Abstract
The effects of cytomegalovirus (CMV) infection on hematopoietic progenitor cells in vivo were investigated to elucidate the pathogenesis of CMV-induced myelosuppression. BALB/c mice were inoculated with 0.2LD50 of murine CMV (MCMV). Lineage marker negative, c-kit positive (Lin−c-kit+) and Lin−CD34+ cells, which are both phenotypically defined as hematopoietic progenitor cells, showed a significant reduction in number on day 3 postinfection (pi). Moreover, the reduction in the number of day-14 colony-forming units-spleen (CFU-S), another indicator to identify hematopoietic progenitor cells, was noted on day 3 pi. To clarify the mechanism of such depletion, we examined the cells undergoing apoptosis in the Lin− populations and found a 15-fold increase in the apoptosis-induction of these cells. Furthermore, an increase in the expression level of Fas, which mediates apoptosis, was observed in such Lin−c-kit+ and Lin−Sca-1+ cells on day 3 pi. In vitro treatment with the anti-Fas antibody accelerated the apoptosis in Lin− cells, but not in the uninfected control cells, thus indicating that the upregulated Fas on Lin− cells is directly related to the acceleration of apoptosis found in these cells in vivo. These results suggest that MCMV infection reduces the number of hematopoietic progenitor cells in bone marrow at least in part due to Fas-mediated apoptosis, and this phenomenon is thus considered to contribute to CMV-induced myelosuppression.
CYTOMEGALOVIRUS (CMV) produces mild and usually asymptomatic infections in individuals with normal immune responses. However, CMV infection is one of the most common infectious complications among immunocompromised patients, especially recipients of bone marrow and solid organ transplantation1,2 and those with acquired immunodeficiency syndrome (AIDS).3 This virus leads to a variety of diseases such as severe pneumonitis, colitis, and retinitis. It has also been reported that CMV infection induces hematologic abnormalities including mononucleosis, thrombocytopenia, and hemolytic anemia.4,5 Moreover, CMV infection is also associated with delayed marrow engraftment and graft failure following bone marrow transplantation.6-9 Although several mechanisms have been proposed so far, the pathogenic mechanism of inhibition of hematopoiesis following CMV infection has yet to be fully elucidated. There is some evidence that CMV directly inhibits the growth and differentiation of hematopoietic progenitor cells.10-12 On the other hand, other investigators showed that CMV indirectly exerts a suppressive effect on hematopoiesis through the impairment of the bone marrow stromal cells that support growth and differentiation of the hematopoietic progenitor cells.13 14
An infection of mice with murine CMV (MCMV) has been used as an animal model to analyze some aspects of the pathogenesis of its human counterpart, human CMV (HCMV), in regard to primary and latent infection, reactivation, and superinfection.15,16 Regarding hematopoiesis in MCMV-infected mice, it has been recently demonstrated that an acute infection of MCMV interferes with the early process of hematopoiesis by the reductions of colony-forming units-spleen (CFU-S) and CFU-granulocyte/macrophage (CFU-GM).17 Similarly, Mutter et al18 reported that MCMV infection prevented the autoreconstitution of bone marrow cells, thus resulting in its fatal effect after sublethal irradiation. These results clearly indicate that MCMV-induced myelosuppression is in part caused by the inhibition of generating hematopoietic progenitor cells. We previously observed that thymocytes in mice that had been inoculated with MCMV 4 weeks earlier were highly sensitive to apoptosis induction.19 It can thus be hypothesized that the myelosuppressive effects by MCMV could be due to the priming for the apoptosis of bone marrow cells including hematopoietic progenitor cells. Fortunately, recent advances in hematologic and immunologic fields revealed the surface markers for murine bone marrow progenitor cells and cells that are sensitive to apoptosis induction such as: c-kit, Sca-1, and CD34, and Fas antigen. It was also reported that lineage marker negative and c-kit positive (Lin−c-kit+) cells in bone marrow contain a high proportion of hematopoietic progenitor cells.20,21 Moreover, Lin−Sca-1+c-kit+ cells have been shown to be primitive hematopoietic stem cells.22 In addition, the murine homologue of human CD34 has also been recently cloned23 and characterized as a marker for hematopoietic progenitor cells.24 Murine Fas antigen has recently been cloned, and its role in apoptosis has been defined.25 Using these surface markers, the role of apoptosis in hematopoietic progenitor cells in the host infected with MCMV was thus examined in this study.
MATERIALS AND METHODS
Mice and virus inoculation.The female mice of an inbred BALB/c strain were obtained from Nippon Clea Inc (Tokyo, Japan) and maintained in specific pathogen-free conditions. Eight- to 10-week-old mice were used for all experiments. All animals were handled in accordance with the guidelines established by the Animal Experimentation Committee of Tokai University School of Medicine. The Smith strain of MCMV (VR-194) was originally obtained from the American Type Culture Collection (Rockville, MD). The stock of MCMV used for inoculation was harvested from salivary gland tissue as a homogenate after being passed twice in BALB/c mice, and was kept in a medium containing 0.1% dimethylsulfoxide (Sigma, St Louis, MO) at −80°C until use. For a quantitative analysis of infectious MCMV, a plaque assay was performed using a BALB-3T3 monolayer cell culture as described previously,26 and the concentration of the MCMV stock suspension was 8 × 107 PFU/mL. For infection, the mice were inoculated intraperitoneally with MCMV at a dose of 1 × 105 PFU (=0.2 LD50 ). The homogenates of the salivary glands from the uninfected mice or inactivated MCMV were used for inoculation into the uninfected control mice (mock infection). The inactivation of MCMV was performed either by incubating the viral solution at 70°C for 15 minutes or by UV treatment (254 nm wavelength, 5 cm distance for 40 minutes on ice).27
Bone marrow cell collection.Suspensions of bone marrow cells were obtained by flushing femurs and tibias with phosphate-buffered saline (PBS). The suspensions were passed through nylon mesh and then were treated with NH4Cl to lyse red blood cells. The cells were resuspended with PBS and the cell viability was assessed by trypan blue exclusion. All samples showed more than a 95% cell viability.
Peripheral blood cell counts.Blood samples were obtained by an intracardiac puncture. Samples were analyzed by Automated Hematology Analyzer (NE8000; Toa Medical Electronics Corp., Kobe, Japan), and leukocyte differential counts were assessed by microscopic examinations with Giemsa staining.
Staining for cell surface markers.Two × 106 cells were suspended with PBS containing 2% fetal calf serum (FCS) and 0.1% NaN3, and incubation for cell staining was carried out on ice for 30 minutes except when particularly mentioned. As for the detection of lineage markers, rat monoclonal antibodies recognizing Mac-1 (CD11b; M1/70, rat IgG2b), Gr-1 (RB6-8C5, rat IgG2b), B220 (CD45R; RA3-6B2, rat IgG2a), and an erythroid marker (TER119, rat IgG2b) and fluorescein isothiocyanate (FITC) conjugated monoclonal antibody for murine CD3 (145-2C11, hamster IgG) were used. Antibodies for Mac-1, Gr-1, and B220 were purchased from Pharmingen (San Diego, CA), and TER119 was kindly provided by Dr Tatsuo Kina (Kyoto University, Kyoto, Japan). FITC conjugated antirat IgG polyclonal antibody (Jackson Immuno Research Laboratories Inc, West Grove, PA) was used as a secondary antibody for the detection of lineage markers. To assess the hematopoietic progenitor cells, biotinylated anti–c-kit (2B8; Pharmingen), phycoerythrin (PE)-conjugated anti–Sca-1 (E13-161.7; Pharmingen), and biotinylated antimurine CD34 (RAM34; Pharmingen) were used. To assess the expressions of c-kit and Sca-1, cells were further stained with biotinylated anti–c-kit and PE-conjugated anti–Sca-1 antibodies followed by the incubation with streptavidin-RED670 (GIBCO BRL). After the Lin− population was gated, the expressions of c-kit and Sca-1 were observed by detecting RED670 and PE, respectively. To assess the CD34 expression on Lin− cells, the cells stained with antilineage markers were further incubated with biotinylated anti-CD34, washed, and then treated with streptavidin-PE (Becton Dickinson, Mountain View, CA). To assess the Fas antigen expression on Lin−Sca-1+ and Lin−c-kit+ cells, rat antimouse Fas (RMF6, rat IgG2a; Medical & Biological Laboratories Co, Nagoya, Japan), which has no apoptosis-inducing activity, was used. The cells stained with antilineage markers were further incubated with RMF-6. Next, the cells were incubated with biotinylated antirat IgG2a, (MARG2a-1; Zymed Laboratories Inc, San Francisco, CA), washed, and then treated with streptavidin RED670 and PE-conjugated anti–c-kit or Sca-1.
Staining for the detection of apoptosis.To assess the cells undergoing apoptosis, the relative DNA content was observed by the method of Ishida et al28 with some modifications. Briefly, the cells were stained with antilineage markers and fixed with 2% paraformaldehyde. After permeabilization with 0.1% NP-40 (Sigma), the cells were treated with 0.05 mg/mL RNaseA (Sigma) for 30 minutes at 37°C. After washing, the cells were suspended in 0.5 mL of propidium iodide (PI) solution (50 μg/mL of PI with 0.1% Na citrate) and incubated for 30 minutes at 4°C. The cells in a discrete subpopulation of signals under the G0/G1 cell cycle region (subdiploid cells) were designated as cells undergoing apoptosis.29 Apoptosis in Lin− bone marrow cells was further demonstrated by the terminal deoxynucleotidyl transferase (TdT)-mediated dUTP-biotin nick end labeling method (TUNEL).30 Briefly, bone marrow cells were fixed and permeabilized with 1% paraformaldehyde and 0.1% Triton X-100 (Sigma), respectively, following the staining with FITC-labeled antilineage markers. The TUNEL reaction was carried out with 0.3 nmol/L biotinylated-dUTP (Boehringer Mannheim, Tokyo, Japan), 3 nmol/L dATP, 25 mmol/L CoCl2 , 25 U TdT (Boehringer Mannheim), and TdT buffer (30 mmol/L Tris pH7.2, 140 mmol/L sodium cacodylate) in a total volume of 50 μL at 37°C for 1 hour. The reaction was stopped by adding 2 μL of 0.5 mmol/L EDTA. Then, the cells were treated with PE-conjugated streptavidin, and were analyzed by flow cytometry.
Flow cytometry analysis and cell sorting.Flow cytometry was performed using a FACScan (Becton Dickinson) equipped with three filters including a 530-nm band-pass (for FITC), a 585-nm band-pass (for PE and PI), and a 650-nm long-pass filter (for RED670). The data were analyzed on the LYSIS II program (Becton Dickinson). To analyze the cells stained with PI, it was confirmed before the experiments that the coefficient variance of PI-stained thymocytes from an uninfected BALB/c mouse was under 2% on a linear scale. To isolate Lin− cells, the cells stained with antilineage markers were analyzed and sorted by a FACStarplus (Becton Dickinson). Cells were maintained at 4°C throughout the sorting procedure.
CFU-S assay.To determine the value of CFU-S in bone marrow preparations, graded number of bone marrow cells obtained from the mice on day 1, 3, 5, and 7 pi were intravenously transferred into lethally irradiated (7.5 Gy) uninfected mice. On day 14 after the transfer, the mice were killed and the surface colonies of their spleens, each of them comprising the progeny of a single hematopoietic progenitor cell, were counted to determine the total number of CFU-S per donor bone marrow. To rule out the possibility of autologous colony formation after irradiation, the control mice were equally irradiated and also received the same volume of PBS.
Treatment of bone marrow cells in vitro with anti-Fas monoclonal antibody.Bone marrow cells were suspended in RPMI 1640 medium (GIBCO BRL) supplemented with 30% FCS in a 12 well plate (Iwaki Glass, Tokyo, Japan) at a density of 1 × 106 cells/mL (2 mL/well) and incubated for 18 hours with or without hamster antimouse Fas antigen monoclonal antibody (Jo2; Pharmingen) at the concentration of 1 μg/mL. Incubation was carried out under a humidified 5% CO2 atmosphere at 37°C. The ratio of the cells undergoing apoptosis was then determined by flow cytometry as described above.
Infection of bone marrow cells with MCMV in vitro.Whole bone marrow or isolated Lin− cells were incubated with MCMV at different multiplicity of infection (MOI) values, ranging from 1 to 10−2 PFU/cell in RPMI 1640 medium supplemented with 10% FCS for 2 hours at 37°C. The cells were then washed with PBS five times and resuspended at a density of 1 × 106 cells/mL in RPMI 1640 medium containing 10% FCS, 100 U/mL IL-3, IL-6 (Genzyme Corp, Cambridge, MA), and 50 ng/mL stem cell factor (Genzyme Corp). The cells were incubated in a humidified 5% CO2 atmosphere at 37°C. After incubation for 24 and 48 hours, the expression level of Fas on Lin−c-kit+ cells was examined by flow cytometry as described above.
Detection of MCMV DNA by polymerase chain reaction (PCR).To detect MCMV DNA, PCR analysis of MCMV specific immediate early (IE) gene was performed as described before.19 Briefly, sample DNA was prepared from whole or sorted bone marrow cells by a series of phenol-chloroform extractions and ethanol purification. A pair of 24-bp oligonucleotides, selected from exon 4 of the MCMV IE gene 1, was used as primers.19 Amplified DNA samples were electrophoresed on 1.2% agarose gel, and prestained with ethidium bromide for photography. The gels were blotted onto a nylon membrane. A 24-bp oligonucleotide, complementary to a sequence lying between the primers, was used as a probe for Southern blot analysis and labeled by using the ECL 3′-oligolabeling and Detection System (Amersham, Buckinghamshire, UK).
Statistical analysis.A comparison of the mean values of the parameters was performed using the two group paired t-test.
RESULTS
Bone marrow cellularity and peripheral blood cell counts after MCMV infection.Bone marrow cellularity was examined in mice infected with 1 × 105 PFU of MCMV (Table 1). The total number of bone marrow cells rapidly decreased after an MCMV infection. The lowest number was observed on day 5 pi, which was 55.5% of the control (P < .01; Table 1). Thereafter, the bone marrow cellularity began to recover and reached a level higher than the control level on day 14 pi, which was 141.7% of the control (P < .01). In contrast, inoculation with heat- or UV-inactivated MCMV did not exert any significant effect on the bone marrow cellularity, 3.51 × 107 and 3.70 × 107 on day 3 pi, respectively. These results thus indicated that bone marrow cellularity decreased in the acute phase of MCMV infection and this effect was induced by infectious virus. To investigate the effects of reduced bone marrow cellularity on the peripheral blood cell counts, hematocrits, platelet counts, total leukocytes, granulocytes, lymphocytes, and monocytes were examined (Table 2). As expected by the reduction of bone marrow cellularity, hematocrits and platelet counts also decreased after infection, a 15% reduction on day 7 pi (from 54% to 46%) and an 88% reduction on day 5 pi (from 72 × 104 to 9 × 104), respectively (Table 2). These reduced hematocrits and platelet counts almost completely returned to the control levels by day 14 pi. In contrast, the total number of leukocytes increased after MCMV infection and the highest number (3-fold increase) was observed on day 7 pi (Table 2). Such increases were also observed in the granulocyte, lymphocyte, and monocyte counts as well. These results were also closely consistent with a previous report,31 regardless of the injection dose of MCMV (0.2 v 0.5 LD50 ). Although the hematocrit and platelet counts changed in parallel with the reduction of bone marrow cellularity after MCMV infection, leukocyte counts were inversely elevated. However, the elevation of the leukocyte counts in the acute phase of MCMV infection could be explained by the mobilization of the leukocytes from the marginal pools or bone marrow to the peripheral blood as has been reported elsewhere.31
Effects of MCMV Infection on the Number of Hematopoietic Progenitor Cells in Bone Marrow
| Days After . | No. of Bone . | Lin−c-kit+ Cells . | Lin−CD34+ Cells . | ||
|---|---|---|---|---|---|
| Infection . | Marrow Cells . | Percent No. . | Total No. . | Percent No. . | Total No. . |
| . | (×107) . | (%) . | (×105) . | (%) . | (×105) . |
| 0 | 3.62 ± 0.20 | 1.58 ± 0.18 | 5.62 ± 0.74 | 1.77 ± 0.05 | 6.41 ± 0.35 |
| 1 | 3.23 ± 0.31 | 1.53 ± 0.06 | 4.95 ± 0.57 | ND | ND |
| 3 | 2.05 ± 0.27* | 1.24 ± 0.06 | 2.55 ± 0.28* | 1.59 ± 0.05 | 3.26 ± 0.42* |
| 5 | 2.01 ± 0.08* | 1.36 ± 0.11 | 2.76 ± 0.13* | ND | ND |
| 7 | 3.67 ± 0.28 | 1.51 ± 0.03 | 5.55 ± 0.30 | ND | ND |
| 14 | 5.13 ± 0.43 | 1.58 ± 0.10 | 8.02 ± 0.66† | ND | ND |
| Days After . | No. of Bone . | Lin−c-kit+ Cells . | Lin−CD34+ Cells . | ||
|---|---|---|---|---|---|
| Infection . | Marrow Cells . | Percent No. . | Total No. . | Percent No. . | Total No. . |
| . | (×107) . | (%) . | (×105) . | (%) . | (×105) . |
| 0 | 3.62 ± 0.20 | 1.58 ± 0.18 | 5.62 ± 0.74 | 1.77 ± 0.05 | 6.41 ± 0.35 |
| 1 | 3.23 ± 0.31 | 1.53 ± 0.06 | 4.95 ± 0.57 | ND | ND |
| 3 | 2.05 ± 0.27* | 1.24 ± 0.06 | 2.55 ± 0.28* | 1.59 ± 0.05 | 3.26 ± 0.42* |
| 5 | 2.01 ± 0.08* | 1.36 ± 0.11 | 2.76 ± 0.13* | ND | ND |
| 7 | 3.67 ± 0.28 | 1.51 ± 0.03 | 5.55 ± 0.30 | ND | ND |
| 14 | 5.13 ± 0.43 | 1.58 ± 0.10 | 8.02 ± 0.66† | ND | ND |
The mice were inoculated with 1 × 105 PFU of MCMV. The population of the Lin−c-kit+ and Lin−CD34+ cells in the bone marrow cells were examined by flow cytometry. The results from three to four mice were expressed as the mean ± SEM.
Abbreviation: ND, not done.
Significant decrease versus uninfected control mice (P < .01).
Significant increase versus uninfected control mice (P < .01).
Effects of MCMV Infection on the Peripheral Blood Cell Counts
| Days After . | Hematocrit . | Platelet . | Total Leukocyte . | Granulocyte . | Lymphocyte . | Monocyte . |
|---|---|---|---|---|---|---|
| Infection . | (%) . | (×104/μL) . | (/μL) . | (/μL) . | (/μL) . | (/μL) . |
| 0 | 54 ± 2 | 72 ± 6 | 3,600 ± 415 | 116 ± 30 | 2,624 ± 59 | 43 ± 9 |
| 1 | 50 ± 2 | 63 ± 3 | 6,100 ± 207 | 1,408 ± 164* | 3,704 ± 364 | 572 ± 39* |
| 3 | 50 ± 1 | 27 ± 5† | 5,240 ± 470 | 1,480 ± 316* | 2,597 ± 311 | 927 ± 32* |
| 5 | 49 ± 3 | 9 ± 1† | 9,625 ± 964* | 1,748 ± 262* | 5,765 ± 735* | 1,494 ± 128* |
| 7 | 46 ± 1† | 45 ± 4† | 10,800 ± 868* | 2,692 ± 660* | 6,004 ± 771* | 1,254 ± 109* |
| 14 | 52 ± 1 | 71 ± 3 | 4,900 ± 409 | 379 ± 101 | 3,993 ± 320 | 95 ± 15 |
| Days After . | Hematocrit . | Platelet . | Total Leukocyte . | Granulocyte . | Lymphocyte . | Monocyte . |
|---|---|---|---|---|---|---|
| Infection . | (%) . | (×104/μL) . | (/μL) . | (/μL) . | (/μL) . | (/μL) . |
| 0 | 54 ± 2 | 72 ± 6 | 3,600 ± 415 | 116 ± 30 | 2,624 ± 59 | 43 ± 9 |
| 1 | 50 ± 2 | 63 ± 3 | 6,100 ± 207 | 1,408 ± 164* | 3,704 ± 364 | 572 ± 39* |
| 3 | 50 ± 1 | 27 ± 5† | 5,240 ± 470 | 1,480 ± 316* | 2,597 ± 311 | 927 ± 32* |
| 5 | 49 ± 3 | 9 ± 1† | 9,625 ± 964* | 1,748 ± 262* | 5,765 ± 735* | 1,494 ± 128* |
| 7 | 46 ± 1† | 45 ± 4† | 10,800 ± 868* | 2,692 ± 660* | 6,004 ± 771* | 1,254 ± 109* |
| 14 | 52 ± 1 | 71 ± 3 | 4,900 ± 409 | 379 ± 101 | 3,993 ± 320 | 95 ± 15 |
The mice were inoculated with 1 × 105 PFU of MCMV. The peripheral blood cell counts were examined on days 1, 3, 5, 7, and 14 after infection. The results from four to five mice were expressed as the mean ± SEM.
Significant increase versus uninfected control mice (P < .01).
Significant decrease versus uninfected control mice (P < .01).
The effects of MCMV infection on the population of hematopoietic progenitor cells.To investigate the mechanism of MCMV-induced myelosuppression, the percentage of c-kit+ cells in the Lin− cells, which represent hematopoietic progenitor cells,20,21 was examined by flow cytometry (Fig 1), and then the percentage and total number of Lin−c-kit+ cells in the bone marrow cells were calculated according to these data (Table 1). The percentage of c-kit+ cells in the Lin− cells, the number of which is shown in the panel in Fig 1, decreased on day 3 pi (75.6% to 60.4%, P < .05) and thereafter almost completely recovered to the uninfected control level on day 7 pi. Likewise, the total number of Lin−c-kit+ cells in the bone marrow showed a 55% reduction on day 3 pi (5.62 × 105 to 2.55 × 105, P < .01; Table 1), while the number thereafter increased and reached a level significantly higher than the control on day 14 pi (P < .01; Table 1). In addition, more than 80% of the Lin− cells in the bone marrow expressed CD34 after infection with MCMV as well as the uninfected control (data not shown). The absolute number of Lin−CD34+ cells in the bone marrow calculated according to these data exhibited an almost 50% reduction on day 3 pi compared with the control (6.41 × 105 to 3.26 × 105, P < .01; Table 1). To determine whether the reduction of hematopoietic progenitor cells is due to the mobilization of these cells to the peripheral organs, we examined the population of Lin−c-kit+ cells in the spleen. The inoculation of MCMV did not induce any significant increase in the percentage of c-kit+ cells in the Lin− cells of the spleen, 6.10% in the uninfected control and 5.10% on day 3 pi. These results thus demonstrated that the number of phenotypically defined hematopoietic progenitor cells such as Lin−c-kit+ and Lin−CD34+ cells in the bone marrow decreases to less than one half the original number after infection with MCMV, not due to the mobilization of these cells to the spleen. It is of interest that the percentage of Sca-1+c-kit+ cells in the Lin− cells, however, increased markedly on day 1 pi (Fig 2), while the percentage of c-kit single positive cells in the Lin− cells remained unchanged (Fig 1). Although it has been shown that Lin−Sca-1+c-kit+ cells represent the primitive hematopoietic stem cells,22 it still needs further clarification to determine whether such Lin−Sca-1+c-kit+ cells in the present study are really primitive hematopoietic stem cells or just Lin−c-kit+ cells that aberrantly induce the expression of Sca-1 antigen by an infection with MCMV.
The expression level of c-kit on Lin− cells after an MCMV infection. The mice were inoculated with 1 × 105 PFU of MCMV on day 0. On various days after infection, as shown in the figure, the mice were killed in order to remove their bone marrow cells. Using flow cytometry, the expression level of c-kit on these cells was examined after the Lin− populations were gated. The numbers in the figure represent the mean percentage of c-kit+ cells in the Lin− cell populations. *Significant decrease versus uninfected control (P < .05).
The expression level of c-kit on Lin− cells after an MCMV infection. The mice were inoculated with 1 × 105 PFU of MCMV on day 0. On various days after infection, as shown in the figure, the mice were killed in order to remove their bone marrow cells. Using flow cytometry, the expression level of c-kit on these cells was examined after the Lin− populations were gated. The numbers in the figure represent the mean percentage of c-kit+ cells in the Lin− cell populations. *Significant decrease versus uninfected control (P < .05).
The expression level of Sca-1 and c-kit on Lin− cells after MCMV infection. The mice were inoculated with 1 × 105 PFU of MCMV. On various days after infection, as shown in the figure, the mice were killed in order to remove their bone marrow cells. Using flow cytometry, the expression level of Sca-1 and c-kit on these cells was examined after the Lin− populations were gated. The numbers in the figure represent the percentage of Sca-1+c-kit+ cells in the Lin− cell populations.
The expression level of Sca-1 and c-kit on Lin− cells after MCMV infection. The mice were inoculated with 1 × 105 PFU of MCMV. On various days after infection, as shown in the figure, the mice were killed in order to remove their bone marrow cells. Using flow cytometry, the expression level of Sca-1 and c-kit on these cells was examined after the Lin− populations were gated. The numbers in the figure represent the percentage of Sca-1+c-kit+ cells in the Lin− cell populations.
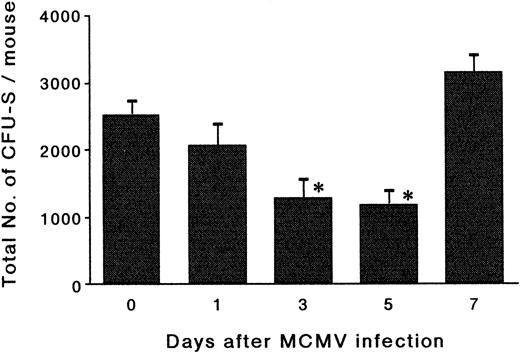
The effects of MCMV infection on CFU-S.To determine whether the reduction in the number of phenotypically defined hematopoietic progenitor cells after MCMV infection actually reflects the reduction of the hematopoietic progenitor cells, a CFU-S assay was performed (Fig 3). The total number of day-14 CFU-S in the bone marrow showed reductions of 49% and 51% on day 3 and day 5 pi, respectively, compared with the number on day 0 (P < .01). The number then increased thereafter and almost completely recovered to the control level on day 7 pi. It was thus confirmed that the decrease in the number of phenotypically defined hematopoietic progenitor cells is accompanied with a reduction in the total number of CFU-S in the bone marrow.
The effects of MCMV infection on CFU-S. The mice were inoculated with 1 × 105 PFU of MCMV. Bone marrow cells were obtained on day 1, 3, 5, and 7 pi and then the graded numbers of bone marrow cells were transferred intravenously into lethally irradiated mice. On day 14 after transfer, the recipient mice were killed in order to remove their spleens. Surface colonies of the spleens were counted to determine the total number of day-14 CFU-S in the donor bone marrow. The results of four mice for each group are expressed as the mean ± SEM. *Significant decrease versus uninfected control (P < .01).
The effects of MCMV infection on CFU-S. The mice were inoculated with 1 × 105 PFU of MCMV. Bone marrow cells were obtained on day 1, 3, 5, and 7 pi and then the graded numbers of bone marrow cells were transferred intravenously into lethally irradiated mice. On day 14 after transfer, the recipient mice were killed in order to remove their spleens. Surface colonies of the spleens were counted to determine the total number of day-14 CFU-S in the donor bone marrow. The results of four mice for each group are expressed as the mean ± SEM. *Significant decrease versus uninfected control (P < .01).
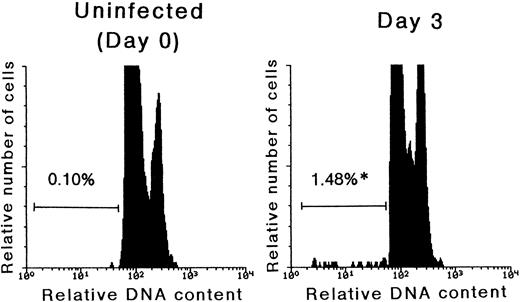
Detection of apoptosis in Lin− cells after MCMV infection.As mentioned above, we have previously reported that immature thymocytes of MCMV infected mice were highly sensitive to apoptosis induction.19 It was therefore examined as to whether or not the decrease in the number of hematopoietic progenitor cells after MCMV infection was due to the acceleration of apoptosis. The ratio of cells with subdiploid DNA, representing apoptotic cells, in the Lin− populations was examined by flow cytometry (Fig 4). On day 3 pi, the percentage of cells undergoing apoptosis in the Lin− population remarkably increased compared with the uninfected control (0.10% ± 0.02% to 1.48% ± 0.14%, P < .01). Similar results were also obtained by a TUNEL assay, and it was thus found that 0.78 ± 0.20% of Lin− bone marrow cells were undergoing apoptosis on day 3 pi, while the same rate was only 0.19% ± 0.04% in uninfected control mice (n = 5, P < .01). These results thus suggested that apoptosis plays a crucial role in reducing the number of hematopoietic progenitor cells in the acute phase of MCMV infection.
The detection of cells undergoing apoptosis in the Lin− population. Mice were inoculated with 1 × 105 PFU of MCMV. On day 3 pi, the mice were killed in order to remove their bone marrow cells. Using flow cytometry, the signals of PI, which represent the DNA contents, were examined after the Lin− populations were gated. The G0/G1-peak was adjusted at channel 100 and subdiploid cells were designated as cells undergoing apoptosis. Before the experiments, it was confirmed that a coefficient variance in the PI-stained thymocytes was ca. 2%. The numbers in the figure represent the percentage of cells undergoing apoptosis in the Lin− cells. *Significant increase versus uninfected control (P < .01).
The detection of cells undergoing apoptosis in the Lin− population. Mice were inoculated with 1 × 105 PFU of MCMV. On day 3 pi, the mice were killed in order to remove their bone marrow cells. Using flow cytometry, the signals of PI, which represent the DNA contents, were examined after the Lin− populations were gated. The G0/G1-peak was adjusted at channel 100 and subdiploid cells were designated as cells undergoing apoptosis. Before the experiments, it was confirmed that a coefficient variance in the PI-stained thymocytes was ca. 2%. The numbers in the figure represent the percentage of cells undergoing apoptosis in the Lin− cells. *Significant increase versus uninfected control (P < .01).
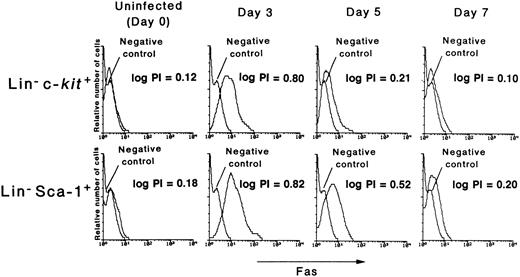
Fas expression on hematopoietic progenitor cells after MCMV infection.Recently, Fas antigen (CD95, APO-1) has been recognized to mediate apoptosis.25 32 Since it was found that the cells undergoing apoptosis in the Lin− population increase after MCMV infection in the present study, the role of Fas antigen in this apoptosis induction was examined. The expression level of Fas on Lin−c-kit+ cells increased after infection with MCMV, reaching the highest level on day 3 pi (logarithm peak intensity channel [log PI]: 0.12 on day 0 to 0.80 on day 3 pi), and decreasing thereafter (Fig 5, upper panels). It almost returned to control levels on day 7 pi (log PI: 0.10). The same dynamics of the expression level of Fas were obtained when Lin−Sca-1+ cells were examined (Fig 5, lower panels). Of note is that the decrease in the number of Lin−c-kit+ and Lin−CD34+ cells (Table 1) is closely correlated with the expression level of the surface Fas on these populations. These data thus suggested that the apoptosis and subsequent reduction of the hematopoietic progenitor cells in the MCMV-infected mice is mediated by Fas antigen.
The expression level of Fas antigen on hematopoietic progenitor cells. The mice were inoculated with 1 × 105 PFU of MCMV. On various days after infection, as shown in the figure, the mice were killed in order to remove their bone marrow cells. Using flow cytometry, the expression level of Fas on these cells was examined using either the Lin−c-kit+ (upper panels) or Lin− Sca-1+ (lower panels) cells. The number of logarithm peak intensity channels (log PI) was described in the figure.
The expression level of Fas antigen on hematopoietic progenitor cells. The mice were inoculated with 1 × 105 PFU of MCMV. On various days after infection, as shown in the figure, the mice were killed in order to remove their bone marrow cells. Using flow cytometry, the expression level of Fas on these cells was examined using either the Lin−c-kit+ (upper panels) or Lin− Sca-1+ (lower panels) cells. The number of logarithm peak intensity channels (log PI) was described in the figure.
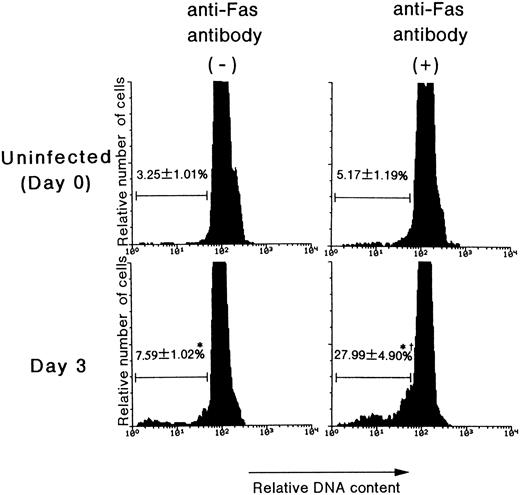
The effects of anti-Fas monoclonal antibody on apoptosis in the Lin− populations.To examine whether the Fas antigen expressed on hematopoietic progenitor cells can actually mediate apoptosis, an apoptosis-inducing anti-Fas monoclonal antibody, Jo2, was applied to the culture of bone marrow cells. The antibody was used at a concentration of 1 μg/mL, which has been reported to exert a sufficient apoptosis-inducing effect.33 As shown in Fig 6, anti-Fas antibody induced marked apoptosis in the Lin− population of bone marrow cells on day 3 pi compared with antibody nontreated cells (27.99% v 7.59%, P < .01; Fig 6), whereas this antibody did not induce any significant apoptosis in the uninfected control (5.17% v 3.25%, P = .29; Fig 6). These results thus suggested that MCMV infection indeed induces Fas-mediated apoptosis in the hematopoietic progenitor cells by the expression of functional Fas antigen on these progenitor cells.
The effects of anti-Fas antibody on the apoptosis induced in Lin− cells. The mice were inoculated with 1 × 105 PFU of MCMV. On day 3 pi, the mice were killed in order to remove their bone marrow cells. The bone marrow cells were treated with Jo2 antibody for 18 hours. Using flow cytometry, the signals of PI, which represent the DNA contents, were examined after the Lin− populations were gated. Subdiploid cells were designated as cells undergoing apoptosis. The number in the figure represents the percentage of cells undergoing apoptosis in the Lin− cells (mean % ± SEM, n = 4). *Significant increase versus uninfected control (P < .01). †Significant increase versus cells incubated without anti-Fas antibody (P < .01).
The effects of anti-Fas antibody on the apoptosis induced in Lin− cells. The mice were inoculated with 1 × 105 PFU of MCMV. On day 3 pi, the mice were killed in order to remove their bone marrow cells. The bone marrow cells were treated with Jo2 antibody for 18 hours. Using flow cytometry, the signals of PI, which represent the DNA contents, were examined after the Lin− populations were gated. Subdiploid cells were designated as cells undergoing apoptosis. The number in the figure represents the percentage of cells undergoing apoptosis in the Lin− cells (mean % ± SEM, n = 4). *Significant increase versus uninfected control (P < .01). †Significant increase versus cells incubated without anti-Fas antibody (P < .01).
The effect of MCMV infection in vitro on the expression of Fas on hematopoietic progenitor cells.To examine whether or not Fas is induced by a direct infection of MCMV, we infected whole bone marrow or sorted Lin− cells with MCMV in vitro. After the infection with MCMV in vitro, these cells were incubated for 48 hours as described above. In both cell fractions infected at different MOI values ranging from 10−2 to 1, MCMV specific IE gene was detected by PCR (data not shown). However, no differences in the expression level of Fas on Lin−c-kit+ cells between MCMV-infected and noninfected cells after incubation for 24 and 48 hours was observed (Fig 7). These results thus indicated that a direct infection of hematopoietic progenitor cells in vitro with MCMV could not induce the expression of Fas on these cells.
The effects of MCMV infection in vitro on the expression of Fas on hematopoietic progenitor cells. After being treated with MCMV for 2 hours at different MOI (multiplicity of infection) values, ranging from 10−2 to 1, bone marrow cells were washed, suspended in RPMI 1640 medium containing 10% FCS, 100 U/mL IL-3, IL-6, and 50 ng/mL stem cell factor and then incubated at 37°C. Forty-eight hours after incubation, the expression level of c-kit and Fas on these cells was examined after the Lin− populations were gated. The numbers in the figure represent the percentage of c-kit+Fas+ cells in the Lin− cell populations.
The effects of MCMV infection in vitro on the expression of Fas on hematopoietic progenitor cells. After being treated with MCMV for 2 hours at different MOI (multiplicity of infection) values, ranging from 10−2 to 1, bone marrow cells were washed, suspended in RPMI 1640 medium containing 10% FCS, 100 U/mL IL-3, IL-6, and 50 ng/mL stem cell factor and then incubated at 37°C. Forty-eight hours after incubation, the expression level of c-kit and Fas on these cells was examined after the Lin− populations were gated. The numbers in the figure represent the percentage of c-kit+Fas+ cells in the Lin− cell populations.
DISCUSSION
In the present study, we have shown that Lin−c-kit+ and Lin−CD34+ cells in the bone marrow, which represent hematopoietic progenitor cells, decrease in number after infection with MCMV. This decrease is closely compatible with the results of a CFU-S assay, in which the reduction in the day-14 CFU-S number closely paralleled the decrease in the number of phenotypically defined hematopoietic progenitor cells. One possible mechanism for this reduction of the hematopoieitc progenitor cells in bone marrow is the mobilization of these cells to other compartments such as the spleen. However, we observed no increase in the number of Lin−c-kit+ cells in spleen after an MCMV infection. Instead, another possibility was proposed in the present study. Decreased number of hematopoietic progenitor cells was also correlated with the expression level of Fas on these cells, and accelerated apoptosis was actually induced in these cells by treatment with anti-Fas antibody. We therefore suggest that the decrease in the number of hematopoietic progenitor cells after infection with MCMV is at least in part due to the acceleration of Fas-mediated apoptosis.
It has been reported that CMV exerts myelosuppressive effects when infected both in vivo and in vitro.6-14,31,34 In previous studies, it was demonstrated that the pathogenesis of the myelosuppressive effects is due to a direct inhibition of the function of hematopoietic progenitor cells10-12 or an impairment of bone marrow stromal cells to support hematopoiesis.13,14 With regard to the inhibitory effect of MCMV infection on hematopoietic progenitor cells, it has recently been demonstrated that CFU-S and CFU-GM decrease after an infection with MCMV.17 A more marked myelosuppression by MCMV was demonstrated in immunocompromised hosts by Mutter et al,18 such that an MCMV infection completely inhibited the recovery of CFU-S after sublethal irradiation. These results thus indicate that an infection with MCMV results in the inhibition of the early process of hematopoiesis, namely a decrease in the number of hematopoietic progenitor cells, which are also compatible with our results. However, in previous studies, the mechanism of the reduction of hematopoietic progenitor cells remains to be elucidated. On the other hand, we demonstrated in the present study that such a reduction is in part due to the induction of Fas-mediated apoptosis.
It is of interest that the number of Lin−Sca-1+c-kit+ cells, which were shown to be primitive hematopoietic stem cells,22 increased in the bone marrow after an MCMV infection in the present study. Since it was demonstrated that various cytokines such as TNFα and IFNγ can augment the expression level of Sca-1,35,36 and that a viral infection including CMV infection induces the release of such cytokines,37,38 it is possible, based on the present findings, that these cytokines induced the expression of Sca-1 on bone marrow cells after an infection with MCMV. Moreover, an increase in the number of Lin−Sca-1+c-kit+ cells was obviously noted very early on day 1 pi (6-fold increase; Fig 2), although the cell division time of murine hematopoietic stem cells was reported to be 18 to 24 hours.39 Taken together, these results suggest that the increase in the number of Lin−Sca-1+c-kit+ cells is not due to the substantial expansion of the primitive hematopoietic stem cell population but merely the augmentation of the expression level of Sca-1 on Lin−c-kit+ cells.
It still needs to be determined as to whether the induction of Fas antigen on hematopoietic progenitor cells of mice infected with MCMV is due to a direct infection of MCMV to these cells, or an indirect effect through host responses to an MCMV infection. Our results presented herein thus support the latter possibility for the below mentioned reasons. First, to examine the possible existence of MCMV in bone marrow cells, a PCR to detect the specific DNA sequence of IE gene of MCMV was performed using DNA extracted from bone marrow cells.16 As a result, it was thus demonstrated that MCMV genome was not detected on day 1 pi, but instead was detected beyond day 3 pi, and exhibited the highest level on day 5 pi (data not shown), when the expression level of Fas had already decreased. Moreover, according to the analysis using Lin− and Lin+ bone marrow cells sorted by flow cytometry, the viral genome was not detected in the Lin− bone marrow cells, but was detected in the Lin+ bone marrow cells on day 5 pi (data not shown). These results thus indicate that the viral genome does not exist in the Lin− bone marrow cells, which contain hematopoietic progenitor cells. Second, an infection of whole or Lin− bone marrow cells with MCMV in vitro does not result in the induction of Fas expression. Taken together, it appears unlikely that the induction of Fas expression on hematopoietic progenitor cells after MCMV infection is primarily due to the direct infection of the virus to these cells. Furthermore, in a study using human hematopoietic progenitor cells, it was reported that the Fas expression and the apoptosis of hematopoietic progenitor cells are induced by TNFα and IFNγ.40,41 The release of such cytokines has already been demonstrated in HCMV infection.37 38 It thus appears likely that the induction of Fas expression on hematopoietic progenitor cells after MCMV infection is associated with the cytokines released as a consequence of host responses to MCMV infection.
Several clinical studies have reported that the infection with HCMV occurred in 20% to 80% of the recipients of allogeneic bone marrow transplantation.1,42-44 The platelet and neutrophil recoveries were slower in such recipients when they were infected with HCMV.8,9 It was also reported that thrombocytopenia, neutropenia, and graft failure were observed in the recipients associated with HCMV infection in spite of satisfactory engraftment.6-8 While these reports underscored the significant role of HCMV infection in the occurrence of hematologic disorders in recipients of bone marrow transplantation, the pathogenesis of the myelosuppression exerted by HCMV infection has yet to be fully elucidated. The results in the present study thus raise the possibility that Fas-mediated apoptosis of hematopoietic progenitor cells may play a crucial role in such CMV-associated inhibition of hematopoiesis in addition to other mechanisms. Nonetheless, further studies are still needed to apply these results observed in immunocompetent mice to the HCMV-associated hematologic complications following bone marrow transplantation.
ACKNOWLEDGMENT
We thank Dr Tatsuo Kina (Kyoto University) for kindly providing us the TER119 antibody and Hideo Tsukamoto (Tokai University) for his skillful technical assistance in coupling the antibodies to FITC or PE.
Address reprint requests to Takehiko Mori, MD, Division of Hematology, Department of Internal Medicine, Keio University School of Medicine, 35 Shinanomachi, Shinjuku-ku, Tokyo 160, Japan.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal