Abstract
Follicular lymphomas (FLs) rarely induce clinically significant T-cell–mediated responses. We showed that freshly isolated tumor infiltrating T cells (T-TILs) lack tumor-specific cytotoxicity. Stimulation of these T cells with FL cells in the presence of interleukin-2 (IL-2) and/or costimulation via CD28 does not lead to T-cell activation and expansion. In contrast, when stimulated with FL cells preactivated via CD40, autologous T-TILs can be expanded by the addition of exogenous IL-2. These T cells can be further expanded in vitro by the addition of exogenous IL-4, IL-7, or interferon-γ, but not IL-12. Once activated, these T cells showed FL-directed cytotoxicity in four of five patients tested. We concluded that autologous cytotoxic anti-FL–specific T cells exist, but can only be detected in vitro under optimized conditions for T-cell stimulation and expansion. This suggests that their frequency in vivo is either very low or that the microenvironment does not provide the necessary signals to activate these T cells. This model system allows dissection of the requisite conditions for activation and expansion of lymphoma-directed cytotoxicity and may permit expansion of previously activated cytotoxic T cells for adoptive transfer.
DESPITE THE well-documented existence of tumor antigens, there is little evidence that clinically significant immune responses are elicited against lymphoma cells. Although antitumor-specific T cells can be isolated from tumor infiltrating T lymphocytes (T-TILs) in B-cell non-Hodgkin's lymphoma, these T cells do not show any significant cytotoxicity towards autologous B-lymphoma cells.1-4 In one study, a T-cell clone could be derived from T-TILs that had specific cytotoxicity against an autologous follicular lymphoma (FL) cell line.5 These data suggest that, whereas autologous antitumor-specific T cells exist, their frequency is either very low or they are insufficiently activated in vivo to induce clinically significant immune responses against autologous tumor. This failure to elicit an immune response to autologous tumor might be caused by defects in the T cell6 or due to a failure of the tumor cell to function as an efficient antigen-presenting cell (APC).
At least two different strategies can be applied to induce and expand autologous antitumor-specific T cells. First, if the tumor antigen is known, it can be presented by professional APCs to activate T cells to kill the autologous tumor cells.7-12 In B-cell malignancies, the Ig idiotype provides a unique tumor antigen that can be exploited in immunologic therapeutic strategies.13-15 A recent report using dendritic cells pulsed with autologous tumor-specific idiotype antigen as a vaccine indicated that a T-cell–mediated anti-FL–specific immune response might be involved in tumor regression.16 An alternative approach is to induce the tumor cell to function as an efficient antigen-presenting cell. This approach may be advantageous in those cases in which the tumor-specific antigen is not known or poorly characterized and may enable a more efficient immune response if there are multiple tumor antigens present. We and others have previously shown that normal and malignant B cells become efficient allo APCs after ligations of CD40.17-20 After culture on transfectants expressing CD40 ligand (CD40L), FL cells upregulated expression of both adhesion and costimulatory molecules, and these cells became efficient allo-APCs. Moreover, after repeated stimulation with FL cells cultured on CD40L (CD40-FL) allogeneic T cells were now capable of proliferating in response to unstimulated FL cells.20
Based on our previous findings in an allogeneic model, we sought to determine whether FL or CD40-FL can present tumor-associated antigens to autologous T cells in addition to alloantigen. We show that T-TILs freshly isolated or cultured in interleukin-2 (IL-2) showed no FL-specific cytotoxicity. In contrast, CD40-FL cells can activate autologous, FL-specific T-TIL cells in the presence of exogenous IL-2. Once activated, these T cells showed FL-directed cytotoxicity. Moreover, these T cells can be further expanded in vitro by the addition of exogenous IL-4, IL-7, or interferon-γ (IFN-γ), but not IL-12. Our data indicate that T cells with ani-FL–specific cytotoxicity exist and that these cells can be amplified in vitro. The development of this model system allows dissection of the requisite conditions for induction and expansion of lymphoma-directed cytotoxicity and may permit expansion of previously activated cytotoxic T cells for adoptive transfer.
MATERIALS AND METHODS
Normal and neoplastic tissues.Lymph nodes were obtained from patients with FL undergoing diagnostic biopsies. All samples were of follicular, small-cleaved cell type (working formulation, type B). Five patients were selected with tumor cells that expressed a t(14; 18) translocation detectable by polymerase chain reaction.21 This provided a marker that could be used to show that cultured FL cells belonged to the malignant clone. For control studies, peripheral blood was obtained from all patients to obtain normal T and B cells. These cells were only used as controls if found to be negative for the t(14; 18) translocation. Normal autologous B cells from peripheral blood could only be obtained in sufficient quantities from two patients (FL4 and FL6). Allo T cells from healthy donors were purified from peripheral blood. All discarded specimens and blood samples were obtained after approval by Institution Review Committees.
Purification of B and T cells.Tissue samples were mechanically homogenized, and mononuclear cells were isolated by Ficoll-Isopaque density centrifugation. Purification of B-cell and T-cell populations was performed as previously described,20 and purity was assessed by immunophenotyping. Isolated FL cells were uniformly greater than 90% CD20+ CD40+, greater than 97% light chain restricted, and less than 5% CD3, CD14, or CD56. CD3+ T cells were obtained as previously described.20 22 CD3+ T cells were greater than 98% CD3+. The total number of purified CD3+ T-TILs after isolation and purification ranged between 1.9 × 107 (FL10) and 2.5 × 108 cells (FL4) depending on the size of biopsy material available.
Immunofluorescence studies.Surface expression of molecules was detected using the following monoclonal antibodies (MoAbs) conjugated with fluorescein isothiocyanate (FITC) or phycoerythrin (PE): CD3-FITC, CD4-PE, CD8-PE, CD19-PE, CD20-PE, CD56-PE (Coulter, Miami, FL); CD80-FITC (YB2.C4; Repligen Corp, Cambridge MA); CD86-PE (B70-PE, clone IT2.2). MoAbs for the α, β, and γ chain of the IL-2 receptor were a kind gift from Dr J. Ritz (Dana-Farber Cancer Institute, Boston, MA).
Cytokines.Optimal concentrations of cytokines were established in proliferation assays using allogeneic T cells (105/well) as effector cells and CD40-activated FL cells (2 × 104/well) as stimulator cells in the presence of increasing concentrations of the cytokines. IL-2 was a generous gift of Dr Ritz and was used at 100 IU/mL. Recombinant human IL-4 was a generous gift of Dr MB Widmer (Immunex, Seattle, WA) and used at 5 ng/mL. Human IL-12 (1 ng/mL) and IFN-γ (10 ng/mL) were purchased from Genzyme (Cambridge, MA). IL-7 was purchased from Sigma (St Louis, MO) and used at 5 ng/mL.
Activation and culture of B cells onto t-CD40L.Purified normal or malignant B cells were stimulated by culture on murine NIH3T3 fibroblasts transfected with the human CD40 ligand (t-CD40L)20 at a concentration of 5 × 105 cells/mL in Iscove's modified Dulbecco's medium (IMDM; GIBCO BRL, Gaithersburg, MD) supplemented with 2% fetal calf serum, 0.5% bovine serum albumin (Sigma), and 50 μg/mL human transferrin (Boehringer Mannheim, Mannheim, Germany), 5 μg/mL bovine insulin (Sigma), and 15 μg/mL Gentamicin (GIBCO BRL) at 37°C in 5% CO2 . B cells were transferred at day 3 to plates with fresh irradiated t-CD40L cells and harvested on day 5 for functional studies. After harvesting, B cells were washed two times in IMDM, kept on ice for 1 hour in IMDM, and then finally washed and resuspended in RPMI 1640 supplemented with 5% human serum, 2 mmol/L Glutamine, 15 mg/mL Gentamicin (RPMI-5).
In vitro cytotoxic T-lymphocyte response induction.Purified T cells from lymph nodes with follicular lymphoma (T-TILs) were stimulated with irradiated (64 Gy) CD40-FL cells or with FL cells at different effector:target (E:T) ratios ranging from 4:1 to 1:2. For CD40-FL cells, an optimal ratio for T-cell expansion at 4:1 was established. T cells were stimulated with irradiated stimulator cells on day 0, 7, and 14. Cytokines were either added at initiation of culture or first added at day 3 and every third day thereafter until cytotoxicity was assessed. Where indicated, CD28 MoAb (10 μg/mL), irradiated (64 Gy) B7-1 or B7-2 transfectants (t-B7-1, t-B7-2) were added on day 0, 7, and 14 together with FL cells. Cocultures were cultured in RPMI 1640 supplemented with 5% human AB serum, 2 mmol/L Glutamine, 50 μg/mL penicillin-streptomycin (GIBCO BRL), 15 μg/mL Gentamicin (GIBCO BRL) at 37°C in 5% CO2 at a concentration of 1 to 3 × 106 cells/mL. Before each restimulation and cytotoxicity test, T cells were Ficoll-density centrifuged to increase cell viability.
Cytotoxicity assay.A previously published method to assess T-cell–mediated cytotoxicity23 was adapted to measure T-cell cytotoxicity against human FL. CD40-FL cells, FL cells, autologous normal CD40-B cells, allogeneic normal CD40-B cells or FL cells, or autologous phytohemagglutinin (PHA) blasts were used as target cells. CD40-B cells were harvested from culture, washed twice by centrifugation in phosphate-buffered saline, and resuspended in RPMI-5. The target cells were incubated with [3H]thymidine overnight at 37°C. Target cells were again washed by centrifugation and mixed with various numbers of effectors in a final volume of 0.2 mL of RPMI-5 in round-bottom microtiter plates (2 × 104 target cells/well). Where indicated, T-cell–mediated lysis was measured in the presence of antibodies to major histocompatibility complex (MHC) class I (W6/32, 10 μg/mL) and/or MHC class II (949, 10 μg/mL) to block the interaction of T cells and target cells. After 6 to 8 hours of incubation, the plates were harvested and the radioactivity was determined in a β counter. Spontaneous lysis was determined by incubating the targets alone in the absence of effector T cells. Maximum lysis was determined by incubating the target cells with 0.2% Triton X-100 (Sigma). All determinations were performed at least in triplicate and the standard errors of the means were always less than 10% of the value of mean. The percentage of specific cytotoxicity was determined using the following equation: Percentage of Specific DNA Loss = (S − E)/S × 100, where E is experimentally retained DNA in the presence of T cells (in cpm) and S is retained DNA in the absence of T cells (spontaneous). The JAM test was validated with allogeneic T-cell lines (TCLs) specifically killing FL cells, as well as with an autologous TCL established from patient FL4 by performing standard chromium release assays. The results from these chromium release assays were almost identical to the results obtained in JAM tests performed at the same time (see Results). To further confirm that the target cells lysed were indeed FL cells, cells were analyzed by flow cytometry using directly conjugated MoAbs and HOECHST 33342 staining to identify which cell population was killed after 4 hours of coculture with the autologous TCL. This analysis showed that 50% of CD19+ CD38+ CD10+ cells had undergone apoptosis or were already dead (data not shown), whereas FL cells cocultured with unstimulated T cells showed less than 10% cell death during this time period (data not shown).
Statistical analysis.Differences between experimental groups were analyzed by the χ2 test and the Student's t-test.
RESULTS
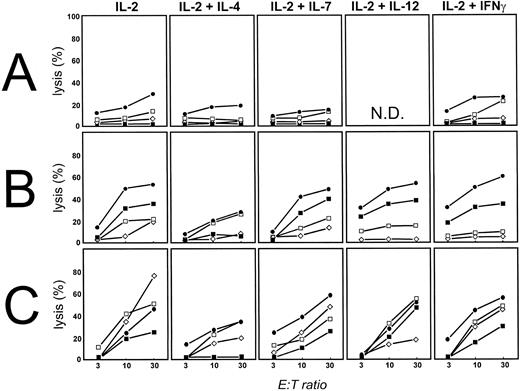
FL cells can be killed by allogeneic T cells.We showed previously that allogeneic T cells could be induced to proliferate against FL cells only after priming with FL cells activated on CD40L.20 We now determined whether these allogeneic T cells had cytotoxic activity against FL cells. Because FL cells are characterized by reduced cell death rather than increased proliferation, these experiments are especially important to determine if FL cells are susceptible to T-cell–induced cell death. Alloantigen-specific TCLs were generated from purified CD3+ allogeneic T cells (>98% CD3+) obtained from normal donors by stimulating them repetitively with FL cells activated by CD40 cross-linking before coculture (CD40-FL). No additional cytokines were provided during this initial coculture. Cytotoxicity of these TCLs was assessed by standard chromium release assay (Fig 1A) and by the JAM test23 (Fig 1B) against both unmodified (▪) and CD40 activated (•) allogeneic FL of patient FL4. For both the chromium release assay and the JAM test a concentration of 2 × 104 target cells/well was used. The thymidine incorporation used as a measurement of DNA-fragmentation, and therefore, cell death in the JAM test ranged between 800 and 2,300 cpm for FL cells, 2,000 to 15,000 cpm for CD40-activated FL and normal B cells, and 1,500 and 13,000 cpm for PHA blasts. Because there was no statistically significant difference between chromium release (Fig 1A) and JAM test (Fig 1B) assays, the results of the JAM test are shown throughout this report. Cytotoxicity was MHC restricted, because allogeneic third party CD40-FL cells (CD40-FL6; ▵), FL cells (FL6; ○), or normal resting (□) or activated B cells (⋄) had greatly reduced cytotoxicity by these TCLs (Fig 1A and B). Antibodies against both MHC class I and/or II decreased cytotoxicity (Fig 1C). We conclude that FL cells are highly susceptible to T-cell–mediated killing in vitro and that activation with CD40-FL cells induces not only T-cell proliferation, but also T-cell–mediated cytotoxicity against primary FL cells.
Human FL cells can be killed by allogeneic T cells, but not freshly isolated or IL-2–activated T-TIL. (A) Chromium release assay of allogeneic T cells stimulated three times with CD40-FL cells and cytotoxicity assessed at day 28 after initiation of culture. Results shown are from patient FL4. A total of 2 × 104 FL cells (▪), CD40-FL cells (•), or control targets including FL6 (○), CD40-FL cells from FL6 (▵) were placed in 96-well round-bottom plates and T cells added at E:T ratios of 3:1, 10:1, and 30:1 in a final volume of 0.2 mL. A TCL from one of three healthy donors is depicted. (B) The same TCL was examined at the same time for cytotoxicity against FL4 tumor cells by JAM test. Target cells included FL cells (▪), CD40-FL cells (•), or control targets including CD40-FL cells from FL6 (▵), unstimulated FL6 cells (○), normal B cells (□), or CD40-activated normal B cells (⋄). (C) Blockade of MHC class I (⋄) and MHC class II (□) by MoAbs decreases anti-FL–directed T-cell cytotoxicity, and the combination of both (○) almost abrogates cytotoxicity. T-TILs were isolated from FL4 and analyzed for cytotoxicity against FL4 (D) immediately after isolation, or (E) after culture in IL-2 for 7 days (FL cells [▪], CD40-FL cells [•], or normal CD40-activated B cells [□]). Comparable results were obtained with FL cells from three additional patients.
Human FL cells can be killed by allogeneic T cells, but not freshly isolated or IL-2–activated T-TIL. (A) Chromium release assay of allogeneic T cells stimulated three times with CD40-FL cells and cytotoxicity assessed at day 28 after initiation of culture. Results shown are from patient FL4. A total of 2 × 104 FL cells (▪), CD40-FL cells (•), or control targets including FL6 (○), CD40-FL cells from FL6 (▵) were placed in 96-well round-bottom plates and T cells added at E:T ratios of 3:1, 10:1, and 30:1 in a final volume of 0.2 mL. A TCL from one of three healthy donors is depicted. (B) The same TCL was examined at the same time for cytotoxicity against FL4 tumor cells by JAM test. Target cells included FL cells (▪), CD40-FL cells (•), or control targets including CD40-FL cells from FL6 (▵), unstimulated FL6 cells (○), normal B cells (□), or CD40-activated normal B cells (⋄). (C) Blockade of MHC class I (⋄) and MHC class II (□) by MoAbs decreases anti-FL–directed T-cell cytotoxicity, and the combination of both (○) almost abrogates cytotoxicity. T-TILs were isolated from FL4 and analyzed for cytotoxicity against FL4 (D) immediately after isolation, or (E) after culture in IL-2 for 7 days (FL cells [▪], CD40-FL cells [•], or normal CD40-activated B cells [□]). Comparable results were obtained with FL cells from three additional patients.
Freshly isolated or IL-2–activated T-TILs are not cytotoxic for FL cells.We sought to determine whether autologous T cells could recognize and kill autologous FL cells. To address this issue, T-TILs from patients with FL were purified and cytotoxicity assessed against autologous FL cells (▪), normal CD40-activated B cells (□), or CD40-activated FL cells (•) from the same lymph node. In keeping with previous data with T-TIL24 we found that freshly isolated T-TIL had no cytotoxic activity in vitro against purified autologous tumor cells as well as CD40-activated FL or normal B cells in any of the patients studied with FL (Fig 1D and E). As can be seen, no specific cytotoxicity against autologous FL cells was observed using freshly isolated T-TIL (Fig 1D) or with T-TILs cultured for 7 days in IL-2 (100 IU/mL Fig 1E). These results are representative of seven experiments performed using FL cells from four patients.
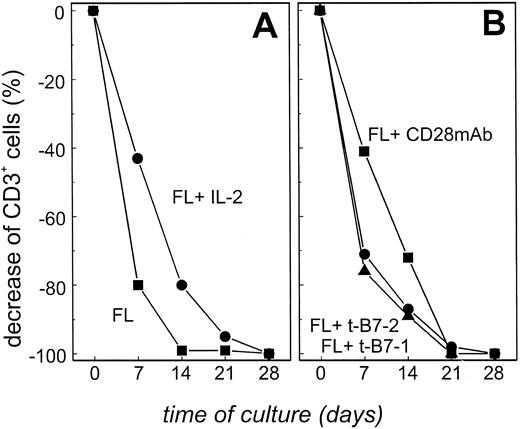
We next sought to determine if T-TILs could be stimulated in vitro with autologous tumor cells. T-TILs were isolated from patient FL4, highly purified (CD3+ T cells >98%) and stimulated weekly (day 0, day 7, and day 14) with isolated, irradiated FL cells at a ratio of 2:1. Because a large number of FL cells and T-TILs were available from patient FL4, cells from this patient were used to identify optimal culture conditions under which T cells were activated, expanded, and showed cytotoxicity. Using only irradiated FL cells as stimulators, the number of viable cells (which equaled the number of CD3+ T cells) in the culture decreased dramatically during the first 7 days, and no viable cells at all could be detected after 14 days of culture (Fig 2A, ▪). The addition of exogenous IL-2 led to a decrease in the rate of cell death, but no viable cells could be detected by 28 days using this culture system (Fig 2A, •). Addition of costimulation provided by B7 transfectants (B7-1, ▴; B7-2, •) or CD28 MoAbs (▪) did not provide the necessary additional signals for T-TILs to expand and no viable cells were detected after 21 days of culture (Fig 2B). These observations were confirmed using FL cells and T-TILs from three other patients. Therefore, under none of these culture conditions was it possible to isolate viable T cells during culture to determine whether stimulated T cells could kill autologous FL cells. This was not simply caused by the culture conditions used, because allogeneic T cells stimulated with FL cells, IL-2, and/or CD28 MoAbs could be expanded consistently (23-fold increase of CD3+ T cells after 28 days) and showed cytotoxicity towards the FL cells (52% at an E:T ratio of 30:1) after 28 days of culture (data not shown). We conclude from these data that the addition of costimulatory signals is not sufficient to induce autologous T-cell proliferation by the primary FL cells that express MHC but lack efficient expression of adhesion molecules.20
Highly enriched T-TILs could not be expanded when stimulated with purified FL cells, even in the presence of IL-2 or costimulation. (A) T-TILs (<98% CD3+) from patient FL4 were stimulated with either unstimulated FL cells (▪) or unstimulated FL cells in the presence of 100 IU/mL IL-2 (•). (B) T-TILs (<98% CD3+) from patient FL4 were stimulated with either FL cells in the presence of B7-1 transfected NIH3T3 cells (▴), B7-2 transfected NIH3T3 cells (•), or FL cells in the presence of CD28 MoAb (10 μg/mL; ▪). The number of CD3+ cells equals the total number of viable cells during culture as determined by phenotypic analysis on days 7, 14, 21, and 28. Results are representative of three experiments with cells from patient FL4. Comparable results were obtained with FL cells from three additional patients.
Highly enriched T-TILs could not be expanded when stimulated with purified FL cells, even in the presence of IL-2 or costimulation. (A) T-TILs (<98% CD3+) from patient FL4 were stimulated with either unstimulated FL cells (▪) or unstimulated FL cells in the presence of 100 IU/mL IL-2 (•). (B) T-TILs (<98% CD3+) from patient FL4 were stimulated with either FL cells in the presence of B7-1 transfected NIH3T3 cells (▴), B7-2 transfected NIH3T3 cells (•), or FL cells in the presence of CD28 MoAb (10 μg/mL; ▪). The number of CD3+ cells equals the total number of viable cells during culture as determined by phenotypic analysis on days 7, 14, 21, and 28. Results are representative of three experiments with cells from patient FL4. Comparable results were obtained with FL cells from three additional patients.
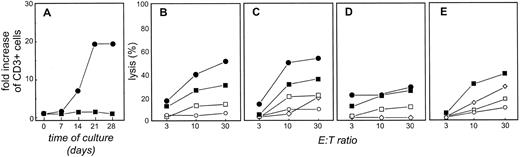
T-TILs are expanded using CD40-activated FL cells in the presence of IL-2 and can kill autologous FL cells.The inability to expand T-TILs efficiently using primary FL cells was not unexpected because the expansion of allogeneic T cells with FL cells and CD28 MoAbs was much less efficient than using CD40-FL cells.20 Based on these results, we next determined whether CD40-FL cells could be used to expand T-TILs. T-TILs were purified and stimulated with CD40-FL cells weekly. Viable CD3+ cells isolated throughout these cultures are shown in Fig 3A. Unlike the allogeneic system, no expansion occurred when the T-TILs were cultured without the addition of cytokines. However, under these culture conditions, the addition of exogenous IL-2 from day 3 of culture induced a more than 20-fold increase of CD3+ T cells after 28 days. Cytotoxicity of T cells isolated from day 28 of culture with CD40-FL and exogenous IL-2 are shown in Fig 3B (chromium release assay) and Fig 3C (JAM test). At this time point, T cells lysed autologous CD40-FL cells (•) as well as unstimulated FL cells (▪). There was only minor cytotoxicity against allogeneic CD40-activated B cells (⋄) at high E:T ratios and no cytotoxicity against allogeneic CD40-activated FL cells (○) cultured under the same culture conditions. More importantly, autologous CD40-activated normal B cells (□) cultured under the exact same culture conditions as the CD40-FL cells were not lysed at low E:T ratios and significantly less than the CD40-FL cells at the highest E:T ratio. This indicates that the cytotoxicity is directed against tumor-associated antigens and rules out that CD40-FL cells presented antigens captured during culture. Moreover, these results indicate that common B-cell antigens do not seem to be a major target for these T cells. When TCLs were established that were depleted of CD8+ cells before stimulation and expansion, cytotoxicity against both FL cells (24% at E:T 30:1) as well as CD40-FL cells (26% at E:T 30:1) was diminished but not abrogated, indicating that both CD4+ and CD8+ T cells were responsible for cell death of the FL cells (Fig 3D). Comparable with the allogeneic TCLs, blockade by MHC class I or II MoAbs decreased FL-specific cytotoxicity, and the combination of both almost completely abrogated cytotoxicity (Fig 3E), again indicating that both CD4+ and CD8+ T cells are exhibiting FL-directed cytotoxicity.
Highly enriched T-TILs expanded in the presence of CD40-FL and exogenous IL-2 exhibit cytotoxicity against autologous lymphoma cells. (A) T-TILs from patient FL4 were stimulated with CD40-FL cells (▪) or CD40-FL cells in the presence of 100 IU/mL IL-2 (•). The total number of viable cells was assessed by trypan blue exclusion test and the number of CD3+ T cells by immunophenotyping on day 0, 7, 14, 21, and 28 of culture. Viable cells were greater than 97% CD3+ T cells as assessed by phenotypic analysis. Cytotoxicity as examined by (B) chromium release assay or (C) JAM test of TCL cells after three stimulations with CD40-FL with addition of IL-2 at day 3 of coculture. Cytotoxicity was assessed on day 28 of culture. As targets, autologous CD40-FL cells (•), FL cells (▪), autologous CD40-B cells (□), allogeneic CD40-B cells (⋄), and allogeneic CD40-FL cells (○) were used in (B) and (C). Similar results were obtained using cells from patient FL6. (D) TCLs derived from T-TILs depleted of CD8+ T cells exhibit reduced cytotoxicity against FL and CD40-FL cells. (E) Blockade of MHC class I (⋄) and MHC class II (□) by MoAbs decreases anti-FL–directed T-cell cytotoxicity and the combination of both (○) almost abrogates cytotoxicity of autologous TCL to FL4.
Highly enriched T-TILs expanded in the presence of CD40-FL and exogenous IL-2 exhibit cytotoxicity against autologous lymphoma cells. (A) T-TILs from patient FL4 were stimulated with CD40-FL cells (▪) or CD40-FL cells in the presence of 100 IU/mL IL-2 (•). The total number of viable cells was assessed by trypan blue exclusion test and the number of CD3+ T cells by immunophenotyping on day 0, 7, 14, 21, and 28 of culture. Viable cells were greater than 97% CD3+ T cells as assessed by phenotypic analysis. Cytotoxicity as examined by (B) chromium release assay or (C) JAM test of TCL cells after three stimulations with CD40-FL with addition of IL-2 at day 3 of coculture. Cytotoxicity was assessed on day 28 of culture. As targets, autologous CD40-FL cells (•), FL cells (▪), autologous CD40-B cells (□), allogeneic CD40-B cells (⋄), and allogeneic CD40-FL cells (○) were used in (B) and (C). Similar results were obtained using cells from patient FL6. (D) TCLs derived from T-TILs depleted of CD8+ T cells exhibit reduced cytotoxicity against FL and CD40-FL cells. (E) Blockade of MHC class I (⋄) and MHC class II (□) by MoAbs decreases anti-FL–directed T-cell cytotoxicity and the combination of both (○) almost abrogates cytotoxicity of autologous TCL to FL4.
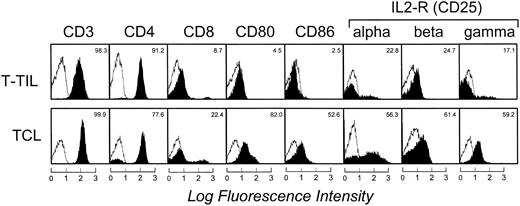
Phenotypic analysis showed that the CD3+ T cells expanded with CD40-FL cells in the presence of IL-2 were a mixed population of activated CD4+ and CD8+ T cells (Fig 4). There was a relative increase of CD8+ T cells from 8.7% before (T-TIL) to 22.4% after culture (TCL). All T cells expressed the adhesion molecules lymphocyte function-associated antigen 1 (LFA-1; CD11a) and LFA-3 (CD58), and more than 40% expressed intracellular adhesion molecule-1 (CD54) comparably before and after culture. The expanded T cells uniformly expressed activation markers including CD95 and CD38 (data not shown) and the majority expressed B7-1 (CD80), B7-2 (CD86), and all three chains of the IL-2 receptor.
Cell-surface expression of T-cell markers, activation markers, and chains of the IL-2 receptor complex on freshly isolated T-TILs and TCL1 cells (day 28) from FL4. The unshaded area indicates fluorescence of isotype-matched antibody. Similar results were seen in samples from patient FL6.
Cell-surface expression of T-cell markers, activation markers, and chains of the IL-2 receptor complex on freshly isolated T-TILs and TCL1 cells (day 28) from FL4. The unshaded area indicates fluorescence of isotype-matched antibody. Similar results were seen in samples from patient FL6.
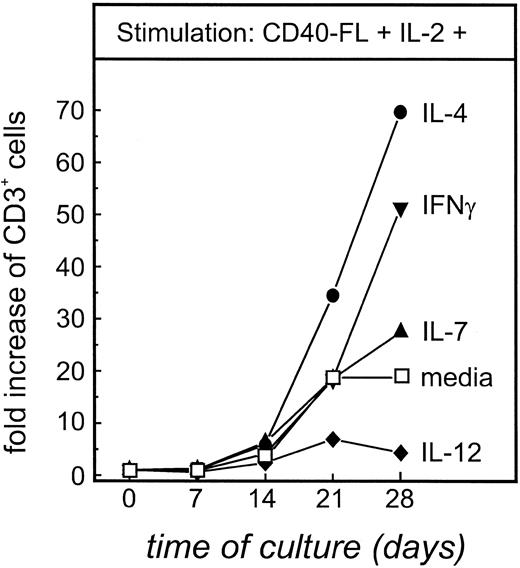
Expansion of autologous T cells can be enhanced by IL-4, IFN-γ, and IL-7, but not IL-12.T-cell expansion induced by CD40-FL cells in the presence of IL-2 (Fig 3A) would likely be insufficient for adoptive transfer strategies. Therefore, using cells from patient FL4, we examined whether the addition of other exogenous cytokines could enhance T-cell proliferation and/or cytotoxicity. When autologous T cells were stimulated with CD40-FL cells in the presence of either IL-4, IL-7, IL-12, or IFN-γ, no expansion could be detected (data not shown), suggesting that IL-2 was a prerequisite for expansion of these T cells. However, as seen in Fig 5, when T-TILs were cultured with CD40-FL and exogenous IL-2 alone, their expansion was increased by further addition of IL-4 (70-fold increase), IFN-γ (52-fold increase), or IL-7 (28-fold increase). In contrast, the further addition of IL-12 led to a fivefold decreased expansion compared with IL-2 alone. Surprisingly, when these T cells were analyzed for the expression of CD4 and CD8, no significant difference between the different culture conditions could be measured (data not shown).
Calculated maximal expansion of T-TILs stimulated with CD40-FL and IL-2 with other cytokines. T-TILs (5 × 106 cells) from FL4 were isolated, purified, and stimulated with CD40-FL cells in the presence of IL-2 (100 IU/mL) either alone or in combination with IL-4 (2 ng/mL), IL-7 (5 ng/mL), IL-12 (1 ng/mL), or IFN-γ (10 ng/mL). Total number of viable cells was assessed by trypan blue exclusion and the number of CD3+ T cells by immunophenotyping on days 0, 7, 14, 21, and 28 of culture. Expanded viable cells were always greater than 97% CD3+ T cells, and therefore, expansion of total cells is equal to expansion of T cells.
Calculated maximal expansion of T-TILs stimulated with CD40-FL and IL-2 with other cytokines. T-TILs (5 × 106 cells) from FL4 were isolated, purified, and stimulated with CD40-FL cells in the presence of IL-2 (100 IU/mL) either alone or in combination with IL-4 (2 ng/mL), IL-7 (5 ng/mL), IL-12 (1 ng/mL), or IFN-γ (10 ng/mL). Total number of viable cells was assessed by trypan blue exclusion and the number of CD3+ T cells by immunophenotyping on days 0, 7, 14, 21, and 28 of culture. Expanded viable cells were always greater than 97% CD3+ T cells, and therefore, expansion of total cells is equal to expansion of T cells.
Cytotoxicity against FL cells is enhanced by IL-12 and IFN-γ but abrogated by IL-4.We next assessed cytotoxicity of the T cells expanded under the various culture conditions described above. After a single stimulation with CD40-FL cells (Fig 6A), cytotoxicity was observed against CD40-FL (•), but there was no effective cytolysis induced against unstimulated FL cells (▪) under any of these conditions in any of the patients studied. In contrast, after 28 days of culture and three consecutive stimulations with CD40-FL cells (Fig 6B), TCL expanded with IL-2 resulted in killing of CD40-FL cells at 52% (•) and FL cells at 31% (▪) at an effector to stimulator ratio of 30:1, whereas there was significantly less killing of autologous (□) and allogeneic activated B cells (⋄). Although the combination of IL-4 and IL-2 was the most efficient combination to expand T cells stimulated with CD40-FL cells, somewhat surprisingly this led to greatly diminished cytotoxicity against CD40-FL cells (28% at an E:T of 30:1) and loss of cytotoxic activity against unstimulated FL cells (Fig 6B). Moreover, there was loss of specificity because they now killed autologous activated B cells (27% at an E:T of 30:1) but not allogeneic activated B cells. TCLs expanded in the presence of IL-2 and either IL-7 or IL-12 killed CD40-FL, unstimulated FL cells, and normal B cells comparably to T cells expanded with IL-2 alone. The most specific cytotoxicity was induced by TCLs expanded with the combination of IL-2 and IFN-γ. Although these TCLs did not kill more efficiently than TCLs expanded in IL-2 alone, there was now negligible nonspecific killing of normal B cells (Fig 6B). Preliminary experiments had suggested that the addition of cytokines from the first day of culture might increase cell expansion, but result in increased nonspecific killing of target cells. This was addressed formally in the experiments shown in Fig 6C, which show that addition of cytokines from day 1 of culture results in increased nonspecific killing compared with that induced when cytokines were added from day 3 (Fig 6B). These findings were confirmed in multiple TCLs derived using FL and T-TILs from several different lymph nodes obtained from patient FL4. Taken together, these results suggest that culture conditions for efficient expansion of lymphoma-specific cytotoxic T cells include culture of the T-TILs with CD40-FL followed by addition of exogenous IL-2 and IFN-γ.
Cytotoxicity of TCL cells under different culture conditions. TCLs were stimulated with CD40-FL in the presence of different cytokine combinations, and cytotoxicity was measured by JAM test. As targets, autologous CD40-FL cells (•), FL cells (▪), autologous CD40-B cells (□), and allogeneic CD40-FL cells (⋄) were used. (A) Cytotoxicity after one stimulation with CD40-FL, (B) after three stimulations with exogenous cytokines added at day 3 of culture, and (C) after three stimulations with exogenous cytokines added at commencement of culture.
Cytotoxicity of TCL cells under different culture conditions. TCLs were stimulated with CD40-FL in the presence of different cytokine combinations, and cytotoxicity was measured by JAM test. As targets, autologous CD40-FL cells (•), FL cells (▪), autologous CD40-B cells (□), and allogeneic CD40-FL cells (⋄) were used. (A) Cytotoxicity after one stimulation with CD40-FL, (B) after three stimulations with exogenous cytokines added at day 3 of culture, and (C) after three stimulations with exogenous cytokines added at commencement of culture.
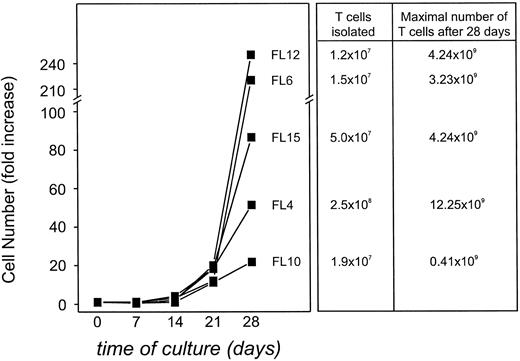
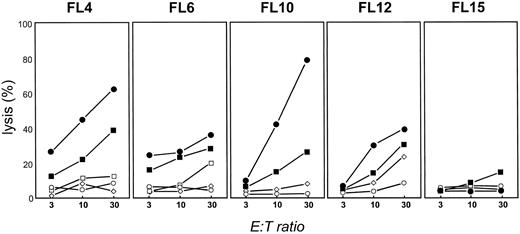
Stimulation with CD40-activated FL cells in the presence of IFN-γ and IL-2 induces FL-specific T cells in four of five patients.Using these optimized culture conditions, we next assessed whether T-TILs could be isolated and expanded from four additional patients with FL. As can be seen in Fig 7, T cells could be expanded from T-TILs in each patient after 28 days of culture from 21.8- to 250.2-fold (calculated values). Because we could purify T cells from these patients ranging from 1.9 × 107 cells up to 2.5 × 108 cells, this would translate into a potential number of expanded T cells ranging from 4.14 × 108 to 1.23 × 1010 cells, if all cells isolated would be used entirely for expansion (Fig 7). Because these tumor samples were not obtained for therapeutic use, these numbers might be even higher in a therapeutic setting. Cytotoxicity of these cells after 28 days in culture was assessed (Fig 8). At an E:T ratio of 30:1, specific cytotoxicity from four patients ranged from 38% to 82% against autologous CD40-FL (•) and from 25.6% to 39.1% against the autologous FL cells (▪). No detectable cytotoxicity against the unstimulated autologous FL cells was generated from patient FL15. T-TILs expanded from FL12 had slightly elevated cytotoxicity against allogeneic CD40-activated FL cells (⋄) at an E:T ratio of 30:1. Nonspecific killing against autologous B cells (□) could not be tested from FL10, FL12, and FL15. However, autologous PHA blasts (○) or autologous T cells (data not shown) were not lysed by the expanded TCLs from these patients. The killing of autologous unstimulated or CD40-activated FL cells was always significantly (P > .05) higher than cytotoxicity against autologous control targets, with one exception for FL6 in which cytotoxicity against unstimulated FL cells was not significantly different to autologous CD40-activated B cells at an E:T ratio of 30:1. However, killing against the CD40-FL cells at the same E:T ratio was significantly higher compared with the autologous normal CD40-activated B cells, suggesting that killing against the tumor cell was indeed specific. The percentage of CD8+ T cells in purified T-TIL cell preparations from these 5 patients ranged from 9% (FL4) to 31% (FL10) after isolation and purification (FL6, 24%; FL12, 29%; and FL15, 17%). After 28 days of culture, the relative number of CD8+ T cells increased slightly in patients FL4 (22%), FL6 (29%), and FL10 (38%) but was only insignificant in patients FL12 (31%) and FL15 (19%).
Calculated maximal expansion of T-TILs from FL4, FL6, FL10, FL12, and FL15 stimulated with autologous CD40-FL cells with addition of IL-2 and IFN-γ from day 3 of coculture if all T cells isolated from the tumor specimen would have been cultured only for expansion and usage in a therapeutic setting. Total number of viable cells was assessed by trypan blue exclusion test and number of CD3+ T cells by immunophenotyping on day 0, 7, 14, 21, and 28 of culture. Expanded cells were always greater than 97% CD3 + T cells, and therefore, expansion of total cells equals the expansion of T cells.
Calculated maximal expansion of T-TILs from FL4, FL6, FL10, FL12, and FL15 stimulated with autologous CD40-FL cells with addition of IL-2 and IFN-γ from day 3 of coculture if all T cells isolated from the tumor specimen would have been cultured only for expansion and usage in a therapeutic setting. Total number of viable cells was assessed by trypan blue exclusion test and number of CD3+ T cells by immunophenotyping on day 0, 7, 14, 21, and 28 of culture. Expanded cells were always greater than 97% CD3 + T cells, and therefore, expansion of total cells equals the expansion of T cells.
Cytotoxicity of TCLs from FL4, FL6, FL10, FL12, and FL15 expanded by stimulation with autologous CD40-FL in the presence of IL-2 and IFN-γ. TCLs were stimulated three times with autologous CD40-FL, and cytotoxicity was measured by JAM test at day 28. Cytokines were added after 3 days of initiation of coculture of T cells and CD40-FL cells. Autologous CD40-FL cells (•), FL cells (▪), autologous CD40-B cells (□), autologous PHA blasts (○), and allogeneic CD40-FL cells (⋄) were used as targets.
Cytotoxicity of TCLs from FL4, FL6, FL10, FL12, and FL15 expanded by stimulation with autologous CD40-FL in the presence of IL-2 and IFN-γ. TCLs were stimulated three times with autologous CD40-FL, and cytotoxicity was measured by JAM test at day 28. Cytokines were added after 3 days of initiation of coculture of T cells and CD40-FL cells. Autologous CD40-FL cells (•), FL cells (▪), autologous CD40-B cells (□), autologous PHA blasts (○), and allogeneic CD40-FL cells (⋄) were used as targets.
DISCUSSION
We have previously shown that FL cells can be induced after activation with CD40-L to become efficient allo-APCs and that once activated, these cells express sufficient adhesion, MHC, and costimulatory molecules to induce alloantigen-specific T-cell proliferation.20 We now show that autologous CD40-FL cells also function as APC to induce cytotoxic anti-FL–specific T cells from T-TILs. Once activated, these T cells are cytotoxic, not only for CD40-FL cells, but also for unstimulated FL cells. Unlike the allogeneic system, in which there is no requirement for the addition of exogenous cytokines, expansion of autologous anti-FL–specific cytotoxic T cells required the addition of exogenous IL-2. Further addition of IL-4 enhanced expansion of T cells significantly, but resulted in loss of specific cytotoxicity for FL cells. In contrast, IL-12 reduced T-cell expansion. Preliminary data suggest that killing is mediated by both CD4 and CD8 cells, and this is further supported by the finding that both MHC class I and II MoAbs decreased cytotoxicity and that TCL depleted of CD8+ T cells showed diminished but still significant tumor-directed cytotoxicity. After initial activation using CD40-FL, specificity of the response was maximized by addition of IL-2 and IFN-γ on day 3 of culture. Using these conditions for T-cell expansion, T-TILs could be expanded from five patients with FL and tumor directed cytotoxicity against the autologous primary tumor shown in four of these five patients. Taken together, these data suggest that the induction of FL-specific autologous T cells in vitro requires a series of signals including costimulation and adhesion provided by the tumor cells and that additional cytokines are necessary for subsequent expansion of specific cytotoxic T cells.
These data show that it is possible to provide a physiological signal to modify a tumor cell so that it can function as an APC in an autologous model. Previous studies on APC function in vivo and in vitro24-33 would have predicted that delivery of a second signal through CD28 or addition of exogenous IL-2 along with presentation of antigen by MHC molecules would be sufficient to induce an immune response. This is sufficient for lymphoma cells to induce proliferation of allogeneic T cells20 or T cells transgenic for influenza A when this antigen is transfected into the lymphoma cells.34 However, B7 transfection alone into lymphoma cells did not translate into a significant antitumor response in vivo.34 The data presented here show that unmodified FL cells are not capable of stimulating and expanding autologous T-TILs in the presence of costimulatory signals provided by CD28 MoAb, B7 transfectants, or exogenous IL-2. This suggests that activation of the FL cells with CD40-L is necessary to function as an APC and that it is unlikely that induction of expression of B7-1 or B7-2 would be in itself sufficient modification of the tumor cell to function as an APC in an autologous system. These phenomenologic observations require mechanistic interpretation. In the absence of modifications induced by signaling though CD40, FL cells may be unable to process and present tumor peptide antigens or they may express insufficient adhesion molecules to maximize antigen presentation, particularly in the setting of a low frequency of anti-FL–specific cytotoxic T-cell precursors in the T-TILs. Regardless of the mechanism, CD40 activation is sufficient to repair the defect of FL cells to serve as APC.
We modified the lymphoma cell to function as an APC to present tumor antigen to autologous T cells. Tumor cells can also be modified by fusing with professional APC.35 Where the tumor antigen is known, an alternative strategy is to use professional APC from the patient to present tumor antigen peptide to initiate an immune response. This strategy has recently been employed successfully where an efficient immune response against the lymphoma could be induced in vivo by presentation of idiotype peptide by peripheral blood dendritic cells.16 However, determination of the sequence of the idiotype is costly and time consuming, and in other malignancies tumor-specific antigens are unknown, not so well characterized, or not tumor specific. There are, therefore, potential advantages in using modified tumor cells themselves to act as APC.
Even if FL cells were not defective APC in our model system, our data in tumor-bearing patients support the numerous human and murine models showing that T-TILs exhibit numerous defects in T-cell recognition and effector function.6 36-43 Although CD40 activation was sufficient for FL cells to present alloantigen to normal allogeneic T cells, this, in itself, was not sufficient to induce either T-cell proliferation or cytolysis in the autologous setting. We and others have observed that T-TILs have quantitative deficiency in expression of the critical T-cell receptor ζ signaling chain. We have preliminary evidence that this defect can be repaired by 24 hours of culture in IL-2 (Schultze et al, manuscript in preparation). The capacity of IL-2 to repair this defect supports the notion that T-cell receptor ζ may be one of the defects associated with the paucity of the antitumor immune response. The mechanism(s) by which IL-2 repairs this and other defects is presently unknown, although it is unlikely to be mediated through the common γ chain of the IL-2 receptor because IL-4 and IL-7, which share this signaling chain, do not repair the defect. How IFN-γ improves proliferation and specificity is unknown but may contribute to the capacity of the CD40-activated FL to present antigen or directly induce proliferation of T cells. That addition of cytokines from day 1 of culture results in greatly reduced cytolytic specificity, whereas the addition at day 3 markedly improves specificity and requires further comment. We postulate that antigen-specific T cells receive both a T-cell receptor and costimulatory signal on day 1, and in the absence of exogenous cytokines nonspecific T cells die. Therefore, when added on day 3, IL-2 promotes the expansion of antigen-specific T cells in preference to the nonspecific T cells. Taken together, our results support the hypothesis that T-TILs are defective in vivo, and this defect can be repaired by the sequential addition of cytokines that favor repair of defective signaling, selection of antigen-specific cells, promotion of expansion, and induction of cytotoxicity.
Our in vitro culture system to expand FL-specific T cells by stimulating them repetitively with modified tumor cells in the presence of immunoregulatory cytokines provides a method for induction and expansion of anti-FL-specific cytotoxic T cells. Because T cells could be expanded up to 250-fold, this system provides a basis for the development of novel immunotherapies for non-Hodgkin's lymphoma patients, such as adoptive transfer and tumor vaccine. Autologous T cells from either T-TILs or peripheral blood could be multiply stimulated and expanded in vitro and then transfused into the patient as adoptive immunotherapy. One technical limitation of culturing FL cells in the CD40L system is their growth disadvantage compared with normal cells. In our experience, and in contrast to a former report,44 we were able to induce proliferation in FL cells after CD40 crosslinking but we were not successful in establishing long-term cultures of FL cells in this system as is possible with normal B cells,18 (Schultze et al, manuscript in preparation). This is mainly caused by the outgrowth of normal B cells after long culture periods (>14 days). Therefore, the number of FL cells obtainable might limit the number of stimulations possible to expand FL-specific T cells for adoptive transfer. However, because we theoretically could expand T cells up to 109 cells from diagnostic biopsy samples, we strongly believe that these cell numbers should be easily reached with larger tissue samples available in a therapeutic setting. The advantage of using TCLs over T-cell clones is based on the hypothesis that these lines might recognize a variety of different tumor antigens with different affinities so that the immune response is not only directed against a single antigenic determinant. However, there are several disadvantages to using TCLs compared with T-cell clones. First, the percentage of tumor reactive T cells in the T-cell preparation is not clearly defined and may vary between preparations and between patients. Second, especially for B-cell malignancies, these TCLs might include a subpopulation of T cells, which could provide help to the tumor cells, thus leading to increased tumor growth. Further studies will be necessary to determine whether this is truly the case. The model system used here allows us to further study the interactions of infiltrating T cells with the malignant B cells and the mechanisms of T-cell mediated induction of tumor-cell death, and allows us to further elucidate the role of other signals provided by CD40-FL cells that activated T-TILs in vitro. In addition, by cloning those cytotoxic T cells, we might be able to identify the antigens recognized on B-cell lymphoma cells.
ACKNOWLEDGMENT
We thank Brita Schultze for her support.
Supported by National Institutes of Health Grants No. 5P01, CA66996-01 to L.M.N. J.L.S was supported by the Deutsche Forschungsgemeinschaft (Schu-950/1-1). S. M. is supported by the Carl Duisberg Gesellschaft.
Address reprint requests to Joachim L. Schultze, MD, Division of Hematologic Malignancies, Dana-Farber Cancer Institute, 44 Binney St, D742, Boston, MA 02115.

![Fig. 1. Human FL cells can be killed by allogeneic T cells, but not freshly isolated or IL-2–activated T-TIL. (A) Chromium release assay of allogeneic T cells stimulated three times with CD40-FL cells and cytotoxicity assessed at day 28 after initiation of culture. Results shown are from patient FL4. A total of 2 × 104 FL cells (▪), CD40-FL cells (•), or control targets including FL6 (○), CD40-FL cells from FL6 (▵) were placed in 96-well round-bottom plates and T cells added at E:T ratios of 3:1, 10:1, and 30:1 in a final volume of 0.2 mL. A TCL from one of three healthy donors is depicted. (B) The same TCL was examined at the same time for cytotoxicity against FL4 tumor cells by JAM test. Target cells included FL cells (▪), CD40-FL cells (•), or control targets including CD40-FL cells from FL6 (▵), unstimulated FL6 cells (○), normal B cells (□), or CD40-activated normal B cells (⋄). (C) Blockade of MHC class I (⋄) and MHC class II (□) by MoAbs decreases anti-FL–directed T-cell cytotoxicity, and the combination of both (○) almost abrogates cytotoxicity. T-TILs were isolated from FL4 and analyzed for cytotoxicity against FL4 (D) immediately after isolation, or (E) after culture in IL-2 for 7 days (FL cells [▪], CD40-FL cells [•], or normal CD40-activated B cells [□]). Comparable results were obtained with FL cells from three additional patients.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/10/10.1182_blood.v89.10.3806/3/m_bl_0034f1.jpeg?Expires=1763553832&Signature=rvks7fW6Xu72b6kqi-guZR5nabCtVcv2UhC-CX9cqTw5EEqxE1qG2MPJnFkh5zBFFclQ~vZ~vwxvy0N5TuJFQK8gLXdamTXTxEDbsC5mz6iak9KvWfRNLhMpbaAYXqfXqCF8nytxawGIgcZmIuWmAMSTK03h38uXaGCcxC9b6PU1E0sx0uEUTxHLB4DkBrrBGQ2BppEg-JFey8jXpiFah2ei7YZBpHGNoGWJmjAhxbQsPdpj2YrnUXINtAD~Amp2EbmGxCo5iCbVbwIPRfX0g~rzqAvnyN8F0g9OFhtHbg3pm4ZVCySeu6NHG-DND5y5vh0FiiY7s2IjKBhJwfvgeQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal