Abstract
Most patients requiring allogeneic bone marrow transplant (allo-BMT) do not have an HLA-matched sibling donor. A phenotypically matched unrelated donor graft has been made available for approximately 50% of Caucasians and less than 10% of ethnic and racial minorities in need. However, almost all patients have a readily available partially mismatched related donor (PMRD). We summarize our experience with 72 patients who ranged from 1 to 50 years of age (median, 16 years) and who were recipients of a PMRD allo-BMT from haploidentical family members following conditioning therapy using total body irradiation (TBI) and multiagent, high-dose chemotherapy. T-cell depletion and post-BMT immunosuppression were combined for graft-versus-host disease (GVHD) prophylaxis. The probability of engraftment was 0.88 at 32 days. Six of 10 patients who failed to engraft achieved engraftment after secondary transplant. Grade II to IV acute GVHD was seen in 9 of 58 (16%) evaluable patients; extensive chronic GVHD was seen in 4 of 48 (8%) evaluable patients. There was a statistically significant difference in 2-year survival probability between low-risk and high-risk patients (0.55 v 0.27, P = .048). Prognostic factors that affected outcomes in multivariate analysis were (1) a lower TBI dose and 3-antigen rejection mismatch decreased stable engraftment (P = .005 and P = .002, respectively); (2) a higher T-cell dose increased acute GVHD (P = .058); (3) a higher TBI dose increased chronic GVHD (P = .016); and (4) a high-risk disease category increased treatment failure from relapse or death (P = .037). A PMRD transplant can be performed with acceptable rates of graft failure and GVHD. Using sequential immunomodulation, the disease status at the time of transplant is the only prognostic factor significantly associated with long-term successful outcome after PMRD allo-BMT. When allogeneic rather than autologous BMT is indicated, progression in disease status before transplant can be avoided using a PMRD with equal inclusion of all ethnic or racial groups.
BONE MARROW transplantation (BMT) from genotypically HLA-matched siblings has improved long-term survival in patients with hematologic malignancies and marrow failure syndromes.1 However, more than 70% of patients who could benefit from allogeneic-BMT (allo-BMT) do not have a matched sibling donor (MSD). Attention has turned, therefore, to alternative donors2-5 primarily, partially mismatched related donors (PMRD),6-9 and phenotypically matched unrelated donors (PMUD).10-16 The chance of receiving a PMUD varies with the race of the patient, ranging from approximately 50% for Caucasians to less than 10% for ethnic minorities, and often requires waiting months to identify the donor and obtain the graft.17 However, allowing for unusual circumstances in which no biological relative is available, there is a greater than 90% chance to promptly identify a haploidentical donor within the family.
Use of alternative donors for allo-BMT involves crossing histocompatibility barriers and, therefore, carries a greater risk of nonengraftment,8,12,13,18,19 severe acute and chronic graft-versus-host disease (aGVHD, cGVHD),7,8,11-14,20-23 and prolonged immunodysregulation increasing the risk of fatal infections and lymphoproliferative disorders.24-26 We sought to reduce these complications through sequential immunomodulation of the recipient, donor marrow, and resultant chimera when using a readily available PMRD for patients with malignant and nonmalignant hematologic conditions.
MATERIALS AND METHODS
Clinical Protocol
Between February 1993 and November 1994, 72 patients who lacked an MSD underwent PMRD allo-BMT on a protocol approved by the Richland Memorial Hospital Institutional Review Board (Table 1). Among patients with leukemia, those classified as low risk included patients with acute leukemia in first or second complete remission and chronic myeloid leukemia (CML) in primary chronic phase. Patients classified as high risk had acute leukemia in greater than second remission or in relapse or CML in accelerated or blastic phase. Patients with marrow failure syndromes were considered high risk if they had HLA antibodies, abnormal cytogenetics, or disease for more than 6 months.
Diagnosis, Risk Category, and HLA Disparity at Time of PMRD allo-BMT
| Patients . | Low Risk . | High Risk . |
|---|---|---|
| n = 72 | 20 (28%)* | 52 (72%) |
| Diagnosis | ||
| ALL | 10 (14%) | 18 (25%) |
| AML | 3 (4%) | 16 (22%) |
| CML/CLL | 5 (7%) | 14 (19%) |
| SAA/MDS | 2 (3%) | 4 (6%) |
| Disease-status linked to degree of HLA disparity | ||
| Donor mismatch (rejection direction)† | ||
| 1 antigen (n = 13) | 4 (6%) | 9 (13%) |
| 2 antigen (n = 35) | 13 (18%) | 22 (31%) |
| 3 antigen (n = 24) | 3 (4%) | 21 (29%) |
| Recipient mismatch (GVHD direction)‡ | ||
| 1 antigen (n = 11) | 5 (7%) | 6 (8%) |
| 2 antigen (n = 30) | 9 (13%) | 21 (29%) |
| 3 antigen (n = 31) | 6 (8%) | 25 (35%) |
| Patients . | Low Risk . | High Risk . |
|---|---|---|
| n = 72 | 20 (28%)* | 52 (72%) |
| Diagnosis | ||
| ALL | 10 (14%) | 18 (25%) |
| AML | 3 (4%) | 16 (22%) |
| CML/CLL | 5 (7%) | 14 (19%) |
| SAA/MDS | 2 (3%) | 4 (6%) |
| Disease-status linked to degree of HLA disparity | ||
| Donor mismatch (rejection direction)† | ||
| 1 antigen (n = 13) | 4 (6%) | 9 (13%) |
| 2 antigen (n = 35) | 13 (18%) | 22 (31%) |
| 3 antigen (n = 24) | 3 (4%) | 21 (29%) |
| Recipient mismatch (GVHD direction)‡ | ||
| 1 antigen (n = 11) | 5 (7%) | 6 (8%) |
| 2 antigen (n = 30) | 9 (13%) | 21 (29%) |
| 3 antigen (n = 31) | 6 (8%) | 25 (35%) |
Abbreviations: n, number of patients; ALL, acute lymphoblastic leukemia; AML, acute myelogenous leukemia; CML, chronic myelogenous leukemia; CLL, chronic lymphocytic leukemia; SAA, severe aplastic anemia; MDS, myelodysplastic syndrome; GVHD, graft-versus-host disease.
Proportion of the entire patient sample.
P = .035 (one-tailed) for the likelihood of receiving a 3-Ag rejection mismatched graft for high-risk versus low-risk category.
P = .131 (one-tailed) for likelihood of receiving a 3-Ag GVHD mismatched graft for high-risk versus low-risk category.
The conditioning regimen and GVHD prophylaxis administered to all patients (except as noted for nonmalignant disease) are described in Fig 1. Patients were housed in a positive-pressure high efficiency particulate air (HEPA)-filtered BMT unit. All patients received granulocyte colony-stimulating factor (G-CSF ) from day +1 until the white blood cell (WBC) count exceeded 5,000/μL. Prophylactic and therapeutic antimicrobial therapy included broad-spectrum antibiotics, intravenous Ig, inhaled Pentamidine (or trimethoprim-sulfamethoxazole, if required), and antiviral and antifungal agents as indicated.
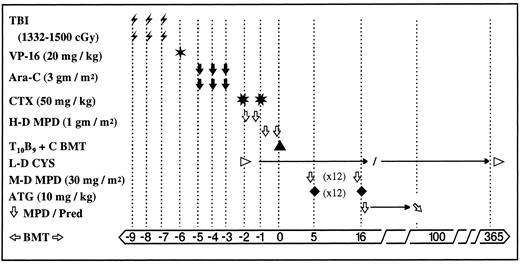
Conditioning therapy and GVHD prophylaxis in 72 recipients of PMRD allo-BMT. In an effort to improve engraftment, the total dose of TBI was increased from 1,332 cGy (administered to the first 45 patients) to 1,500 cGy (administered to the subsequent 27 patients). Cyclosporin and prednisone were gradually tapered and discontinued in patients free of acute and/or chronic GVHD. Abbreviations: TBI , total body irradiation administered twice daily via AP/PA fields with ∼50% pulmonary transmission with lung shielding and electron beam boosting of chest wall, and testicles (ALL, biphenotypic) using an instantaneous dose rate of 13 to 22 cGy/min and interfraction interval of ∼8 hours; VP-16 ✶, etoposide administered once (omitted for nonmalignant disease); Ara-C ⬇, cytosine arabinoside administered twice daily × 6 doses; CTX ✳, cyclophosphamide administered daily × 2 doses; H-D MPD ⇩, high-dose methylprednisolone administered every 12 hours × 4 doses; T10B9 + C BMT ▴, marrow graft T-cell depleted with T10B9 and complement, L-D CYS ▹, low-dose cyclosporin started day −1 at 3 mg/kg constant infusion and maintained at levels between 100 to 200 as measured by monoclonal antibody technique, switched to orally after day +21 and weaned gradually through the first year post-BMT; M-D MPD ⇩, moderate-dose methylprednisolone administered before ATG; ATG ♦, antithymocyte globulin administered daily × 12 doses on day +5 to +16; ⇩ MPD/Pred; steroid dose tapered 10% weekly and switched to prednisone orally after day +21; ❁BMT➭, days before and after BMT; →, expanded time period.
Conditioning therapy and GVHD prophylaxis in 72 recipients of PMRD allo-BMT. In an effort to improve engraftment, the total dose of TBI was increased from 1,332 cGy (administered to the first 45 patients) to 1,500 cGy (administered to the subsequent 27 patients). Cyclosporin and prednisone were gradually tapered and discontinued in patients free of acute and/or chronic GVHD. Abbreviations: TBI , total body irradiation administered twice daily via AP/PA fields with ∼50% pulmonary transmission with lung shielding and electron beam boosting of chest wall, and testicles (ALL, biphenotypic) using an instantaneous dose rate of 13 to 22 cGy/min and interfraction interval of ∼8 hours; VP-16 ✶, etoposide administered once (omitted for nonmalignant disease); Ara-C ⬇, cytosine arabinoside administered twice daily × 6 doses; CTX ✳, cyclophosphamide administered daily × 2 doses; H-D MPD ⇩, high-dose methylprednisolone administered every 12 hours × 4 doses; T10B9 + C BMT ▴, marrow graft T-cell depleted with T10B9 and complement, L-D CYS ▹, low-dose cyclosporin started day −1 at 3 mg/kg constant infusion and maintained at levels between 100 to 200 as measured by monoclonal antibody technique, switched to orally after day +21 and weaned gradually through the first year post-BMT; M-D MPD ⇩, moderate-dose methylprednisolone administered before ATG; ATG ♦, antithymocyte globulin administered daily × 12 doses on day +5 to +16; ⇩ MPD/Pred; steroid dose tapered 10% weekly and switched to prednisone orally after day +21; ❁BMT➭, days before and after BMT; →, expanded time period.
When patients required regrafting, the same or an alternative PMRD donor was used. Second grafts were processed using a single incubation with complement to limit the efficiency of T-cell depletion effectively increasing the T-cell dose. Reconditioning therapy included the use of cyclophosphamide, antithymocyte globulin, and methylprednisolone. Patients who developed greater than grade I acute GVHD were treated with high-dose pulsed methylprednisolone (MPD; 500 mg/m2 every 12 hours for 2 doses and repeated every 48 to 72 hours for up to 4 pulses). Second-line therapy for recurrence of GVHD included a second course of antithymocyte globulin (ATG) and/or azathioprine.
Donors
Family members were assessed for degree of mismatch by HLA serologic typing.27 When necessary, molecular techniques were used to define class II antigens (Ag).28 Donors were prioritized on the basis of the greatest HLA matching, cytomegalovirus (CMV) seronegativity for seronegative recipients, younger age, same sex, nonparity, and better health.29
Marrow Graft Preparation
Donors were harvested by aspirating 4.5 to 6 × 108 nucleated cells per kilogram of recipient body weight. Grafts were depleted of mature T lymphocytes using T10B9.1A-31 (T10B9 courtesy of Dr John Thompson, University of Kentucky, Lexington, KY), an IgM murine monoclonal antibody (MoAb) directed against the αβ chains of the T-cell receptor heterodimer, and rabbit complement as described previously.30 The degree of T-cell depletion was assessed by limiting dilution analysis.31 Recovery of hematopoietic precursors was assessed by predepletion and postdepletion colony-forming unit–granulocyte-macrophage (CFU-GM) and CD34+ cell assays. Marrow grafts were cultured for possible microbial contaminants.
Statistical Design
This study was conducted as a single-arm prospective trial in which consecutive patients who met inclusion criteria were entered. Data were collected prospectively on case report forms and retrospectively by medical record review. Graft characteristics were calculated excluding second/third transplants. Patients were monitored for toxicity, engraftment, acute and chronic GVHD, relapse, and survival.
Endpoints.Toxicity was graded according to standard criteria for organ systems. All patients were considered evaluable for engraftment. The day of engraftment was taken as the first of three consecutive days on which the WBC count was 1,000/μL. Patients were considered to have achieved stable engraftment if they maintained their blood counts with DNA evidence of donor-derived hematopoiesis. Patients who failed to establish engraftment within 30 days of BMT, as evidenced by a WBC count less than 200/μL and absence of donor-specific DNA properties, were considered to have graft failure. Achievement of a WBC count of 500/μL with subsequent decline was criteria for rejection, which was considered to have occurred if a corresponding marrow was mostly acellular. Patients were considered evaluable for aGVHD if they engrafted successfully and were considered evaluable for cGVHD if they also survived at least 80 days post-BMT. Acute GVHD was graded according to accepted criteria during the first 100 days post-BMT.32 Time-to-GVHD was defined as the time interval from allo-BMT to onset of any grade of aGVHD. Patients were monitored for cGVHD beginning at day 80 post-BMT, and cases were graded according to accepted definitions of limited and extensive disease.33 Tissue biopsies were obtained to distinguish toxicity, infection, and GVHD whenever possible. All leukemia patients were considered evaluable for relapse and disease-free survival.
Statistical methods.Distributions for time-to-engraftment, time-to-acute/chronic GVHD, time-to-relapse, survival, and disease-free survival (DFS) were evaluated using the method of Kaplan and Meier.34 Such variables were measured beginning on day 0. Values were censored for engraftment at time of death if the patient did not engraft, for GVHD at time of death or at time of second transplant, and for survival and relapse at last follow-up. Comparisons were implemented using the log-rank test. Cox proportional hazards multivariate regression was used to investigate relationships with prognostic variables including patient age, donor age, racial group, disease status at transplant, sex mismatch, CMV status, rejection and GVHD Ag disparities, total body irradiation (TBI) dose, acute/chronic GVHD, and chronic leukemia/other diagnosis. For second transplants, values for prognostic variables were those associated with the initial transplant, except that all donors were considered jointly for CMV seropositivity. Frequencies for categorical variables were compared using Fisher's exact test. Unless otherwise specified, all reported P values are for two-sided hypothesis tests. Confidence intervals (CI) were computed using standard techniques and reflect a confidence coefficient of 95% in each case.
RESULTS
Patient-Donor Characteristics
Twenty patients were considered low risk and 52 were high risk at the time of transplant (Table 1). Frequencies of HLA disparities in the donor and recipient show a positive association between high-risk disease category and the use of a 3-Ag mismatched graft. Table 2 shows characteristics of recipients and donors.
Recipient and Donor Characteristics
| Parameters . | Values* . |
|---|---|
| Median age in yr (range) | |
| Recipient | 16 (1-50) |
| Donor | 27 (4-55) |
| Recipient/donor CMV status | |
| Seropositive/seropositive | 34 (47%) |
| Seropositive/seronegative | 12 (17%) |
| Seronegative/seropositive | 13 (18%) |
| Seronegative/seronegative | 13 (18%) |
| Recipient race | |
| Caucasian | 50 (69%) |
| African American | 16 (22%) |
| Asian | 3 (4%) |
| Indian | 3 (4%) |
| Sex | |
| Recipient: M/F | 49 (68%)/23 (32%) |
| Donor: M/F | 33 (46%)/39 (54%) |
| Donor-recipient sex pairs | |
| Same: F → F/M → M | 15 (21%)/25 (35%) |
| Opposite: F → M/M → F | 8 (11%)/24 (33%) |
| Donor relationship to recipient | |
| Parent | 32 (44%) |
| Sibling | 27 (37%) |
| Child | 11 (16%) |
| Cousin | 2 (2%) |
| Parameters . | Values* . |
|---|---|
| Median age in yr (range) | |
| Recipient | 16 (1-50) |
| Donor | 27 (4-55) |
| Recipient/donor CMV status | |
| Seropositive/seropositive | 34 (47%) |
| Seropositive/seronegative | 12 (17%) |
| Seronegative/seropositive | 13 (18%) |
| Seronegative/seronegative | 13 (18%) |
| Recipient race | |
| Caucasian | 50 (69%) |
| African American | 16 (22%) |
| Asian | 3 (4%) |
| Indian | 3 (4%) |
| Sex | |
| Recipient: M/F | 49 (68%)/23 (32%) |
| Donor: M/F | 33 (46%)/39 (54%) |
| Donor-recipient sex pairs | |
| Same: F → F/M → M | 15 (21%)/25 (35%) |
| Opposite: F → M/M → F | 8 (11%)/24 (33%) |
| Donor relationship to recipient | |
| Parent | 32 (44%) |
| Sibling | 27 (37%) |
| Child | 11 (16%) |
| Cousin | 2 (2%) |
Abbreviation: CMV, cytomegalovirus.
Values are rounded to nearest whole number.
Graft Characteristics
The median degree of T-cell depletion was 1.8 logs (range, 1.2 to 2.8). Patients received a median of 1.5 × 108 per kilogram of mononuclear cells (range, 0.6 to 3.7) and 7.5 × 104 per kilogram of T cells (range, 0.02 to 1.61). Median CFU-GM and CD34+ doses were 7.0 × 104 per kilogram (range, 1.3 to 40.5) and 1.36 × 106 per kilogram (range, 0.14 to 8.92), respectively. Median CFU-GM dose in patients who engrafted after the first BMT was 7.26 × 104 versus 5.11 × 104 in those who did not (P = .391), whereas median CD34+ dose was 1.3 × 106 and 1.51 × 106, respectively (P = .229). Only T-cell dose was shown to have a significant effect on transplant outcomes in multivariate analysis (Table 3).
Prognostic Factors Associated With Outcomes Following PMRD Allo-BMT
| Outcome . | Prognostic Factor3-150 . | Reference . | Risk Level . | RR3-151 . | 95% CI . | P Value . |
|---|---|---|---|---|---|---|
| . | . | Level . | (unfavorable) . | . | . | . |
| . | . | (favorable) . | . | . | . | . |
| WBC 1,000 engraftment3-152 | TBI dose | 1,500 cGy | 1,332 cGy | 0.33 | (0.18, 0.59) | <.001 |
| Rejection mismatch | <3-Ag | 3-Ag | 0.29 | (0.14, 0.57) | <.001 | |
| Degree of TCDρ | Less | More | 0.45 | (0.21, 0.97) | .043 | |
| Stable engraftment∥ | TBI dose | 1,500 cGy | 1,332 cGy | 0.46 | (0.27, 0.80) | .005 |
| Rejection mismatch | <3-Ag | 3-Ag | 0.38 | (0.29, 0.70) | .002 | |
| Acute GVHD III-IV3-154 | T-cell doseρ | Lower | Higher | 7.17 | (0.94, 54.98) | .058 |
| Chronic GVHD3-154 | TBI dose | 1,332 cGy | 1,500 cGy | 3.54 | (1.26, 9.89) | .016 |
| Relapse3-154 | Disease category | Low risk | High risk | 3.57 | (1.04, 12.20) | .043 |
| Treatment failure¶# | Disease category | Low risk | High risk | 2.17 | (1.05, 4.51) | .037 |
| Death3-154 | Disease category | Low risk | High risk | 2.10 | (1.01, 4.35) | .046 |
| Outcome . | Prognostic Factor3-150 . | Reference . | Risk Level . | RR3-151 . | 95% CI . | P Value . |
|---|---|---|---|---|---|---|
| . | . | Level . | (unfavorable) . | . | . | . |
| . | . | (favorable) . | . | . | . | . |
| WBC 1,000 engraftment3-152 | TBI dose | 1,500 cGy | 1,332 cGy | 0.33 | (0.18, 0.59) | <.001 |
| Rejection mismatch | <3-Ag | 3-Ag | 0.29 | (0.14, 0.57) | <.001 | |
| Degree of TCDρ | Less | More | 0.45 | (0.21, 0.97) | .043 | |
| Stable engraftment∥ | TBI dose | 1,500 cGy | 1,332 cGy | 0.46 | (0.27, 0.80) | .005 |
| Rejection mismatch | <3-Ag | 3-Ag | 0.38 | (0.29, 0.70) | .002 | |
| Acute GVHD III-IV3-154 | T-cell doseρ | Lower | Higher | 7.17 | (0.94, 54.98) | .058 |
| Chronic GVHD3-154 | TBI dose | 1,332 cGy | 1,500 cGy | 3.54 | (1.26, 9.89) | .016 |
| Relapse3-154 | Disease category | Low risk | High risk | 3.57 | (1.04, 12.20) | .043 |
| Treatment failure¶# | Disease category | Low risk | High risk | 2.17 | (1.05, 4.51) | .037 |
| Death3-154 | Disease category | Low risk | High risk | 2.10 | (1.01, 4.35) | .046 |
Abbreviations: PMRD, partially mismatched related donor; allo-BMT, allogeneic bone marrow transplant; RR, relative risk; WBC, white blood cell; TBI, total body irradiation; Ag, antigen; TCD, T-cell depletion; GVHD, graft-versus-host disease.
Only prognostic factors that were significant at the 0.05 level in multivariate analysis are shown.
Relative risk (RR) applies to the unfavorable level of the prognostic factor compared with the favorable level used as the reference group.
Engraftment to WBC count of ≥1,000/μL uses data from initial transplants only. RR < 1 indicates lower probability of engraftment and/or delayed engraftment for patients with an unfavorable level of the given prognostic factor.
ρ Degree of T-cell depletion and T-cell dose were entered as continuous variables in log10 units.
∥ Stable engraftment refers to achievement of WBC count of ≥1,000/μL using data for all patients receiving one or more transplants. RR < 1 indicates lower probability of engraftment and/or delayed engraftment for patients with an unfavorable level of the given prognostic factor.
RR > 1 indicates higher risk of the adverse event for patients with an unfavorable level of the indicated prognostic factor.
Treatment failure refers to relapse or death.
Engraftment
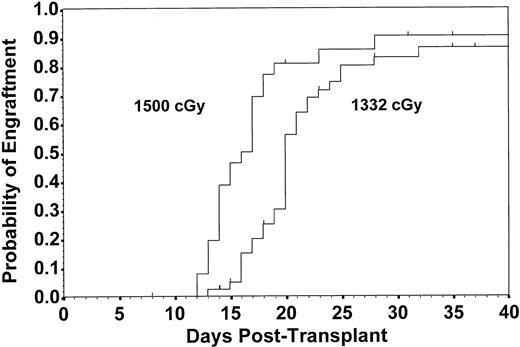
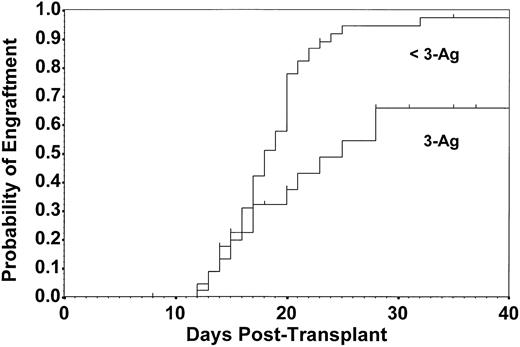
Fifty-nine patients established successful engraftment after initial transplantation at a median of 20 days, resulting in an estimate of the probability of engraftment at 32 days of 0.88 (CI, 0.80 to 0.97). Patients receiving the higher TBI dose engrafted more quickly than those receiving the lower dose (median, 16.5 v 20.0 days, respectively; P = .003; Fig 2). Engraftment rates did not differ by sex mismatch between donor and recipient (P = .880). Patients receiving 3-Ag rejection mismatched grafts had diminished engraftment compared with those with less than 3-Ag rejection mismatched grafts (P = .002; Fig 3). Of those who failed to engraft or maintain engraftment, 10 received a second transplant, and 6 of these engrafted. Thus, a total of 62 patients (87%) achieved stable engraftment. The estimate of the probability of engraftment by day 70 for all patients was 0.96 (CI, 0.90 to 1.00).
Estimated probability of engraftment to WBC count of 1,000/μL for patients receiving 1,332 cGy (n = 46) and 1,500 cGy (n = 26) of TBI. Patients receiving the higher TBI dose experienced earlier engraftment with an estimated probability of engraftment by 32 days of 0.90 compared with 0.86 in patients receiving 1,332 cGy (P = .003). Data are derived from first transplants only.
Estimated probability of engraftment to WBC count of 1,000/μL for patients receiving 1,332 cGy (n = 46) and 1,500 cGy (n = 26) of TBI. Patients receiving the higher TBI dose experienced earlier engraftment with an estimated probability of engraftment by 32 days of 0.90 compared with 0.86 in patients receiving 1,332 cGy (P = .003). Data are derived from first transplants only.
Estimated probability of engraftment to WBC count of 1,000/μL for patients with 3-Ag rejection mismatch (n = 24) and those with less than 3-Ag rejection mismatch (n = 48). Delayed engraftment and lower probability of engraftment by 32 days occurred in the 3-Ag mismatched group (P = .002) in which the median time to engraftment was 25 days versus 18 days in the less than 3-Ag mismatched group. Data are derived from first transplants only.
Estimated probability of engraftment to WBC count of 1,000/μL for patients with 3-Ag rejection mismatch (n = 24) and those with less than 3-Ag rejection mismatch (n = 48). Delayed engraftment and lower probability of engraftment by 32 days occurred in the 3-Ag mismatched group (P = .002) in which the median time to engraftment was 25 days versus 18 days in the less than 3-Ag mismatched group. Data are derived from first transplants only.
Acute and Chronic GVHD
Grade I to IV aGVHD occurred in 18 of 58 (31%) evaluable patients. Grade II to IV aGVHD was seen in 9 (16%) patients between days 15 and 38 (median, 20 days), whereas grades III to IV occurred in 4 (7%) patients. The estimated risk of greater than grade I aGVHD by 100 days was 0.16 (CI, 0.06 to 0.25), and 0.07 for greater than grade II (CI, 0.00 to 0.14; Fig 4). There was not a statistically significant difference in probabilities of aGVHD among patients receiving 1-, 2-, or 3-Ag GVHD mismatched grafts, the number of cases being 2 of 11, 3 of 25, and 4 of 22, respectively, for greater than grade I (P = .852), and 1 of 11, 1 of 25, and 2 of 22, respectively, for greater than grade II (P = .771).
Estimated risks of grade II to IV (9 cases) and grade III to IV (4 cases) aGVHD among 58 evaluable patients; probability of aGVHD by 100 days was 0.16 and 0.07, respectively.
Estimated risks of grade II to IV (9 cases) and grade III to IV (4 cases) aGVHD among 58 evaluable patients; probability of aGVHD by 100 days was 0.16 and 0.07, respectively.
Chronic GVHD occurred in 17 (35%) of 48 evaluable patients 3 to 17 months post-BMT, with an estimated risk of 0.51 (CI, 0.33 to 0.68) within 18 months. Four patients developed extensive cGVHD, all with onset times of less than 6 months, resulting in a 6-month risk of 0.10 (CI, 0.01 to 0.20; Fig 5).
Estimated risks of limited and extensive chronic GVHD. By 2 years, the cumulative risks were 44% and 10%, respectively.
Estimated risks of limited and extensive chronic GVHD. By 2 years, the cumulative risks were 44% and 10%, respectively.
Complications and Regimen-Related Toxicity
Nonhematologic grade 4 toxicity involved the gastrointestinal (25%), hepatic (19%) renal (10%), pulmonary (8%), cardiovascular (6%), and central nervous (4%) systems. There was a 7% risk of regimen-related early (<60 days) mortality, which occurred exclusively in high-risk patients.
Survival
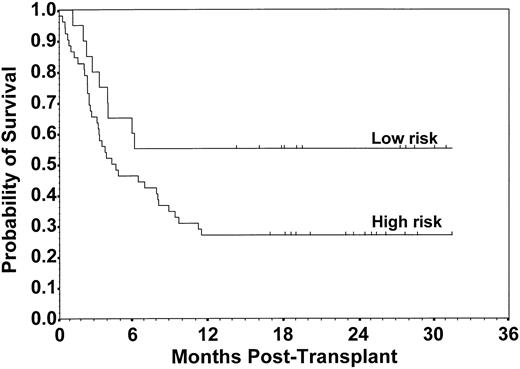
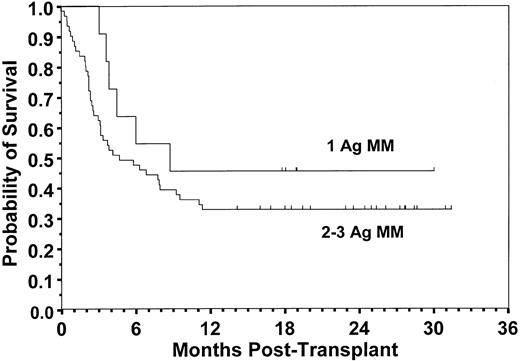
Of 72 patients, 25 are surviving at a median follow-up of 24 months (range, 14 to 31 months). The overall median survival was 5.9 months (CI, 3.4 to 9.6), with an estimated 2-year survival probability of 0.35 (CI, 0.24 to 0.46). There was a statistically significant difference between survival distributions for risk groups in which there were 9 deaths among 20 low-risk patients compared with 38 of 52 high-risk patients with a 2-year survival probability of 0.55 (CI, 0.33 to 0.77) and 0.27 (CI, 0.15 to 0.39), respectively (P = .048; Fig 6). The relative risk (RR) of death for high-risk versus low-risk patients unadjusted for other factors was 2.0 (CI, 1.0 to 4.2; P = .053). An analysis to compare patients who received a 1-Ag GVHD mismatched graft with those who received a 2- or 3-Ag GVHD mismatched graft showed no statistically significant difference in the probability of survival (0.45 v 0.33, respectively; P = .272; Fig 7). There was also not a significant difference in survival according to TBI dose (P = .450), patient age ≤18 versus >18 years (P = .974), diagnosis (P = .994), racial group (P = .091), sex mismatch (P = .538), or CMV seropositivity (P = .860). Among surviving patients, 23 of 25 had Karnofsky clinical performance scores ≥90. The primary cause of death seen most frequently was relapse, occurring in 16 of 47 (34%) patients, followed by engraftment failure (n = 10), fungal infection (n = 6), nonfungal infection (n = 6), interstitial pneumonitis (n = 3), GVHD (n = 2), Epstein-Barr virus lymphoma (n = 1), veno-oclusive disease (n = 1), and other (n = 2).
Probability of survival for patients with low-risk and high-risk disease status. Estimated survival at 2 years was 0.55 and 0.27, respectively (P = .048).
Probability of survival for patients with low-risk and high-risk disease status. Estimated survival at 2 years was 0.55 and 0.27, respectively (P = .048).
Probability of survival for recipients of a 1-Ag versus 2- or 3-Ag GVHD mismatched graft. Estimated survival at 2 years was 0.45 and 0.33, respectively (P = .272).
Probability of survival for recipients of a 1-Ag versus 2- or 3-Ag GVHD mismatched graft. Estimated survival at 2 years was 0.45 and 0.33, respectively (P = .272).
Relapse
Twenty-two of 68 evaluable patients (32%) relapsed, which occurred before 16 months in all but 1 patient, who relapsed at 27 months; estimated risk of relapse at 2 years was 0.47 (CI, 0.31 to 0.62). Three of 19 evaluable patients in the low-risk group (16%) relapsed, compared with 19 of 49 in the high-risk group (39%), with estimated risks of relapse at 2 years of 0.21 and 0.58, respectively. Thus, there was a significant difference between risk groups (P = .031), but not between acute lymphoblastic leukemia (11/28), acute myelogenous leukemia (6/18), and chronic myelogenous leukemia (3/17) patients (P = .495).
DFS
Of 67 evaluable patients, 20 (30%) were alive and free of disease at median follow-up of 21.5 months. Median DFS was 4.2 months (CI, 3.0 to 6.2), and estimated 2-year DFS probability was 0.31 (CI, 0.20 to 0.42). There was a significant difference in 2-year DFS between low-risk and high-risk groups (0.53 and 0.23, respectively; P = .032). There was not a significant difference in DFS by diagnosis (P = .964).
Multivariate Analysis
Table 3 shows the results of multivariate analysis. Relative risk values less than 1.0 correspond to lower probability of engraftment, and values greater than 1.0 correspond to increased rates of aGVHD, cGVHD, relapse, treatment failure, and death. Lower TBI dose, 3-Ag rejection mismatch, and higher degree of T-cell depletion adversely affected engraftment. Higher T-cell dose was associated with severe aGVHD and higher TBI dose with cGVHD. High-risk disease category increased treatment failure from relapse and death; other factors were not significant after adjustment for risk category.
DISCUSSION
Almost a decade ago, for patients with leukemia it was shown that major HLA barriers would significantly interfere with success of allo-BMT from other than a 1-Ag mismatched haploidentical familial donor.7,18,21 However, in their series comparing PMRD and MSD recipients, Beatty et al7 found no difference in survival when patients were transplanted in remission. Nonetheless, rates of rejection and severe aGVHD, both of which carried a high mortality risk, were regarded as unacceptable, and transplants from a PMRD with more than one major HLA disparity were not generally recommended. Therefore, the attention of most investigators turned to using PMUDs. Unfortunately, this approach is costly, time-consuming, and has been attained for less than half of the patients who seek an alternative donor, usually excluding patients from ethnic and racial minorities. Our study defines techniques that circumvent major HLA barriers, thus expanding access to allo-BMT for almost all patients. We estimate that greater than 90% of patients will have a haploidentical family member who is immediately available.
During this past decade, investigations into new approaches for immunomodulation after PMRD transplantation have achieved higher rates of engraftment and control of aGVHD.9,35-37 Henslee-Downey et al9 explored a strategy that combined ex vivo and in vivo T-cell lysis using two experimental MoAb products targeted to T-cell–specific antigens for sequential immunotherapy. For marrow T-cell depletion, T10B9 was used,38 which was shown by Ash et al39 to be effective in reducing the risk of severe aGVHD after PMUD transplants. An immunotoxin, H65-RTA,40 was used for in vivo T-cell lysis. Patients experienced a 93% engraftment rate and a 36% risk of grade II to IV aGVHD. In our larger study, T10B9 was again used for T-cell depletion, ATG replaced H65-RTA, and low-dose cyclosporin was added in an effort to improve outcome using full haplodisparate PMRDs.
Engraftment kinetics significantly improved after more intensive host conditioning from an increase in the dose of TBI. This may be the result of creating a more empty marrow space and/or decreasing immunologic resistance to engraftment. We did not detect a significant difference in engraftment based on the CD34+ cell dose, contrary to a report from Aversa et al36 showing improved engraftment in haploidentical recipients receiving T-cell–depleted, G-CSF–mobilized peripheral blood preparations in combination with T-cell–depleted bone marrow. However, it is possible that our patients benefited from a similar mechanism through systemic administration of G-CSF immediately after graft infusion. Both studies indicate the need for sufficient immunologic ablation of the recipient to achieve successful engraftment, the toxicity of which must be carefully evaluated and constrained.
In our study, a more highly T-cell–depleted marrow graft was associated with diminished engraftment in multivariate analysis, which agrees with other studies41,42 and cautions against potent depletion methods. Consistent with Anasetti et al18 and others,8 our data showed that the degree of major HLA mismatch for rejection also had an adverse effect on engraftment. Because there are often multiple familial donors available for any given patient, one could preferentially choose donors who exhibit the least mismatch and, when possible, are homozygous for major HLA antigens.
Moderate-to-severe aGVHD was reduced in our study to an estimated risk of 16%, which is lower than that often published for major HLA “compatible” grafts from a PMUD or even from an MSD, which ranges between 33% to 78% and 23% to 42%, respectively.11-16,20-22,39,43-48 Furthermore, the aGVHD rate was not associated with the degree of recipient major HLA mismatch, which differs from previous experience with alternative donors.7,8,39,43 This could be caused by the low overall incidence of aGVHD as a result of highly effective prophylaxis. Another factor may include the role that nonmajor HLA and/or minor histocompatibility antigens plays in the genesis of aGVHD.49 Most PMUDs are not molecularly identical for major HLAs50 and are unlikely to share other histocompatibility antigens, whereas PMRDs share genotypic major histocompatibility complex (MHC) haplotype identity, including HLA variant and MHC non-HLAs. In addition, PMRDs may share genotypic identity for minor histocompatibility antigens, which could result in a broader immunologic compatibility.5,51,52 Our results challenge the requisite for major HLA “matching” to define an acceptable allogeneic alternative donor.
In multivariate analysis, a greater number of T cells in the donor marrow graft was associated with a higher risk of severe aGVHD, supporting the concept that minimizing the dose of T cells administered at the time of graft infusion is an important step in the control of aGVHD. It remains a challenge to produce a graft with optimal quantitative and qualitative properties that will ensure engraftment while minimizing the risk of severe aGVHD. Our study suggests that depletion should not exceed approximately 2.8 logs if a high probability of engraftment is to be attained and that post-BMT immunotherapy must then be used for control of aGVHD.9 For this purpose, the combination of lympholytic and suppressive agents used in our study was successful.
The majority of our patients were in a high-risk disease category, which, in multivariate analysis, was the only significant prognostic factor associated with treatment failure from relapse or death. This association is well established after all types of BMT using either autologous or allogeneic stem cells. Avoidance of haploidentical donors has been based primarily on concerns regarding transplant-related mortality, and yet the leading cause of death in our patients was relapse, particularly among those at high risk. One might ask whether the successful avoidance of aGVHD activity in our patients might have interfered with a graft-versus-leukemia effect shown to be operative in the cure of leukemic, allo-BMT recipients.53 However, the risk of relapse falls within the range reported from large series of similar-risk patients.54 Estimates of 2-year survival and DFS, based on disease-risk category, are comparable to results after either PMUD11-16,20,22,39,43-46 or MSD BMT.54-58
Prospective randomized trials comparing PMRDs, PMUDs, and cord blood stem cells59-62 are needed to determine whether there is a preference among available alternative donor sources. In the absence of differences, pragmatic and logistic issues surface, those being primarily cost and time. In this respect, the use of a PMRD becomes advantageous by creating immediate donor availability and reducing costs associated with obtaining the graft, including substantial federal and private funding required to develop and maintain donor registries and banks. The timing of transplant, preferably when patients are in remission, is the one factor most likely to improve long-term outcome. The ability to use haploidentical family donors provides near-unrestricted access to allo-BMT.
ACKNOWLEDGMENT
The authors thank the BMT program staff, nursing service, and laboratory team for the dedicated and skilled care given to the patients and their families. We are indebted to the clinical research data management team, biostatistics group, and secretarial staff for their invaluable service in the preparation of this manuscript.
Supported in part by grants from the Center for Cancer Treatment and Research Board of Directors and the South Carolina Cancer Center.
Address reprint requests to P. Jean Henslee-Downey, MD, Division of Transplantation Medicine, 7 Richland Medical Park, Columbia, SC 29203.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal