Abstract
To study prognostic factors in infant acute myeloid leukemia (AML), we analyzed 44 children treated on Childrens Cancer Group protocols for MLL gene rearrangement by Southern blot, cytogenetic 11q23 abnormalities, and reactivity with monoclonal antibody 7.1. This antibody detects the human homologue of the rat NG2 chondroitin sulfate proteoglycan molecule, which has previously been reported to be expressed on human melanoma. NG2 has been found to be expressed on human leukemic blasts but not on other hematopoietic cells. In childhood AML, NG2 cell surface expression correlated with poor outcome and with some but not all 11q23 rearrangements. In childhood acute lymphoblastic leukemia, NG2 expression correlated with poor outcome and with balanced 11q23 translocations. In this study, 29 of 44 (66%) of infants with AML showed MLL rearrangement and, as expected, this group had a high incidence of French-American-British M4/M5 morphology (22/29). Of the cases tested, 35.1% (13/37) were NG2 positive. All (13/13) NG2-positive cases were rearranged at MLL, whereas only 46% (11/24) of NG2-negative cases had MLL rearrangement. NG2 expression did not correlate with poor outcome (P = .31); there was a trend towards a worse outcome with MLL rearrangement (P = .13). Thus monoclonal antibody 7.1 does not detect all cases of MLL rearrangement in infant AML.
COMPARED WITH childhood acute lymphoblastic leukemia (ALL), few clinical or laboratory prognostic indicators exist for childhood acute myeloid leukemia (AML). In ALL, cell surface markers (ie, CD10), chromosomal abnormalities (ie, 4; 11 or 9; 22 translocations), and specific gene rearrangements (ie, MLL) have strong prognostic significance.1 Specifically, in infant ALL, MLL gene rearrangement at chromosome band 11q23 confers a poor prognosis and correlates with CD10− immunophenotype, age of less than 6 months, and the chromosomal translocation t(4; 11).2,3 When the MLL gene is rearranged, it can be involved in balanced translocations with partners other than 4q21 (1p32 and 19p13 in ALL) or in isolated inversions or deletions of 11q23.4 When the translocation partner is 4q21, the prognosis is worse.5
MLL gene rearrangement is also seen in AML. In infant AML, it is correlated with hyperleukocytosis and French-American-British (FAB) M4 or M5 subtypes.6 MLL rearrangement is seen in 23%7 to 58%6 of infants with AML; translocation partners include 1q21, 2p21, 6q27, 9p22, 10p12, 17q25, and 19p13.8 The prognostic implications of MLL rearrangement and of specific 11q23 translocation partners have not been clear in infant AML.
Blast cell immunophenotype has shown no prognostic significance in AML.9 In an effort to identify cell surface markers with prognostic significance in AML, Smith et al10 generated a monoclonal antibody (MoAb) known as 7.1 that recognized the human homologue of the rat NG2 chondroitin sulfate proteoglycan molecule. Originally reported to be expressed on melanoma cells,11 it was absent from normal hematopoietic cells and from cell lines of hematopoietic origin. This molecule has been found on the surface of leukemic blasts in childhood AML10 and ALL.12 An unexpected finding was that NG2 expression correlates with MLL gene rearrangement and poor prognosis in these leukemias. In ALL, NG2 expression correlated with t(4; 11) and t(11; 19).12 In childhood AML, NG2 expression correlated with FAB M5 morphology, a subgroup of children with poor treatment outcome, and some 11q23 abnormalities. These 11q23 abnormalities were balanced translocations involving 9p22, 19p13, and 17q11, although this association was less clear; some leukemias with unbalanced 11q23 cytogenetic abnormalities also showed NG2 positivity.10
We report here our efforts to determine the prognostic significance of 11q23/MLL gene rearrangement and NG2 expression in infant AML. We also analyzed the cytogenetic data on these infants to determine whether certain 11q23/MLL rearrangements correlate with NG2 expression.
MATERIALS AND METHODS
Patients and specimens.Bone marrow or peripheral blood samples were obtained from 44 newly diagnosed infants (≤12 months) who were treated according to Childrens Cancer Group (CCG) protocols 2861 and 2891 (2861 was the pilot for 2891), the two most recently completed AML protocols. These protocols have been described elsewhere.13 Briefly, treatment consisted of 5-drug induction with daunomycin, Ara-C, 6-thioguanine, etoposide, dexamethasone, and intrathecal Ara-C. Four courses were administered, with patients receiving standard AML timing or intensive timed-sequential therapy with 6 days rest between courses 1 and 2 and between 3 and 4. Intensification consisted of bone marrow transplantation or intensive chemotherapy (Ara-C, L-asp, 6-TG, vincristine, 5-azacytidine, cytoxan, etoposide, daunomycin, dexamethasone, and intrathecal Ara-C). The diagnoses of AML were made at participating institutions and confirmed (including FAB subtype) by central CCG review. Cases were selected only on the availability of cryopreserved blasts. All 44 cases were studied for MLL gene rearrangement; of these, 26 were the subject of a report on MLL rearrangement in infant AML.6 Thirty-seven patients had specimens available for NG2 analysis (see below). Twenty-one patients had karyotypes available for review (see below).
Detection of MLL gene rearrangement by Southern analysis.Genomic DNA was extracted, digested with restriction endonucleases, and analyzed by Southern blotting as described.3 Briefly, MLL rearrangements were detected after digestions were performed with EcoRI, HindIII, and BamHI and hybridization to the P/S4 and 98.40 probes (single copy genomic probes located telomeric and centromeric, respectively, to the der ll breakpoint of the RS4; 11 cell line) and the 4.2E probe (a subclone of the EcoRI fragment of MLL located just telomeric to the region recognized by P/S4). Repetetive sequences recognized by the 4.2E probe were blocked with 400 μg/mL of total human DNA (Sigma Chemical Co, St Louis, MO) in the hybridization solution. DNA from RS4; 11 or B1 cells was used as a positive control, and DNA from normal human peripheral blood was used as a germline control.
Immunophenotyping for NG2 expression.The production of MoAb 7.1 has been previously reported.10 Briefly, hybridomas were made from mice immunized with an SV-40–transformed marrow stromal cell line. 7.1 is nonreactive with normal peripheral blood and marrow hematopoietic cells and with several leukemic cell lines, including RS4; 11 (which has 11q23/MLL rearrangement), Jurkat, U937, Nalm 6, and HL-60. It does react with a variety of nonhematopoietic cell lines (HeLa, Readin, Pinkney, and others). Leukemic blasts were stained by indirect immunofluorescence, as previously described.10 Cells were considered positive for NG2 expression if at least 25% of the cells had a fluorescence intensity exceeding 95% of that of cells stained with isotype-matched control antibody.
Cytogenetics.Cytogenetic analyses were performed at local CCG-affiliated institutions. Karyotypes were subsequently centrally reviewed, and a normal case was considered adequate if at least 20 metaphases were analyzed at the 400 band level or higher.
Statistical analysis.Outcome measures were induction rate, death during induction, survival, and event-free survival (EFS). To detect ascertainment bias, clinical parameters (white blood cell count [WBC] and age at diagnosis, FAB classification) and outcomes were compared between those patients whose cells were or were not available for study, using χ2 analysis. Fisher's exact test14 was used to assess induction rate and death in induction. Survival curves were generated using the Kaplan-Meier15 method and assessed with the log-rank test.16
RESULTS
Study population.To examine selection bias introduced by selecting study patients based on availability of cryopreserved cells, we compared the clinical characteristics and outcomes for patients studied herein (n = 44) and all evaluable infants on protocol (n = 102). No statistical difference was found between study patients and the full cohort, as far as induction rate (P = .41), death during induction (P = 1.0), 4-year EFS (log-rank, P = .39), age at diagnosis (P = .28), the number of patients with Down syndrome (P = .53), FAB subclass (P = .93), and the percentage of blasts in marrow (P = .10). A significant difference was found in the WBC at diagnosis, with the median WBC for study patients being 69,300 (range, 4,600 to 467,000) and for the remainder of the cohort 20,600 (range, 2,000 to 286,000; P = .0007). This may represent the ease of storing excess cells when the presenting WBC is high.
Clinical and laboratory characteristics of MLL-rearranged patients.Twenty-nine of 44 (66%) infants showed MLL gene rearrangement by Southern blot. The patients with MLL rearrangement had a high (22/29) incidence of M4/M5 morphology (of the other 7, 2 were not classified, 1 was M1, 2 were M2, and 2 were M7), as previously reported.6 MLL rearrangement correlated with high WBC at diagnosis. The rearranged group had a median presenting WBC of 114,000 (range, 5,300 to 467,000), whereas that of the nonrearranged group was 29,600 (range, 4,600 to 149,000; P = .004). Age less than 6 months at diagnosis was more common in the rearranged group (11/29 [38%]) compared with the nonrearranged group (4/15 [27%]), but this did not reach statistical significance (P = .68). There was a trend towards a higher incidence of central nervous system leukemia in MLL rearranged (14/29 [48.3%]) versus nonrearranged (2/15 [13.3%]) patients (P = .10).
MLL gene rearrangement and NG2 molecule expression.MoAb 7.1 reacted with the leukemic blasts of 13 of 37 tested (35%) cases of infant AML. Eleven of these 13 had M4/M5 morphology. No case with germline MLL gene configuration showed expression of 7.1/NG2 (Table 1). Of the 7.1-tested cases with MLL gene rearrangement (n = 24), 13 (54.5%) expressed NG2 and 11 (45.5%) did not. Thus, all leukemias expressing the NG2 antigen had MLL gene rearrangement, indicating that 7.1-positivity is a specific marker for MLL rearrangement, with a specificity of 100%. Most of these NG2-positive cases were FAB M4/M5. However, cells that were negative for NG2 were more varied in histologic subtype, and sometimes MLL was rearranged, showing that the 7.1 antibody does not detect all cases with MLL/11q23 abnormality in infant AML, with a sensitivity of 54%.
MLL and FAB Status of Infant AML Cases Tested for 7.1/NG2 Expression
| . | MLL . | MLL . | FAB . | Other or . |
|---|---|---|---|---|
| . | Rearranged . | Germline . | M4/M5 . | Undefined . |
| 7.1 positive | 13 | 0 | 11 | 2 |
| 7.1 negative | 11 | 13 | 8 | 16 |
| . | MLL . | MLL . | FAB . | Other or . |
|---|---|---|---|---|
| . | Rearranged . | Germline . | M4/M5 . | Undefined . |
| 7.1 positive | 13 | 0 | 11 | 2 |
| 7.1 negative | 11 | 13 | 8 | 16 |
Cytogenetics.Karyotypes were reviewed and adequate in 21 of 44 (47.7%) patients, and 19 of these had NG2 testing completed as well. These results are shown in Table 2. Of the 6 adequate karyotypes in the MLL-rearranged and NG2-positive group, 4 had balanced 11q23 translocations, including 3 with a t(9; 11) and 2 with interstitial 11q23 abnormalities. Among the MLL-rearranged but NG2-negative cases were 3 balanced 11q23 translocations, including 2 with a t(10; 11) and 1 with t(11; 17), but none with the common t(9; 11). This group also had 1 del(11)(q23) and 1 inv(16). In the MLL-germline group no NG2-positive cases were found, and no cytogenetic 11q23 abnormalities were present. Monosomy 7 and hyperdiploidy (49 to 50 chromosomes) were found only among these NG2-negative and MLL-germline samples.
Cytogenetics According to MLL and 7.1/NG2 Status
| MLL Rearranged, 7.1/NG2 Positive | |
| 46,XY,add(5)(q22),der(11)del(11)(p13)add(11)(q23) | |
| 46,XX,t(7; 11)(p22; q23) | |
| 46,XX,t(9; 11)(p22; q13) | |
| 47,XY,t(9; 11)(p22; q23),+21c | |
| 46,XY,t(9; 11)(p22; q23) | |
| 46,XY,del(11)(q14q23) | |
| 1 inadequate | |
| 6 unavailable for review | |
| MLL Rearranged, 7.1/NG2 Negative | |
| 46,XY,inv(10)(p11.2q22),t(10; 11)(p11.2; q23) | |
| 46,XY,del(11)(q23) | |
| 46,XY,inv(16)(p11.2q24) | |
| 46,XX,t(11; 17)(q23; q21) | |
| 46,XX,t(10; 11)(p11.2; q23) | |
| 2 inadequate | |
| 4 unavailable for review | |
| MLL Germline, 7.1/NG2 Negative | |
| 49,XY,+8,+21,+22 | |
| 45,XY,−7 | |
| 46,XY,t(4; 6; 9)(q31; q25; q34) | |
| 45,XY,−7 | |
| 46,XX,t(7; 11)(q11.2; p15.5),t(11; 12)(q13; p13.2) | |
| 45,XY,−7 | |
| 46,XY,t(10; 11)(p13; q21) | |
| 50,XX,del(3)(q21q27),+19,+21c,+21,+22 | |
| 5 unavailable for review | |
| MLL Germline, 7.1/NG2 Positive | |
| None |
| MLL Rearranged, 7.1/NG2 Positive | |
| 46,XY,add(5)(q22),der(11)del(11)(p13)add(11)(q23) | |
| 46,XX,t(7; 11)(p22; q23) | |
| 46,XX,t(9; 11)(p22; q13) | |
| 47,XY,t(9; 11)(p22; q23),+21c | |
| 46,XY,t(9; 11)(p22; q23) | |
| 46,XY,del(11)(q14q23) | |
| 1 inadequate | |
| 6 unavailable for review | |
| MLL Rearranged, 7.1/NG2 Negative | |
| 46,XY,inv(10)(p11.2q22),t(10; 11)(p11.2; q23) | |
| 46,XY,del(11)(q23) | |
| 46,XY,inv(16)(p11.2q24) | |
| 46,XX,t(11; 17)(q23; q21) | |
| 46,XX,t(10; 11)(p11.2; q23) | |
| 2 inadequate | |
| 4 unavailable for review | |
| MLL Germline, 7.1/NG2 Negative | |
| 49,XY,+8,+21,+22 | |
| 45,XY,−7 | |
| 46,XY,t(4; 6; 9)(q31; q25; q34) | |
| 45,XY,−7 | |
| 46,XX,t(7; 11)(q11.2; p15.5),t(11; 12)(q13; p13.2) | |
| 45,XY,−7 | |
| 46,XY,t(10; 11)(p13; q21) | |
| 50,XX,del(3)(q21q27),+19,+21c,+21,+22 | |
| 5 unavailable for review | |
| MLL Germline, 7.1/NG2 Positive | |
| None |
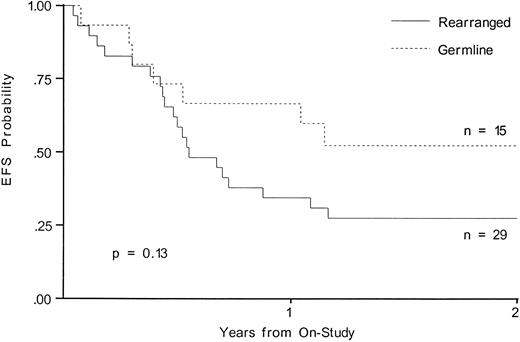
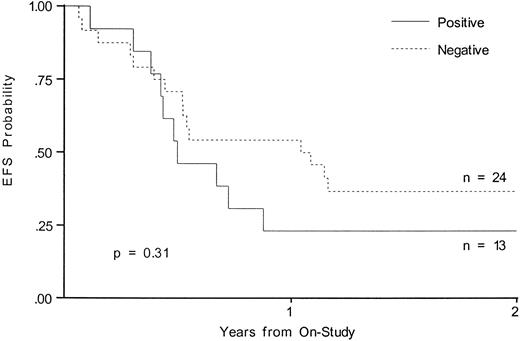
Outcome of therapy.Although neither MLL rearrangement nor NG2 expression reached statistical significance when analyzed for outcome, there was a trend towards a worse outcome in infants with MLL gene rearrangement. EFS at 2 years in the MLL rearranged group was 27.6% and was 52.5% for the germline group (P = .13; Fig 1). NG2 expression did not predict a poor prognosis; EFS at 2 years was 23.1% for the NG2-positive patients and 36.7% for the NG2-negative patients (P = .31; Fig 2).
EFS from time of study entry for patients with and without MLL gene rearrangement.
EFS from time of study entry for patients with and without MLL gene rearrangement.
EFS from time of study entry for patients with and without 7.1/NG2 expression.
DISCUSSION
We studied MLL/11q23 rearrangement, NG2 expression, and cytogenetics in infant AML. We sought to discern whether MLL rearrangement correlated with a poorer prognosis in infant AML and whether infants with NG2-positive AML had a poorer prognosis than those with NG2-negative blasts as in childhood AML10 and ALL.12 We also sought to contribute to the existing data asking whether all or particular cytogenetic 11q23 rearrangements correlate with NG2 expression.
It was the search for cell surface markers shared by hematopoietic precursors and stromal cells that led to the development of MoAb 7.1. This antibody identified, on certain AML blasts, a transmembrane protein homologous to the rat NG2 molecule, a chondroitin sulfate proteoglycan thought to function as an inhibitor of cell adhesion and migration. Previously, we showed that NG2-positivity correlated with MLL gene rearrangement; children with NG2-positive AML treated on CCG protocol 213 had a significantly worse outcome than 7.1/NG2-negative cases.10 In this study, we found that, in the select group of infants with AML treated on CCG protocols 2861/2891 (similar chemotherapy to 213, but with more intensive timing), there was a trend towards a worse outcome in 7.1/NG2-positive cases that was not prognostically significant. The difference in prognostic significance of NG2 positivity between this group of AML patients and that previously studied10 could be due to differences in therapy, the different patient subsets tested (infants v all pediatric patients), and/or small numbers.
There was a strong trend towards a worse outcome for infants with MLL gene rearrangement in this study. This is not a reflection of age alone, because infants on CCG 2861/2891 are doing as well as the cohort overall (W.G.W., personal communication, November 1996), which agrees with Pui et al,17 who showed that infants with AML had an outcome similar to that of other children. MLL rearrangement does correlate with high WBC at diagnosis, which also has been poor prognostic indicator.18,19 These results indicate that MLL rearrangement is more prognostically significant than NG2 expression in infant AML. This is similar to the correlation of MLL rearrangement and CD10-negativity in infant ALL, in which MLL rearrangement has the stronger prognostic significance.3
And as in infant ALL, 11q23/MLL rearrangement in AML takes place with various translocation partners, with the significance of these different partners remaining unclear. The genes from 11q23 translocation partners AF-4 (4q21), AF-9 (9p22), and ENL (19p13) have been sequenced, and they show significant homology, coding for putative transcription factors (having nuclear localization sequences, serine and proline-rich regions, and an abundance of basic amino acids).20,21 In addition, other 11q23 translocation partner genes have been sequenced, with different putative functions ascribed: AF1q at 1q23,22 ELL at 19p13.1,23 Xq13,24 6q2725 (implicated in cell-cell signaling), 10p1226 and 17q2127 (transcriptional regulation), and 1p3228 (contains a domain characteristic of cytoskeletal proteins). The MLL gene is known to encode a transcription factor homologous to Drosophila trithorax.29
The expression of NG2 may depend on the particular gene paired with MLL when it is rearranged.10 The cytogenetics results in this and the two other reports of NG2 in acute leukemia begin to address this issue. Behm et al12 studied 117 children with ALL; 9 (8.6%) were NG2-positive and had a poor outcome and all 9 showed MLL rearrangement. All 9 had balanced cytogenetic 11q23 translocations; 5 had t(4; 11)(q21; q23) and 4 had t(11; 19)(p13; q23). Seven patients with unbalanced 11q23 abnormalities (deletions or inversions) that were negative for molecular MLL gene rearrangement were also NG2 negative. The conclusion was that the MoAb 7.1 detected clinically significant MLL-rearranged ALL cases and that those were the ones with balanced 11q23 translocations involving 4q21 and 19p13.12
Smith et al10 studied 165 children with AML (CCG protocol 213); 18 (11%) were NG2-positive, and they had a poor outcome compared to the rest of the cohort. Although NG2-positivity correlated with MLL gene rearrangement, the correlation was not perfect. NG2-positive cases showed t(11; 19)(q23; p13), t(9; 11)(p22; q23), t(11; 17)(q23; q11.2), and t(10; 11)(p?; q?). However, 4 NG2-negative cases showed t(9; 11)(p22; q23), del(11)(q23), t(11; 17)(q23; q25), and t(1; 11)(p32; q23).10 Molecular testing confirmed MLL rearrangement. The conclusion was that NG2 expression conferred a poor prognosis and that, even though the 7.1 MoAb did not detect all cases of MLL rearrangement, the NG2 molecule may serve as a target for leukemia-specific therapy.
Our results in infant AML patients corroborate the data from Smith et al10 in that MoAb 7.1 does not detect all cases of 11q23/MLL rearrangement detected with molecular or cytogenetic means, even despite a small number with available karyotypes. Unlike the data from childhood ALL, in which NG2-positivity correlated with balanced cytogenetic 11q23 translocations, in our NG2-positive group we found 11q23 deletions in 2 cases, along with 4 balanced translocations [3 with t(9; 11), and 1 with t(7; 11); Table 2]. In our NG2-negative patients, 2 had t(10; 11)(p11.2; q23), 1 had t(11; 17)(q23; q21), 1 had del(11)(q23), and 1 had inv(16). Although the data are preliminary and more cases need to be studied, in the infant AML cases tested thus far, t(9; 11) seems to correlate with NG2 positivity and t(10; 11) with NG2-negativity. Cases with 11q23 deletions show no clear pattern of NG2 expression. Thus, the known heterogeneity in 11q23 translocation partners in AML may be reflected in abnormal expression of the NG2 molecule, depending on which partner gene is changing (or having its expression changed by) the expression of the MLL gene.
At this point, detection of MLL gene rearrangement is more predictive of ultimate outcome than detection of NG2 expression in infant AML. The regulation of expression of the NG2 antigen by changes in MLL expression await more studies with concurrent cytogenetics.
ACKNOWLEDGMENT
The authors thank Roderick Moore for excellent technical assistance and Jan Watterson for manuscript preparation. We also thank the institutional cytogenetecists for the submission of karyotypes from patients included in this study.
Supported in part by a Children's Cancer Research Fund Grant and by the Pine Tree Apple Tennis Classic. J.H.K. is the recipient of an Outstanding Investigator Award (CA49721) from the National Cancer Institute. J.M.H. is the recipient of an American Society of Clinical Oncology Young Investigator Award.
Address reprint requests to Joanne M. Hilden, MD, Children's Health Care-St Paul, 345 N Smith Ave, St Paul, MN 55102.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal