow cytometry after labeling with human MoAbs recognizing human B-cell antigens. Quadrants limits defining positivity and negativity were set up using cells labeled with isotype-matched FITC- (D) and PE-labeled (E) control antibodies. Analysis is shown in the lymphoid gate circled in (A) and (C). (A) and (B) are from one experiment and (C) through (J) are from a separate experiment.
Maturation stage of the lymphoid precursors assessed by immunophenotyping and RT-PCR detection of B-specific transcripts.The maturation stage of B cells generated by CD34+CD38lowCD19− (and CD34+CD38lowCD19−CD10−) cells from cord blood cultured in both lymphoid and switch conditions was assessed by flow cytometry and RT-PCR analysis of B-specific transcripts.
Table 1 summarizes data obtained by flow cytometry analysis of all experiments and Fig 2C through J shows dot plot analysis obtained in one representative experiment in which cells were maintained in lymphoid conditions. Table 1 and Fig 2 show that in all 10 experiments established in lymphoid conditions, all CD19+ cells produced after 6 weeks were CD34− (Fig 2F ) and coexpressed high levels of CD38 (Fig 2G) and CD10 (Fig 2H). Only 4% of CD19+ cells coexpressed CD20 (Fig 2I) and almost none was convincingly stained with CD22 (Fig 2J). Surface IgM were undetectable (not shown) and intracytoplasmic IgM was present on rare CD19+ cells in 2 experiments (not shown). The phenotype of CD19+ cells generated from cultures maintained in switch conditions was similar to that observed in lymphoid conditions, except for a lower proportion of CD20+ cells (1.3% ± 0.3%; Table 1 and data not shown). These CD34−CD10+CD38+CD19+CD20±CD22−cIgM− B cells could be classified as late pro-B stage.32-34
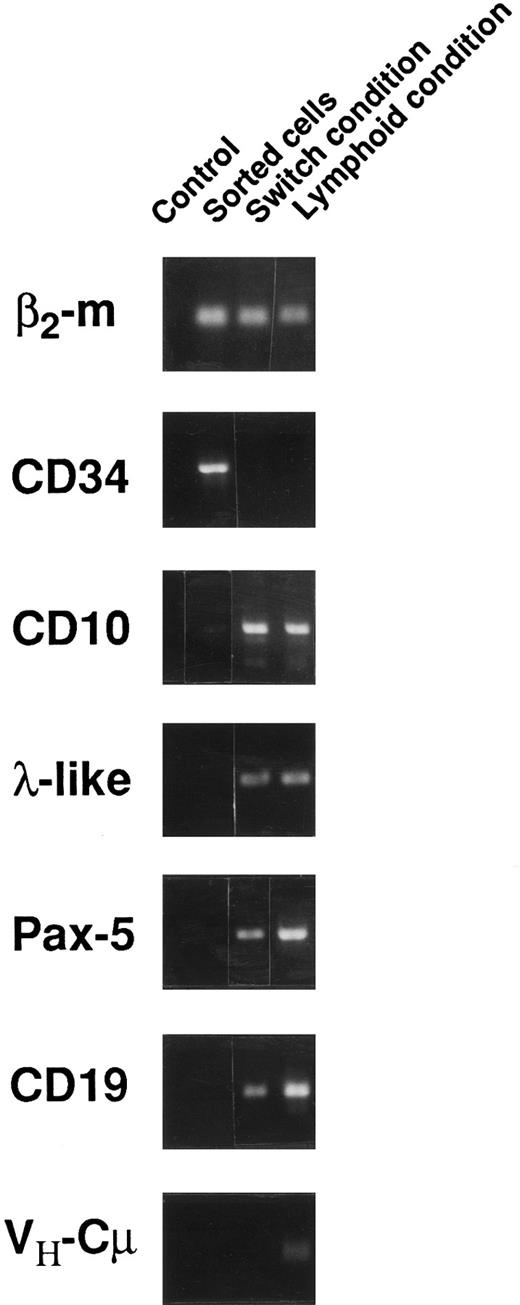
To follow the engagement of CD34+CD38low CD19− cord blood cells into the B lineage, the presence of transcripts specific for the B lineage was looked for by RT-PCR in cells cultured 6 weeks in either switch or lymphoid conditions (Fig 3). Transcripts derived from genes expressed in early progenitors or linked to IgH expression (VH-Cμ) were analyzed. Expression of λ-like35,36 and Pax-5,37,38 which have been shown of importance in early B-cell differentiation, was also tested. CD34+CD38lowCD19− cells at the start of the culture did not express B-specific transcripts (Fig 3). After culture, expression of CD34 transcripts was lost, but transcripts for λ-like, Pax-5, CD10, and CD19 were clearly detected in cells derived from CD34+CD38lowCD19− cells (Fig 3). Finally, rearranged heavy chain transcripts were detected only under lymphoid culture conditions, whereas no light chain transcripts could be amplified (data not shown). Altogether, phenotypic and RT-PCR analysis point to a differentiation that is blocked at a CD34−CD10+CD19+ late pro-B stage34 and show that lymphoid conditions favor the emergence of pre-B cells in a more mature stage, as suggested by the higher proportion of CD20+ cells and the presence of μ transcripts.
Transcriptional activity of CD34+CD38lowCD19− sorted cells before and after 6 weeks of culture in either switch or lymphoid conditions. RT-PCR was performed using primers and conditions as indicated in the Materials and Methods. A control without cDNA was included in the left column of each series.
Transcriptional activity of CD34+CD38lowCD19− sorted cells before and after 6 weeks of culture in either switch or lymphoid conditions. RT-PCR was performed using primers and conditions as indicated in the Materials and Methods. A control without cDNA was included in the left column of each series.
Individual CD34+CD38lowCD19− Human Cord Blood Progenitors Cocultured With MS-5 Cells Generate B-Lymphoid and Granulocytic Cells
CD19+ cells obtained in experiments described above might arise from a B-lymphoid–restricted progenitor or/and from progenitors endowed with the ability to differentiate into B-lymphoid as well as myeloid lineages. To explore these possibilities, in two experiments, multiple wells precoated with MS-5 cells were seeded with single CD34+CD38lowCD19− cord blood cells and kept in either lymphoid or switch conditions (60 wells per experiment in each condition, Table 2). Six weeks later, wells were individually examined for the presence of cells expressing B-lymphoid (CD19) and myeloid (CD11b, CD33) markers. Only those wells with sufficient numbers of cells were harvested (the number of cells, estimated by microscopic observation, ranged from 50 to >300). Table 2 shows that, of the 120 wells seeded with 1 CD34+CD38lowCD19− cell and kept in lymphoid conditions, 24 had proliferated and 8 of these 24 contained both CD19+ and CD11b+ cells after 6 weeks. Numbers of CD19+ cells and CD11b+ cells each ranged from 10 to greater than 200. The specificity of the labeling was indicated first by the intensity of the fluorescence (>102) and second by the scatter properties of CD19+ cells (low FSC, low SSC) and of CD11+ cells (high FSC, medium SSC) characteristic of lymphoid and monocytic/granulocytic cells, respectively. Figure 4A shows an example of the dot plot obtained by flow cytometry analysis after labeling by anti-CD11b and anti-CD19 antibodies of the content of one well seeded with a single CD34+CD38lowCD19− cell. These results identified among CD34+CD38lowCD19− cord blood cells an early progenitor cell at least able to differentiate in both the B-lymphoid and myeloid lineages. The frequency of these cells was calculated to be 7% in the CD34+CD38lowCD19− fraction of cord blood cells, which might be an underestimate because only proliferating wells were analyzed. Table 2 also shows that, in these same experiments, 7 wells contained only CD19+ lymphoid cells and 1 well only CD11b+ myeloid cells, defining lymphoid- and myeloid-restricted progenitor cells.
Phenotype of Cells Generated by Human CD34+CD38lowCD19− Cord Blood Cells Cultured 6 Weeks at Limiting Dilutions on MS-5 Cells
| Experiment No. . | Condition . | Cells per Well . | No. of Wells Initiated . | No. of Wells With Proliferation . | No. of Wells Positive for . | ||
|---|---|---|---|---|---|---|---|
| . | . | . | . | . | CD19+ Only . | CD11b+ Only . | CD19+ CD11b+ . |
| 1 | Myeloid | 1 | 60 | 11 | 0 | 10 | 0 |
| 10 | 7 | 7 | 0 | 7 | 2 | ||
| Switch | 1 | 60 | 16 | 1 | 6 | 4 | |
| 10 | 7 | 7 | 0 | 0 | 7 | ||
| Lymphoid | 1 | 60 | 10 | 3 | 1 | 5 | |
| 10 | 7 | 7 | 0 | 0 | 7 | ||
| 2 | Lymphoid | 1 | 60 | 14 | 4 | 0 | 3 |
| Switch | 1 | 60 | 4 | 1 | 0 | 3 | |
| 10 | 22 | 22 | 0 | 0 | 22 | ||
| Experiment No. . | Condition . | Cells per Well . | No. of Wells Initiated . | No. of Wells With Proliferation . | No. of Wells Positive for . | ||
|---|---|---|---|---|---|---|---|
| . | . | . | . | . | CD19+ Only . | CD11b+ Only . | CD19+ CD11b+ . |
| 1 | Myeloid | 1 | 60 | 11 | 0 | 10 | 0 |
| 10 | 7 | 7 | 0 | 7 | 2 | ||
| Switch | 1 | 60 | 16 | 1 | 6 | 4 | |
| 10 | 7 | 7 | 0 | 0 | 7 | ||
| Lymphoid | 1 | 60 | 10 | 3 | 1 | 5 | |
| 10 | 7 | 7 | 0 | 0 | 7 | ||
| 2 | Lymphoid | 1 | 60 | 14 | 4 | 0 | 3 |
| Switch | 1 | 60 | 4 | 1 | 0 | 3 | |
| 10 | 22 | 22 | 0 | 0 | 22 | ||
Wells from 96-well plates precoated with the MS-5 cells were seeded with 1 to 50 cord blood cells of the selected phenotype using the automatic cell device unit of the FACS vantage. Cells were cultured in myeloid, switch, or lymphoid conditions as described (see the Materials and Methods). At the end of the 6-week culture period, individual wells were visually inspected and those that contained enough cells were harvested and cells labeled with lineage-specific antibodies. Analysis was performed on the FACSort as described.
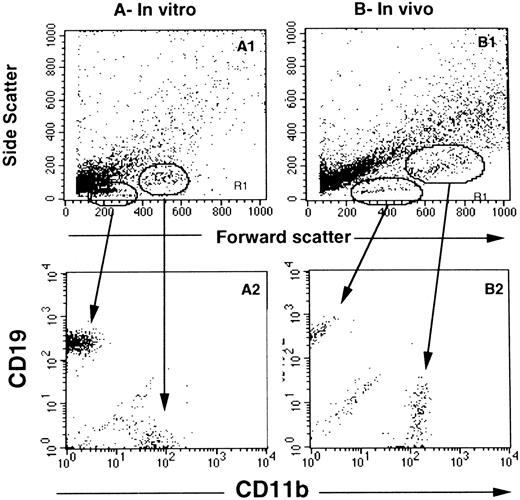
Phenotype of cells generated in wells seeded with single CD34+CD38lowCD19− cells. In (A), wells precoated with MS-5 cells were seeded with single CD34+CD38lowCD19− cells from cord blood samples and grown in switch conditions. After 6 weeks, cells collected from individual wells were labeled with PE-CD19 and FITC-CD11b MoAbs and analyzed by flow cytometry. (A) shows the forward scatter and side scatter properties of cells from one representative well and (A2) shows the expression of CD19 and CD11b on the corresponding cells. CD19+ cells display a lymphoid morphology (low FSC and SSC) and CD11b+ cells have a higher FSC and SSC. (B) and (B2) show the morphologic parameters and simultaneous labeling with CD19 and CD11b of cells collected from one well seeded 6 weeks earlier with a single CD34+CD38low cell sorted from the marrow of an NOD-SCID mouse injected with 20 × 106 CB-MNC.
Phenotype of cells generated in wells seeded with single CD34+CD38lowCD19− cells. In (A), wells precoated with MS-5 cells were seeded with single CD34+CD38lowCD19− cells from cord blood samples and grown in switch conditions. After 6 weeks, cells collected from individual wells were labeled with PE-CD19 and FITC-CD11b MoAbs and analyzed by flow cytometry. (A) shows the forward scatter and side scatter properties of cells from one representative well and (A2) shows the expression of CD19 and CD11b on the corresponding cells. CD19+ cells display a lymphoid morphology (low FSC and SSC) and CD11b+ cells have a higher FSC and SSC. (B) and (B2) show the morphologic parameters and simultaneous labeling with CD19 and CD11b of cells collected from one well seeded 6 weeks earlier with a single CD34+CD38low cell sorted from the marrow of an NOD-SCID mouse injected with 20 × 106 CB-MNC.
Several experimental arguments further show that CD11b+ cells belong to the myeloid lineage. (1) Typical polymorphonuclear cells, which stained positively for myeloperoxidase, were observed on May-Grünwald-Giemsa–stained slides from one well seeded with 1 CD34+CD38lowCD19− cells and from several wells initiated with 10 cells (data not shown). These PMN were mixed with lymphocytes. (2) All CD11b+ cells coexpressed CD33 but none of them coexpressed CD56+ and CD1a+ cells, which ruled out the possibility that CD11b+ cells were natural killer (NK) or dendritic cells. Cells expressing the CD5 antigen were not found. (3) In one experiment initiated with 5 CD34+CD38lowCD19−CD10− cells, 8 of 30 wells analyzed contained both CD19+ and CD11b+ cells and 2 of these 8 wells also included CFU-GM (1 and 5). Although most of these observations were made in wells initiated with 5 to 10 cells/well, they strongly argue that CD11b+ cells found in single-cell cultures identify granulocytic cells.
In the same experiments, single-cell cocultures were also maintained in switch conditions (60 wells per experiment, 2 experiments). Table 2 shows that, of these 120 wells, 20 proliferated; 7 of these 20 contained both CD11b+ and CD19+ cells. The frequency of these progenitors was thus similar in cultures maintained in lymphoid or switch conditions. In contrast, all wells seeded with single cells and maintained in standard myeloid conditions contained only CD11b+ cells (Table 2), and only 2 of the 7 wells seeded with 10 cells contained both CD11b+ and CD19+ cells, thus confirming that pure myeloid conditions, even though they may not totally abrogate expression of the B-lymphoid potential, lead to a decrease in the number of clones with CD19+ cells.
Development of B Cells in Immune-Deficient Mice Transplanted With CD34+CD38lowCD19− Human Cord Blood Progenitor Cells
Both pro-B and pre-B cells were detected in the marrow of engrafted NOD-SCID.To determine whether primitive human cord blood progenitors would also efficiently express their B-lymphoid potential in vivo, CD34+CD19−CD10− human cord blood cells (Table 3, experiments 1 and 2) were injected into sublethally irradiated NOD-SCID mice. After 4 to 6 weeks, human cells present in the bone marrow of the transplanted mice were counted and labeled with the same panel of myeloid- and lymphoid-specific antibodies used in vitro. Human cells were detected in 3 of 6 mice transplanted with 60,000 CD34+CD19−CD10− cells and greater than 50% of these were CD19+ cells (Table 3, experiments 1 and 2). Table 3 summarizes the data obtained in all in vivo experiments and Fig 5 provides an example of the phenotype of human CD19+ cells found in the marrow of a mouse transplanted with 60,000 CD34+CD19−CD10− cells (corresponding to experiment 2 of Table 3). Comparison of the phenotype of CD19+ B cells obtained in vivo and in vitro showed interesting differences. First, 10% to 20% of human CD19+ cells present in the marrow of immune-deficient mice coexpressed the CD34 antigen, reflecting the presence of pro-B cells (Table 3 and Fig 5D). Second, 20% to 40% of CD19+ cells coexpressed the CD20 (Fig 5G) and CD22 (Fig 5H) antigens, and surface IgM was also easily detected on 4% to 5% of the CD19+ cells (Fig 5J). The presence of CD34+CD19+ pro-B and CD19+slgM+ B cells was in striking contrast with the lack of these cells in long-term cultures in vitro (Fig 2).
Proportion and Phenotype of Human CD19+ B Cells Observed in the Marrow of SCID and NOD-SCID Mice Injected With CB-MNC or CD34+CD19−CD10− Cells
| Experiment No. . | Cells . | Positive/Total Mice . | % Human Cells3-150 . | Mice With CD19+ Cells . | % CD19+3-151 . | % of CD19+ Cells Positive for . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | CD34 . | CD38 . | CD10 . | CD20 . | CD22 . | sIgM . |
| 1 | 34+19−10− | 2/3 | 0.5; 0.23-152 | 2/3 | 40; 90 | 15; 0 | 100 | 100 | 42; 79 | 35; 50 | ND |
| 2 | 34+19−10− | 1/3 | 3.2 | 1/3 | 75 | 21 | 100 | 100 | 36 | 17 | 5 |
| 3 | MNC | 3/3 | 22 ± 9 | 3/3 | 27 ± 1.5 | 10 | 100 | 90 ± 1 | 31 ± 7 | 8 ± 4 | ND |
| 4 | MNC | 3/3 | 39 ± 10 | 3/3 | 64 ± 3 | 8.5 ± 1 | 100 | ND | ND | ND | ND |
| 5 | MNC | 4/4 | 21 ± 6 | 4/4 | 40 ± 15 | ND | 100 | 90 ± 1.2 | 40 ± 4 | 26 ± 2 | 4 ± 1 |
| 6 | MNC | 3/3 | 42 ± 13 | 3/3 | 59 ± 13 | 8 ± 1 | 100 | ND | ND | ND | ND |
| Experiment No. . | Cells . | Positive/Total Mice . | % Human Cells3-150 . | Mice With CD19+ Cells . | % CD19+3-151 . | % of CD19+ Cells Positive for . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | CD34 . | CD38 . | CD10 . | CD20 . | CD22 . | sIgM . |
| 1 | 34+19−10− | 2/3 | 0.5; 0.23-152 | 2/3 | 40; 90 | 15; 0 | 100 | 100 | 42; 79 | 35; 50 | ND |
| 2 | 34+19−10− | 1/3 | 3.2 | 1/3 | 75 | 21 | 100 | 100 | 36 | 17 | 5 |
| 3 | MNC | 3/3 | 22 ± 9 | 3/3 | 27 ± 1.5 | 10 | 100 | 90 ± 1 | 31 ± 7 | 8 ± 4 | ND |
| 4 | MNC | 3/3 | 39 ± 10 | 3/3 | 64 ± 3 | 8.5 ± 1 | 100 | ND | ND | ND | ND |
| 5 | MNC | 4/4 | 21 ± 6 | 4/4 | 40 ± 15 | ND | 100 | 90 ± 1.2 | 40 ± 4 | 26 ± 2 | 4 ± 1 |
| 6 | MNC | 3/3 | 42 ± 13 | 3/3 | 59 ± 13 | 8 ± 1 | 100 | ND | ND | ND | ND |
NOD-SCID (experiments no. 1, 2, 3, and 5) and SCID (experiments no. 4 and 6) mice were injected intravenously with either cord blood light-density MNC (20 × 106 per mouse) or CD34+19−10− cells purified by cell sorting from cord blood CD34+ cells (4 to 6 × 104 per mouse). Mice were killed 4 to 8 weeks later and the proportion and phenotype of human cells in the marrow of each transplanted mouse were examined by labeling the cells with a panel of human lineage-specific markers. Numbers represent the mean (±SD) proportion of cells measured in the indicated number of animals.
Abbreviation: ND, not done.
Human cells were identified by labeling with either anti-CD45 or anti-HLA class I antibodies.
The proportion of CD19+ cells was calculated in a gate including most viable nucleated cells.
Data obtained in both mice are indicated.
Phenotype of human CD19+ B cells detected in the marrow of a NOD-SCID mouse transplanted with human CD34+- CD19−CD10− cord blood cells. In this representative experiment, NOD-mice were injected with 60,000 CD34+CD19−CD10− cord blood cells. Six weeks later, mice were killed and the bone marrow cells were labeled with a panel of MoAbs recognizing human antigens. Analysis was performed in the morphologic gate defined in (A), which includes all human lymphoid cells. Quadrant limits were set as defined in Fig 1 using PE-labeled (B) and FITC-labeled (C) control antibodies.
Phenotype of human CD19+ B cells detected in the marrow of a NOD-SCID mouse transplanted with human CD34+- CD19−CD10− cord blood cells. In this representative experiment, NOD-mice were injected with 60,000 CD34+CD19−CD10− cord blood cells. Six weeks later, mice were killed and the bone marrow cells were labeled with a panel of MoAbs recognizing human antigens. Analysis was performed in the morphologic gate defined in (A), which includes all human lymphoid cells. Quadrant limits were set as defined in Fig 1 using PE-labeled (B) and FITC-labeled (C) control antibodies.
In another set of 4 experiments, 13 mice were injected with total human cord blood MNC (Table 3, experiments 4 through 6) and all mice were reconstituted with human cells. Fifty percent (±8%) of human cells detected in the mouse marrow were CD19+ B cells. Interestingly, the number of CD34+CD19+ pro-B cells among output cells exceeded by at least a factor 10 the number of input pro-B cells, thus demonstrating that amplification of early lymphoid progenitors had occurred in the murine marrow environment.
CD34+CD38lowCD19− progenitors generating both CD19+ and CD11b+ cells are present in vivo in the marrow of NOD-SCID mice grafted with cord blood cells.We have recently shown that human CD34+CD38low LTC-IC able to differentiate in the myeloid lineages are present in the marrow of chimeric SCID and NOD-SCID mice transplanted with cord blood MNC.25 To determine if progenitors with the ability to produce B-lymphoid and granulocytic cells exist among these CD34+CD38low cells, CD34+CD38low (3 experiments) or CD34+ (1 experiment) human cells were sorted by flow cytometry from the bone marrow of immune-deficient mice transplanted 4 to 6 weeks earlier with human cord blood cells (Table 4). CD34+CD38low and CD34+ cells were cocultured as described above on MS-5 cells either in 24 wells (1,000 cells per well, experiments 1, 2, 4, and 5 from Table 4) or in 96 wells (1, 10, and 50 cells per well, experiments 1, 3, and 4). Four to 6 weeks later, wells were analyzed for their content of CD19+ and CD11b+ cells. As shown in Table 4, both human CD19+ and CD11b+ (or CD15+) cells were detected in all wells seeded with 50 cells (experiment 4, Table 4) and in 21 of the 36 proliferating wells seeded with 10 cells (experiments 3 and 4). Of 480 wells seeded with single CD34+CD38low cells, 51 proliferated and were analyzed by flow cytometry. Eight of these contained CD11b+ and CD19+ cells, which gives a frequency of 1.5% for these at least bipotent progenitor cells in the CD34+CD38low fraction sorted from the marrow of engrafted mice. Results of the phenotypic analysis of a representative positive well are shown in Fig 4B.
Phenotype of Cells Generated by Human CD34+ or CD34+CD38low Cells Sorted From the Marrow of SCID or NOD-SCID Mice Transplanted With Human CB-MNC or CD34+ Cells and Cultured With MS-5 Stromal Cells
| Experiment No. . | Cells Injected . | Mice . | Time (wk) . | Sorted Cells . | Time in Culture (wk) . | 24 Wells4-150 . | Cultures at Limiting Dilutions . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | CD11b+ or CD15+ . | CD19+ . | Cells/Well . | No. of Wells . | Positive/Total Wells Analyzed4-151 . | . | . | ||
| . | . | . | . | . | . | . | . | . | . | CD19+ . | CD19+ CD15− . | CD19− CD15+ . | . | . |
| . | . | . | . | . | . | . | . | . | . | CD15+/CD11b+ . | . | . | . | . |
| 1 | MNC | SCID | 4 | 34+38low | 4 | 59% | 5% | 1 | 180 | 5/80 | 4/80 | 10/80 | ||
| 2 | MNC | SCID | 7 | 34+38low | 7 | 25% | 10% | ND | ND | ND | ND | ND | ||
| 3 | MNC | NOD-SCID | 4 | 34+38low | 4 | ND | ND | 1 | 240 | 3/13 | 2/13 | 8/13 | ||
| 10 | 60 | 8/18 | 4/18 | 5/18 | ||||||||||
| 4 | CD34+ | NOD-SCID | 7 | 34+38low | 4 | 39% | 5% | 1 | 60 | 0/12 | 9/12 | 3/12 | ||
| 10 | 60 | 13/18 | 3/18 | 2/18 | ||||||||||
| 50 | 60 | 10/10 | 0/10 | 0/10 | ||||||||||
| 5 | CD34+ | NOD-SCID | 5 | 34+ | 7 | 25% | 8% | ND | ND | ND | ND | ND | ||
| Experiment No. . | Cells Injected . | Mice . | Time (wk) . | Sorted Cells . | Time in Culture (wk) . | 24 Wells4-150 . | Cultures at Limiting Dilutions . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | CD11b+ or CD15+ . | CD19+ . | Cells/Well . | No. of Wells . | Positive/Total Wells Analyzed4-151 . | . | . | ||
| . | . | . | . | . | . | . | . | . | . | CD19+ . | CD19+ CD15− . | CD19− CD15+ . | . | . |
| . | . | . | . | . | . | . | . | . | . | CD15+/CD11b+ . | . | . | . | . |
| 1 | MNC | SCID | 4 | 34+38low | 4 | 59% | 5% | 1 | 180 | 5/80 | 4/80 | 10/80 | ||
| 2 | MNC | SCID | 7 | 34+38low | 7 | 25% | 10% | ND | ND | ND | ND | ND | ||
| 3 | MNC | NOD-SCID | 4 | 34+38low | 4 | ND | ND | 1 | 240 | 3/13 | 2/13 | 8/13 | ||
| 10 | 60 | 8/18 | 4/18 | 5/18 | ||||||||||
| 4 | CD34+ | NOD-SCID | 7 | 34+38low | 4 | 39% | 5% | 1 | 60 | 0/12 | 9/12 | 3/12 | ||
| 10 | 60 | 13/18 | 3/18 | 2/18 | ||||||||||
| 50 | 60 | 10/10 | 0/10 | 0/10 | ||||||||||
| 5 | CD34+ | NOD-SCID | 5 | 34+ | 7 | 25% | 8% | ND | ND | ND | ND | ND | ||
Cord blood cells (20 × 106 MNC or 25 to 50 × 104 CD34+ cells) were injected intravenously to either SCID (experiments no. 1 and 2) or NOD-SCID (experiments 3 through 5) mice. After 4 to 7 weeks, mice were killed and marrow cells were analyzed for the presence of human cells (see Table 3). Human CD34+CD38low cells were sorted by FACS and used to initiate long-term cocultures on MS-5 cells as described in the Materials and Methods. Cells were incubated either in 24 wells (2,500 to 5,000 CD34+CD38low cells/well) or at limiting dilutions in 96-well plates. After 5 to 7 weeks in culture, the phenotype of cultured cells was analyzed by labeling with antibodies directed against human CD19 and CD15 (or CD11b).
Abbreviation: ND, not done.
Percentages represent the proportion of nucleated cells of the indicated phenotype.
Total wells analyzed represent the number of wells in which enough cells were detectable to allow reliable phenotypic analysis. Positive wells represent the number of these analyzed wells that contain cells of the indicated phenotype.
DISCUSSION
In this study we show that CD34+CD38lowCD19− primitive progenitors isolated from human cord blood and cultured on a murine stromal cell line in the absence of human cytokines generate high numbers of CD19+ late pro-B cells. Single-cell experiments showed for the first time that a high proportion of these cord blood-derived CD34+CD38lowCD19− progenitors had retained the potential to produce both B lymphocytes and granulocytes. The additional observation that CD34+CD38low progenitors harboring both potentialities were detected in the marrow of chimeric SCID and NOD-SCID mice 6 to 7 weeks after transplantation of CD34+CD19−CD10− cord blood cells further argues that these progenitors represent an early physiologic step of the normal human hematopoietic hierarchy. Interestingly, in in vivo experiments, all stages of the B-cell differentiation pathway were observed from CD34+CD19+ pro-B to CD19+CD20+ sIgM+ mature B cells, whereas in vitro, all cells exhibited the phenotype of a late CD34−CD19+cIgM− pro-B stage with no mature B cells.
Analysis of the critical molecules involved in the regulation of human B lymphopoiesis has been hampered by the difficulty of growing B-cell precursors in vitro.16 Most studies have used CD34+CD19+ or CD34+CD10+ pro-B cells and there is a general consensus that both stromal cells and cytokines, including interleukin-7 (IL-7) and Flt-3 ligand, are required for optimal proliferation of these cells in short-term assays.12,13,15 These studies have also been facilitated by the use of fetal bone marrow, which is highly enriched for lymphoid precursors.10-13,15,39 However, attempts to produce B cells from more primitive human CD19−CD10− progenitor cells with no marker of B-cell commitment have been disappointing until very recently.16 This was in contrast with the successful production of differentiated B cells in standard long-term marrow cultures switched in lymphoid conditions as initially described by Whitlock-Witte. The reasons for the failure to establish a human equivalent of the Whitlock-Witte system are still unclear.
Recently, two groups have succeeded in generating high numbers of B cells from either total CD34+ cord blood cells17,18 or more primitive subsets (CD34+Thy1+ or CD34+Thy1+CD19−CD10+ [and CD10−] cells) from fetal liver, fetal and adult bone marrow, and cord blood.18-20 In these studies, primitive progenitor cells were grown for several weeks in low serum concentration on xenogenic murine stromal cells previously reported to be permissive for murine B-lymphoid and myeloid hematopoiesis42 and human myeloid differentiation.21 It is possible that substituting these murine cells for the human marrow-derived stromal cells used in standard long-term cultures was a key parameter that altered the balance between stimulatory and inhibitory signals in favor of the former in this xenogenic situation.43
Following the same strategy, we show in this study that the murine stromal cells MS-5 were competent to support the commitment of postnatal human primitive stem cells lacking any B-specific transcripts towards B-cell progenitors. MS-5 cells, which are probably functionally equivalent to the Sys-1,21 S-17,17 and 30R12 murine stromal cell lines used by others, also permit the differentiation of these B-cell progenitors, as shown by the production of at least 10,000 CD19+ cells from 1,000 CD34+CD38lowCD19− input cells. However, MS-5 cells may not supply the critical regulatory molecules for the downstream transition between pro-B and cIgM+ cells. Indeed, CD19+ lymphocytes produced in vitro lacked surface expression of CD34 and IgM. Most also lacked intracytoplasmic μ chains, although heavy chain transcripts were detected by RT-PCR. This phenotype fits with that of late pro-B cells33 and has been shown very recently to characterize a rare B-cell subset found in normal human bone marrow that probably represents an intermediate step between pro-B and pre-B cells.34 Accumulation of late pro-B cells with this phenotype has been a common finding in all B-cell culture assays initiated with CD34+Thy1+CD10+ or CD34+ primitive progenitors.17,20 Whether this block indicates that molecules essential for the differentiation in human pre-B cells are not produced by murine stromal cells (or are produced but do not cross species barrier) will have to be further investigated. Of note, the addition of IL-7 to cord blood CD34+ cocultured with the S-17 murine stromal cell line was without effect on the production or maturation of CD19+ cells.17 On the other hand, arguments exist for a stimulatory role of IL-7 in synergy with fit-3 ligand and murine stromal cells on the amplification of CD34+CD19+ human pro-B cells and their expression of intracytoplasmic μ chains,12 although IL-7 was without effect in the absence of stromal cells.44
Interestingly, our in vivo data showed that B-cell differentiation proceeded harmoniously in immune-deficient mice grafted with human cord blood cells in contrast to the in vitro situation. Thus, surface IgM+ B cells were detected in the marrow of mice transplanted with cord blood-derived CD34+CD19−CD10−, arguing that the transition of pro-B cells to more mature stages occurs in vivo in the mouse environment and does not result from an intrinsic inability of these human cells to mature. Of note, CD34+CD19+ pro-B cells were also easily identified in vivo in chimeric mice transplanted with primitive human stem cells, whereas they were undetectable in vitro after 6 weeks. The sustained production of CD34+CD19+ pro-B cells most likely indicates that in vivo, but not in vitro, the B-cell compartment is continuously replenished by a more primitive subset of cells.
We previously demonstrated that MS-5 cells support the differentiation of primitive CD34+CD38low LTC-ICs from both cord blood cells and adult bone marrow cells.25,30 The observation provided here that MS-5 cells also provide a permissive environment for B-cell differentiation of the same primitive progenitor cells prompted us to investigate whether individual CD34+CD38lowCD19− cord blood cells would be able to generate both CD11b+ granulocytes and CD19+ B cells. Indeed, we found that 7% of CD34+CD38lowCD19− cells isolated from fresh cord blood samples produced CD11b+ and CD19+ cells, a property also shared by 1% to 2% of CD34+CD38low human cells detected in the marrow of immune-deficient mice transplanted 4 to 6 weeks earlier with human cord blood. Primitive cells with the ability to differentiate in the B-cell pathway as well as in multiple myeloid lineages have been identified in various tissues of fetal and adult mice by clonal assays using either in vivo gene marking1 or in vitro colony assays45 and long-term cultures.9,46 The human counterparts of these murine lympho-myeloid progenitors have been identified at the clonal level in fetal bone marrow. Thus, single CD34+CD38−HLADr+22 or 1% to 2% CD34+Thy1+Lin−21 fetal bone marrow cells grown in the presence of cytokines with or without a stromal support generated both CD19+ and CD15+ or CD33+ cells. The existence of similar progenitors in cord blood has been suggested from limiting dilution experiments18,20 and have also been detected in adult bone marrow among the rare cytokine-stimulated CD34+ cells that resisted treatment by 5-fluorouracil.23 Because in our study the sum of CD11b+ and CD19+ cells did not represent 100% of the human cells produced by individual CD34+CD38lowCD19− cord blood-derived cells, these progenitors may in fact have more extended potentialities. A more detailed dissection of the potentialities of this CD34+CD38lowCD19− fraction will be facilitated by supplementing the cultures with lineage-specific cytokines such as erythropoietin, IL-15,47 granulocyte colony-stimulating factor, and Mpl-ligand, which might lead to the amplification of mature cells of the different lineages. This might lead to the identification of discrete populations of progenitors combining various degrees of lymphoid and myeloid potentialities, as recently proposed for adult bone marrow cells.20 Preliminary experiments performed with CD34+CD38−CD19−CD10− cells purified from adult marrow and cultured on MS-5 in lymphoid or switch conditions indicate, however, that the frequency of progenitors able to generate both CD19+ and CD11b+ cells is much below that observed in cell suspensions of the same phenotype purified from cord blood (unpublished data).48 49
The availability of such in vivo and in vitro systems in which B cells and myeloid cells can be reproducibly obtained simultaneously from early progenitor cells suggest that it may be further possible to extend these results and design in vitro systems permissive for the development of all the various hematopoietic lineages, including NK47 or even T-cell pathways.50 This will offer an invaluable tool not only to follow, in vitro as in vivo, individual clones after their genetic marking,2 but also to explore molecular mechanisms associated with stem cell commitment51 and their potential regulation by external signals.
ACKNOWLEDGMENT
We are indebted to Prof John Dick from the Hospital for Sick Children in Toronto for providing CB-17 SCID and NOD-SCID mice. Patrice Ardouin and Annie Rouches (Animal Facilities, Institut Gustave Roussy) made an invaluable contribution in breeding and maintaining the CB17-SCID and NOD-SCID strains. We are also grateful to AMGEN and IMMUNEX for their generous gift of human recombinant cytokines, to K. Mori for providing the MS5 cell line, and to the staff of the cord blood preparation unit (Hospital St Vincent de Paul).
Supported by INSERM and grants from ARC (6532 to L.C.), Electricité de France, Agence Française du Sang, ANRS (Agence Nationale Recherche sur le SIDA), and Institut Gustave Roussy. A.C.B. was supported by a fellowship from INSERM.
Presented in part at the European Hematology Meeting (Paris, France, June 1996; Br J Haematol 93:44, 1996 [suppl 2]).
Address reprint requests to Laure Coulombel, MD, PhD, INSERM U 362, Institut Gustave Roussy, 39 av Camille Desmoulins, Villejuif, 94800 France.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal