Abstract
Increasingly, allogeneic and even more often autologous bone marrow transplants are being done to correct a wide variety of diseases. In addition, autologous marrow transplants potentially provide an opportune means of delivering genes in transfected, engrafting stem cells. However, despite its widespread clinical use and promising gene therapy applications, relatively little is known about the mechanisms of engraftment in marrow transplant recipients. This is especially so in the nonablated recipient setting. Our data show that purified lineage negative rhodamine 123/Hoechst 33342 dull transplanted hematopoietic stem cells engraft into the marrow of nonablated syngeneic recipients. These cells have multilineage potential, and maintain a distinct subpopulation with “stem cell” characteristics. The data also suggests a spatial localization of stem cell “niches” to the endosteal surface, with all donor cells having a high spatial affinity to this area. However, the level of stem cell engraftment observed following a transplant of “stem cells” was significantly lower than that expected following a transplant of the same number of unseparated marrow cells from which the purified cells were derived, suggesting the existence of a “nonstem cell facilitator population,” which is required in a nonablated syngeneic transplant setting.
INCREASINGLY, allogeneic and more often autologous bone marrow transplants (BMTs) are being performed in an effort to correct a wide variety of disorders. In addition, autologous marrow transplants potentially provide an opportune means of delivering genes in transfected stem cells capable of engrafting. However, despite widespread clinical use of BMTs, and promising possibilities in gene therapy applications, relatively little is known regarding the mechanisms of engraftment in marrow transplant recipients. This is particularly true in the nonablated recipient setting.
A bone marrow (BM) stem cell is thought to reside in a hematopoietic “niche,” or a microenvironment containing specific stromal and extracellular matrix elements. Successful long-term BMTs rely on stem cells homing to these niches. Micklem et al1 were probably the first to attempt to detect chromosomally marked BM cells injected into normal, syngeneic recipients. Their studies in mice showed low levels (up to 8.5%) of engraftment of T6T6 cells into nonablated CBA host marrow, 3 months after a transplant of 20-million marrow cells from normal CBA-T6T6 donors. Takada et al2,3 also detected only low numbers of T6 marked marrow cells after their injection into normal CBA mice. Experiments such as these drove the hypothesis that there are a limited number of stem cell “niches,” and that these are virtually all occupied in normal animals with only very low levels of exchange from injected stem cells.1 4 As a consequence, marrow transplants have traditionally been given to ablated hosts, with conventional dogma suggesting that cytoablative therapy with irradiation and/or cytotoxic drugs is required to free “niches” from host cells.
More recently, Brecher et al5 and Saxe et al6 successfully engrafted relatively high numbers of donor murine BM into normal recipients. Brecher et al5 showed engraftment levels from 16% to 25%, 2 to 13 weeks posttransplant of 40 million cells per day for 5 days into normal hosts. Saxe et al6 used chromosomally marked syngeneic marrow cells to show donor levels of 0% to 16% in the marrow or spleen, 1 to 6 months posttransplant. Stewart et al7 extended these studies, showing high levels of engraftment (15% to 42%) for over 2 years in the marrow of mice injected with 40 million cells for 5 consecutive days.
However, these studies have only evaluated unseparated marrow, not purified marrow stem cells. In the present study we have shown the successful homing of highly purified, primitive, rhodamine 123/Hoechst 33342 dull (Rh/Hoedull) stem cells into the marrow of nonablated hosts. These cells have multilineage potential and undergo maturation and differentiation to give equivalent proportions in both the mature as well as the primitive stem cell compartment. In addition these cells appear to selectively home to the femoral marrow interface.
MATERIALS AND METHODS
Mice.BALB/c H-2D mice were purchased from Charles River Laboratories (Wilmington, MA) and housed in a conventional clean facility for at least 1 week before experimental use. All mice received mouse chow and acidified water ad libitum.
Cell suspensions.Six to 8-week-old mice were killed by cervical dislocation. BM was collected from femurs, tibiae, and iliac crests. This was routinely done by thorough grinding in phosphate-buffered saline (PBS) supplemented with 5% heat inactivated (HI) fetal calf serum (FCS; Hyclone, Logan, UT) using a mortar and pestle. The bone fragments were washed multiple times, and the supernatant cell suspension and wash fractions were filtered through a 40-μm filter (Becton Dickinson, Franklin Lakes, NJ) to remove large bone particles. High lipid concentrations were reduced by the centrifugation and resuspension of the cells in fresh buffer. The cells were allowed to sit on ice for 5 minutes, so small bone particles would settle out. The cell supernatant, depleted of these fragments, was then diluted to 107 cells/mL PBS 5% HI FCS.
When whole BM cell suspensions were to be injected into a recipient without manipulation, the cells were collected by flushing the femurs and tibiae with cold PBS supplemented with 5% HI FCS. The cells were filtered as described above, washed, and resuspended for injection in 0.5 mL of PBS.
Preparation of lineage negative cells.Lineage negative cells were isolated in a manner very similar to that previously described by Bertoncello et al.8 Briefly, low density cells (<1.0777 g/cm3 ) were isolated by discontinuous density centrifugation using Nycoprep for animals (Accurate Chemical and Scientific Corp, Westbury, NY), washed and resuspended at 108 cells/mL PBS 5% HI FCS. Cells were labeled with a cocktail of primary antibodies: anti-B220 (B cells) Coffman9; anti-Mac-1(macrophages) Springer10; anti-Gr-1 (neutrophils) Hestdal11; anti-Lyt-2 (CD8) and anti-L3T4 (CD4) (T cells) Cobbold12 (Becton Dickinson); YW25.12.7 (erythroid precursors) Watt et al13; and TER119 (erythrocytes) a kind gift from Tatsuo Kina (Chest Disease Research Institute, Kyoto University, Kyoto, Japan). Each batch of antibody was evaluated by flow cytometric analysis, for the concentration which resulted in the greatest shift in mean channel fluorescence and/or the percentage positive cells detected. The optimal dilution for each antibody was in the range of 1:10 to 1:100 (final). After a 15-minute incubation on ice, the labeled cells were washed in PBS 5% HI FCS and resuspended at 108 cells/mL PBS 5% HI FCS. The cells were incubated with sheep anti-rat IgG conjugated immunomagnetic polystyrene spheres (M-450 Dynabeads; Dynal, Lake Success, NY) at 4°C for 3 minutes by adding a 1:1 bead:cell ratio. The beads were suspended in PBS 5% HI FCS, and when added to the cells resulted in 1.5 times the original cell volume. Immunomagnetic bead-rosetted cells were removed using a magnetic particle concentrator (Dynal MPC-6), and the unrosetted cells remaining in suspension were harvested by pipette. The beads were washed 3 times, and the supernatant pooled with the cell suspension. The unrosetted cells were further incubated with anti-rat conjugated immunomagnetic beads at 4°C for 20 minutes by adding a 4:1 bead:cell ratio, resulting in double the original cell volume. Again immunomagnetic bead-rosetted cells were removed using a magnetic particle concentrator, and the unrosetted cells remaining in suspension were harvested by pipette. The beads were washed 3 times, and the supernatant pooled with the cell suspension.
Rhodamine 123 (Rh)/Hoechst 33342 (Hoe) labeled cell separation. Immunomagnetically enriched cell suspensions were washed and resuspended at 106 cells/mL in PBS 5% HI FCS and incubated in a final Rh concentration of 0.1 μg/mL for 20 minutes at 37°C in the dark. A final Hoe concentration of 10 μmol/L was then added, and the cells incubated at 37°C in the dark for a further hour. The cells were washed in ice-cold PBS 5% HI FCS and held on ice in preparation for sorting.
Labeled cells were analyzed on a FACStarplus cell sorter (Becton Dickinson, Mountain View, CA) equipped with a 5-W argon ion laser (Coherent Innova 90, Palo Alto, CA) running at 200 mW of power, and a 5-W argon ion laser (Coherent Innova 90) running at 50 mW of power. Light-scatter signals were collected through a 488-nm band pass filter and a 1-decade logarithmic neutral density filter in the forward light scatter path. Hoe fluorescence was collected through a 424/44-nm band pass filter, and Rh fluorescence was collected through a 530/30-nm band pass filter. Cells with the predetermined light-scatter properties of hematopoietic progenitors, termed “blast cells” and comprising approximately 10% of total BM cells,14 were analyzed and sorted at 4°C on the basis of their Hoe and Rh fluorescence.
The stem cell subset has been characterized as having both low Hoe15-17 and Rh14,18,19 retention. Transplantable stem cells with long-term reconstituting ability were concentrated within the 1st through 3rd percentiles of Hoe fluorescence and then the 1st through 15th percentiles of Rh fluorescence (Rh/Hoedull).20 Sorted cells were collected on ice, counted, and injected into recipients. Approximately 12 hours elapsed between removal of donor marrow and injection into recipients.
Hematopoietic progenitor cell assay.The cloning efficiency of separated marrow populations was determined by analysis for high proliferative potential colony forming cells (HPP-CFC), using a double layer nutrient agar culture system as previously described,21 except for the use of 7 growth factors (interleukin-1 [IL-1], IL-3, colony-stimulating factor-1 [CSF-1], granulocyte-macrophage colony-stimulating factor [GM-CSF], granulocyte colony-stimulating factor [G-CSF], stem cell factor [SCF], and basic fibroblastic growth factor [FGF]) instead of 3 factors. HPP-CFC typically generated colonies greater than 0.5 mm in diameter consisting of tightly packed cells. Functionally these are defined as primitive cells by their relative resistance to 5-FU, their synergistic growth factor requirements, and their copurification with long-term reconstituting cells in vivo.22 23
Transplants.Transplant experiments involved a single intravenous infusion of between 2,600 and 10,000 Rh/Hoedull male marrow cells into nonablated female recipients. After either 6 weeks or 6 months, the presence of donor cells was quantitated using fluorescence in situ hybridization (FISH) and Southern blot analysis. At 6-weeks postengraftment, the spatial distribution of engrafted cells was analyzed on sections of whole marrow using FISH.
Engraftment Quantification
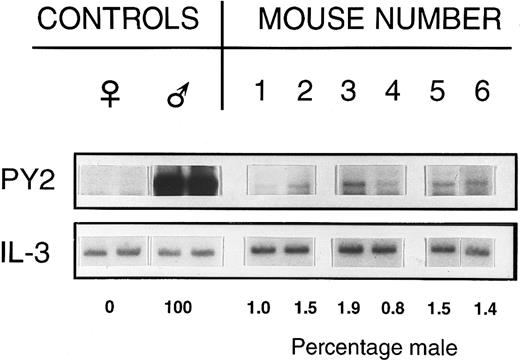
Southern blot analysis.DNA extracts of BM were prepared by lysis in 0.15 mol/L NaCl, 0.02 mol/L Tris, 0.02 mol/L EDTA, and 1% sodium dodecyl sulfate followed by purification using organic extraction with proteinase K, RNAse, and phenol-chloroform and precipitation in ethanol. The presence of Y chromosome-specific DNA sequences was assessed using a pY2-cDNA probe (donated by I. Lemischka, Princeton University, NJ).24 Five micrograms of each DNA sample was digested with the restriction enzyme Dra I, and separated by gel electrophoresis in 0.8% agarose (GIBCO-BRL, Bethesda, MD). DNA fragments were transferred onto Zetaprobe nylon membranes (Biorad, Richmond, CA) according to established Southern blotting techniques. Sample loading variability was assessed and adjusted for by reprobing membranes with a partial or full-length cDNA for IL-3 (donated by J. Ihle and DNAX, Palo Alto, CA). Probes were labeled with 32P using a random primed labeling kit (Boehringer Mannheim, Mannheim, Germany), and an autoradiograph made using Kodak XRP x-ray film (Eastman Kodak, Rochester, NY). Blots were exposed to photostimulatable storage phosphor imaging plates (Molecular Dynamics, Sunnyvale, CA), and the percentage of male and female DNA quantified after scanning the plates with a 400A phosphorimager (Molecular Dynamics).
FISH.Cytospins were fixed in a 50% Carnoys (75% methanol/25% acetic acid) and 50% PBS solution for 10 minutes before being baked at 72°C for an hour. The slides were then further fixed in 100% Carnoys for 5 minutes before permeabilization using proteinase K (0.2 μg/mL) (Sigma Diagnostics, St Louis, MO) enzymatic digestion in 20 mmol Tris buffer at 37°C for 1.5 minutes. The cytospins were dehydrated through graded ethanol, then denatured in 70% formamide (GIBCO-BRL) in 2 × SSC (0.3 mol/L NaCl and 0.03 mol/L sodium citrate, pH 6.4) at 70°C for 3 minutes. The slides were dehydrated once more, and hybridized with a digoxigenin-labeled Y chromosome probe25 at 45°C overnight. Unbound probe was removed by stringent washings in 3 changes of 50% formamide in 2 × SSC and 2 changes of 2 × SSC at 45°C. Following a wash in 4 × SSC (0.6 mol/L NaCl and 0.06 mol/L sodium citrate, pH 6.4) at room temperature, the slides were blocked using a blocking buffer consisting of 5% FCS, 5% nonfat milk (Shaw's, Bridgewater, MA), and 0.05% Triton X-100 (Sigma) in 4 × SSC for 15 minutes. Detection of digoxigenin was done using antidigoxigenin-rhodamine, Fab fragments (Boehringer Mannheim), at a concentration of 6.5 μg/mL in PBS containing 2% bovine serum albumin fraction V (Sigma) for 30 minutes in the dark. Nonbound antibody was removed through 3 extensive, light protected, washings; first in 4 × SSC for 10 minutes, then in 4 × SSC containing 0.05% Triton X-100 for 10 minutes, and finally in 4 × SSC for 10 minutes. Cytospins were counterstained in 0.4 μmol DAPI (4,6-diamidino-2-phenylindole) (Sigma) and mounted in the antifade media Vectashield (Vector, Burlingame, CA). Specific positive label was confirmed under the microscope by a visual check at excitation and emission wavelengths other than that of rhodamine. Samples were analyzed by multiple readers for verification of results.
Engraftment of Rh/Hoedull Marrow Cells in Nonablated Recipients
| No. Donor Cells . | Cloning Efficiency %* . | No. of Recipients . | % Male in Whole Marrow Using Southern Blot Analysis† . | % Male Cells in Whole Marrow Determined Using FISH‡ . |
|---|---|---|---|---|
| Rh/Hoedull | ||||
| 2,600ρ | 82.3 | 12 | 1.4 ± 0.2 | 1.7 ± 0.2 |
| 3,0001-155 | 88.7 | 9 | 0.9 ± 0.07 | 1.1 ± 0.2 |
| 5,500ρ | 49.8 | 3 | 1.2 ± 0.4 | 1.0 ± 0.3 |
| 10,000ρ | 86.7 | 3 | 0.7 ± 0.4 | ND |
| 10,000ρ | 81.3 | 5 | 2.2 ± 0.4 | 3.4 ± 0.4 |
| Whole marrow | ||||
| 100 × 106ρ | ND | 23 | 24.9 ± 1.9 | ND |
| No. Donor Cells . | Cloning Efficiency %* . | No. of Recipients . | % Male in Whole Marrow Using Southern Blot Analysis† . | % Male Cells in Whole Marrow Determined Using FISH‡ . |
|---|---|---|---|---|
| Rh/Hoedull | ||||
| 2,600ρ | 82.3 | 12 | 1.4 ± 0.2 | 1.7 ± 0.2 |
| 3,0001-155 | 88.7 | 9 | 0.9 ± 0.07 | 1.1 ± 0.2 |
| 5,500ρ | 49.8 | 3 | 1.2 ± 0.4 | 1.0 ± 0.3 |
| 10,000ρ | 86.7 | 3 | 0.7 ± 0.4 | ND |
| 10,000ρ | 81.3 | 5 | 2.2 ± 0.4 | 3.4 ± 0.4 |
| Whole marrow | ||||
| 100 × 106ρ | ND | 23 | 24.9 ± 1.9 | ND |
Abbreviation: ND, not done.
The cloning efficiency represents the proportion of 7 factor (IL-1, IL-3, CSF-1, GM-CSF, G-CSF, SCF, and FGF), responsive HPP-CFC after 14 days in a double-layer agar culture.
Values are the means ± SEM. Southern blots were probed with pY2 and the percentage male was determined using phosphorimage analysis, taking the male to be 100% and the female as 0%. Loading variability was corrected using an IL-3 probe.
Values are the means ± SEM representing analysis of at least 300 cells from at least 4 different fields of focus. FISH analysis was done using a Y chromosome-specific painting probe. Male slides were 100%, and female slides 0% following this analysis.
ρ Analyzed 6 weeks posttransplant.
Analyzed 6 months posttransplant.
Analysis of multilineage potential.Peripheral blood was collected from mice injected with 4,500 Rh/Hoedull cells 6 weeks posttransplant. Red cells were lysed using 0.83% ammonium chloride at 37°C for 5 minutes. Residual cells were washed twice, first in PBS and then in PBS 5% HI FCS. Individual cell aliquots were labeled with the lineage specific primary antibodies MAC-1, GR-1, B220, or CD4/CD8 as described above. After a 15-minute incubation on ice, the labeled cells were washed in PBS 5% HI FCS and resuspended in the same initial volume. The cells were incubated with mouse serum-absorbed fluorescein isothiocyanate (FITC)-conjugated goat anti-rat IgG (Southern Biotechnology Associates, Birmingham, AL) at 4°C for 20 minutes in the dark. The cells were washed in PBS 5% HI FCS and held on ice until sorted. Each subpopulation was analyzed and sorted by the same FACStarplus cell sorter described above. Background fluorescence (cell surface binding of FITC-conjugated goat anti-rat second antibody) was less than 5%. Positive cells were collected into individual tubes, and analyzed for donor cells using the FISH method described above.
Analysis of Spatial Distribution
In situ detection of individual transplanted BM cells using FISH on sections of paraffin-embedded whole murine femurs was done as previously described.26 Briefly, mice were perfused with 4% paraformaldehyde into the descending aorta. Femurs were removed, decalcified in EDTA, dehydrated in graded ethanol, and cleared in mineral spirits (Aldrich Chemical Co, Milwaukee, WI) before infiltration and embedding in Paraplast x-tra (Oxford Labware, St Louis, MO).
Five micrometer femoral sections were mounted on 0.01% poly-L-lysine (Mr 150,000-300,000; Sigma) subbed slides, deparaffinized in xylene, and rehydrated in graded ethanol. The formaldehyde from the original fixation was inactivated in 0.1 mol/L glycine, before permeabilization using proteinase K (5 μg/mL) enzymatic digestion. The sections were further fixed in fresh paraformaldehyde, prehybridized, and following denaturation, hybridized with the digoxigenin-labeled Y chromosome probe. Unbound probe was removed by stringent washings.
Nonspecific binding of the detection fluorochrome was blocked using a skim milk blocking buffer. Detection of digoxigenin was done using anti-digoxigenin-rhodamine Fab fragments. To preserve fluorescent labeling, sections were mounted in Vectashield.
RESULTS
To accurately assess the extent of donor chimerism of hematopoietic cells and the histological location of these engrafted cells, we used several techniques that rely on the detection of Y chromosome-specific (donor) DNA sequences. These included Southern analysis and in situ hybridization analysis of both single cell suspensions and paraffin embedded femoral sections.
Stem cell engraftment.Six weeks to 6-months posttransplant, 2,600 to 10,000 male donor stem cells (Rh/Hoedull cells) successfully engrafted into the marrow of nonablated female hosts. Southern blot analysis of whole marrow to detect Y chromosome-specific DNA sequences in female hosts clearly showed the presence of donor cells in multiple experiments (Table 1, Fig 1). This low level engraftment was confirmed by FISH on BM cell suspensions (Table 1).
Percentage of male DNA in BM of individual recipient female mice. Female mice received 2,600 Rh/Hoedull marrow cells and were sampled 6 weeks posttransplant. Southern blots were labeled using a Y chromosome-specific probe and the percentage donor calculated by phosphorimage analysis, taking the male as 100% and the female as 0%. Loading variability was corrected using a probe for IL-3.
Percentage of male DNA in BM of individual recipient female mice. Female mice received 2,600 Rh/Hoedull marrow cells and were sampled 6 weeks posttransplant. Southern blots were labeled using a Y chromosome-specific probe and the percentage donor calculated by phosphorimage analysis, taking the male as 100% and the female as 0%. Loading variability was corrected using a probe for IL-3.
Six weeks posttransplant, the multilineage potential of transplanted cells was accessed by analysis of peripheral white blood cells from mice transplanted with 4,500 Rh/Hoedull cells. White blood cells were sorted into lineage subpopulations, and each subpopulation analyzed for donor cells by FISH, using a Y chromosome-specific painting probe (Table 2). Rh/Hoedull cells were shown to home, proliferate, and differentiate, forming a detectable proportion of the macrophage (MAC-1), granulocyte (GR-1), B-cell (B220), and T-cell (CD4/CD8) subpopulations. In the lineages analyzed, donor cells comprised 2.9%, 13.3%, 13.7%, and 1.2%, respectively, of each lineage subpopulation.
Analysis of Multilineage Potential
| . | % Subpopulation in Peripheral WBC* . | % Male in Each Subpopulation† . |
|---|---|---|
| MAC-1 | 13.8 ± 2.7 | 0.4 ± 0.2 |
| GR-1 | 7.5 ± 0.6 | 1.0 ± 0.6 |
| B220 | 16.1 ± 3.1 | 2.2 ± 0.6 |
| CD4/CD8 | 24.3 ± 2.0 | 0.3 ± 0.3 |
| . | % Subpopulation in Peripheral WBC* . | % Male in Each Subpopulation† . |
|---|---|---|
| MAC-1 | 13.8 ± 2.7 | 0.4 ± 0.2 |
| GR-1 | 7.5 ± 0.6 | 1.0 ± 0.6 |
| B220 | 16.1 ± 3.1 | 2.2 ± 0.6 |
| CD4/CD8 | 24.3 ± 2.0 | 0.3 ± 0.3 |
Mice were injected with 5,500 Rh/Hoedull cells and harvested 6 weeks posttransplant. The percentage donor cells in the BM as determined by FISH was 1.0 ± 0.3%. Peripheral blood was collected, and the red blood cells lysed using ammonium chloride.
White cells were labeled for macrophages (MAC-1), granulocytes (GR-1), B cells (B220), and T cells (CD4/CD8).
Each peripheral white blood cell (WBC) subpopulation was analyzed for donor cells by FISH using a Y chromosome-specific painting probe. Values are the means ± SEM.
Pooled marrow from mice transplanted with either 2,600 or 10,000 Rh/Hoedull cells was resorted for cells with the same Rh/Hoedull characteristics of the cells initially seeded. FISH analysis of the proportion of donor cells within this subpopulation (2.2% and 4.7%, respectively) (Fig 2) was equivalent to that found in the whole marrow. This highlights the maintenance of a significant population of donor cells with a primitive Rh/Hoedull phenotype. In addition, the data show for the first time that the analysis of whole marrow in nonablated hosts is a true reflection of the proportion of donor cells within the primitive Rh/Hoedull stem cell compartment.
A cytospin of pooled marrow from mice transplanted with 10,000 Rh/Hoedull cells and resorted for cells with the same Rh/Hoedull characteristics of the cells initially seeded. (A) Cells are stained using in situ hybridization for a digoxigenin-conjugated Y chromosome-specific “painting” probe and visualized with rhodamine using a 590-nm longpass filter. Positive cell (d) is of donor origin, while unlabeled cells (h) are of host origin. (B) Same as (A) except counterstained with DAPI, excited with UV, and visualized using a 450-nm longpass filter. Original magnification (OM) × 313.
A cytospin of pooled marrow from mice transplanted with 10,000 Rh/Hoedull cells and resorted for cells with the same Rh/Hoedull characteristics of the cells initially seeded. (A) Cells are stained using in situ hybridization for a digoxigenin-conjugated Y chromosome-specific “painting” probe and visualized with rhodamine using a 590-nm longpass filter. Positive cell (d) is of donor origin, while unlabeled cells (h) are of host origin. (B) Same as (A) except counterstained with DAPI, excited with UV, and visualized using a 450-nm longpass filter. Original magnification (OM) × 313.
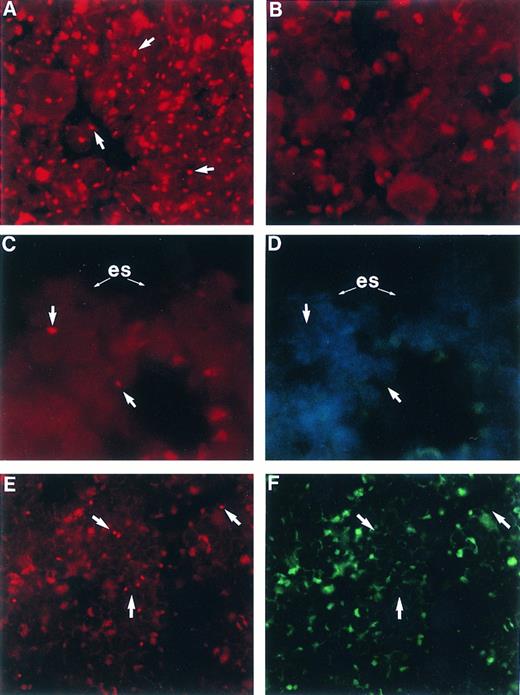
Spatial distribution of engrafted cells.The application of our new FISH technique on sections of paraffin embedded whole femurs allowed the identification of individual engrafted cells ex vivo. Animals receiving Rh/Hoedull cells were analyzed 6 weeks posttransplant. In all animals analyzed, engrafted cells had a strong spatial association for the endosteal surface (Table 3, Fig 3C), and in none of the samples were any donor cells found in the central marrow region. All of the donor cells detected were within 6 cells of the endosteal surface, except for one donor cell which was within 12 cells of the endosteal surface. After a transplant of Rh/Hoedull cells, donor cells almost always occurred as single entities. This distribution was unlike that seen following a transplant of 100 × 106 whole marrow cells, where engrafted cells were seen distributed throughout the marrow as both large clusters as well as single cells (Fig 3E).
Analysis of the Spatial Distribution of Rh/Hoedull Cells and Their Progeny 6 Weeks Posttransplant
| Section No. Analyzed3-150 . | Central Marrow Region . | Endosteal Region . |
|---|---|---|
| 1 | − | + |
| 2 | − | − |
| 3 | − | − |
| 4 | − | + |
| 5 | − | − |
| 6 | − | + |
| 7 | − | + |
| 8 | − | + |
| 9 | − | + |
| 10 | − | − |
| 11 | − | + |
| 12 | − | + |
| 13 | − | + |
| 14 | − | − |
| Section No. Analyzed3-150 . | Central Marrow Region . | Endosteal Region . |
|---|---|---|
| 1 | − | + |
| 2 | − | − |
| 3 | − | − |
| 4 | − | + |
| 5 | − | − |
| 6 | − | + |
| 7 | − | + |
| 8 | − | + |
| 9 | − | + |
| 10 | − | − |
| 11 | − | + |
| 12 | − | + |
| 13 | − | + |
| 14 | − | − |
P < .004 using a two-side paired-sample sign test, based on the assumption that donor cells have an equal probability of being in the central marrow region as at the endosteal surface (defined as 12 cells from the femoral marrow interface).27
Abbreviations: − Represents no donor cells detected; + cells of donor origin detected.
Sections were analyzed from at least 4 different mice from 3 different experiments.
Femoral BM sections from BALB/c mice: (A) male, (B) female, (C) female 6 weeks posttransplant with 2,500 Rh/Hoedull marrow cells, and (E) female 6 weeks posttransplant with 100 × 106 whole marrow cells. Sections are stained using in situ hybridization for a digoxigenin-conjugated Y chromosome-specific “painting” probe and rhodamine anti-digoxigenin (arrows) and visualized using a 590-nm longpass filter. (D) and (F ) same as (C) and (E), respectively, except counterstained with DAPI, excited with UV, and visualized using a 450-nm longpass filter. Arrows point to corresponding Y chromosome-positive cells. (es) Endosteal surface; the actual bone is removed during the denaturing step of the in situ hybridization. Sections are mounted in Vectashield antifade. OM × 746 (A through D); OM × 500 (E and F ).
Femoral BM sections from BALB/c mice: (A) male, (B) female, (C) female 6 weeks posttransplant with 2,500 Rh/Hoedull marrow cells, and (E) female 6 weeks posttransplant with 100 × 106 whole marrow cells. Sections are stained using in situ hybridization for a digoxigenin-conjugated Y chromosome-specific “painting” probe and rhodamine anti-digoxigenin (arrows) and visualized using a 590-nm longpass filter. (D) and (F ) same as (C) and (E), respectively, except counterstained with DAPI, excited with UV, and visualized using a 450-nm longpass filter. Arrows point to corresponding Y chromosome-positive cells. (es) Endosteal surface; the actual bone is removed during the denaturing step of the in situ hybridization. Sections are mounted in Vectashield antifade. OM × 746 (A through D); OM × 500 (E and F ).
DISCUSSION
We have shown that transplanted purified hematopoietic stem cells (Rh/Hoedull cells) engraft into the marrow of nonablated recipients. These cells were shown to proliferate and differentiate with multilineage potential, representing an equivalent proportion of the host primitive Rh/Hoedull compartment to that observed in host unseparated BM. However, the level of engraftment observed was significantly lower than that expected following a transplant of equivalent whole marrow.
Murine hematopoietic stem cells have traditionally been defined by their ability to repopulate the marrow of a lethally irradiated recipient. The stem cell compartment has been characterized as having both low Hoe15-17 as well as low Rh14,18,19 retention. Transplantable stem cells with long-term reconstituting ability were shown to be within the 1st through 3rd Hoe fluorescence percentiles and 1st through 15th Rh fluorescence percentiles (Rh/Hoedull).20 Repopulation of irradiated hosts has been achieved from as few as one or two stem cells with the help of mature support cells enabling the mouse to survive while the stem cells amplify and feed out. As such, the irradiated host represents an amplification unit, providing a high yield from a small number of donor cells. As a consequence, this model was easily applied to the clinic, where irradiation removed diseased cells, and reconstitution only required relatively small numbers of donor cells. However, cytoablative preparative treatment severely damages the hematopoietic microenvironment. In addition, cytoablative treatment is not desirable or feasible for many diseases as it has multiple associated risks to the patient. As a consequence, there are many clinical advantages of a nonablated transplant model.
In our experience, the proportion of primitive cells (Rh/Hoedull cells) in the marrow is approximately 0.003%, or 3,000 in 100 × 106 whole BM cells. Using Southern blot analysis of male into female transplant recipients we have shown approximately 25% donor cells in the marrow 6 weeks after a transplant of 100 × 106 whole BM cells into nonablated hosts (Table 1). Assuming that this long-term engraftment comes from the primitive Rh/Hoedull cell population, we would expect the proportion of Rh/Hoedull cells engrafted into the marrow to reflect that seen following transplants with equivalent numbers of whole marrow. However, our data showed only between 0.8% and 6.4% of these expected values (Table 4).
Evidence for An Accessory Cell Interaction
| No. Donor Rh/Hoedull Cells . | No. of Starting Whole Marrow Cells (×106) . | % Male Expected From Equivalent Whole Marrow Cells4-150 . | Actual % Male in Whole Marrow From Rh/Hoedull Cells Using Southern Blot Analysis . | % of Expected Value . |
|---|---|---|---|---|
| 2,600 | 87 | 22 | 1.4 | 6.4 |
| 3,000 | 100 | 25 | 0.9 | 3.6 |
| 5,500 | 183 | 46 | 1.2 | 3.3 |
| 10,000 | 333 | 83 | 0.7 | 0.8 |
| 10,000 | 333 | 83 | 2.2 | 2.6 |
| No. Donor Rh/Hoedull Cells . | No. of Starting Whole Marrow Cells (×106) . | % Male Expected From Equivalent Whole Marrow Cells4-150 . | Actual % Male in Whole Marrow From Rh/Hoedull Cells Using Southern Blot Analysis . | % of Expected Value . |
|---|---|---|---|---|
| 2,600 | 87 | 22 | 1.4 | 6.4 |
| 3,000 | 100 | 25 | 0.9 | 3.6 |
| 5,500 | 183 | 46 | 1.2 | 3.3 |
| 10,000 | 333 | 83 | 0.7 | 0.8 |
| 10,000 | 333 | 83 | 2.2 | 2.6 |
The expected values for 100 × 106 whole marrow cells was determined from Southern blot analysis of at least 3 previous experiments giving these equivalent numbers of whole marrow cells. The expected values for the other whole marrow cell doses were theoretically calculated based on a linear relationship between the number of cells given and the % donor cells detected at 6 weeks to 6 months.
It is unlikely that this significantly reduced percentage of donor cells is the consequence of an immune mediated rejection against the H-Y (Y chromosome-associated histocompatibility) antigen. BALB/c H-2D mice were specifically chosen for this series of experiments because of their very low degree of immunoreactivity to the H-Y antigen.28 These mice have previously been shown not to reject skin grafts from the opposite sex.29 In addition, previous transplant experiments reported from this laboratory show expected levels of engraftment following the transplant of male whole BM into nonablated female recipients,30 and this remains through 25 months, the normal life span of a mouse.7 Together with the results of the whole BMT reported in the present study, which resulted in the expected levels of donor cell engraftment 6 weeks posttransplant, these data give no indication of any immune mediated rejection in this transplant model.
It is also unlikely that the lower than expected percentage of donor cells 6 weeks after transplant is due to a lag phase of these cells, as very similar results were obtained after 6 months (Table 1). In addition, because of the very low number of cells transplanted, detection of donor cells relies on the proliferation of those initially injected. Not only were cells detected, but they comprised an equivalent proportion of mature circulating peripheral blood cells, whole BM cells, as well as Rh/Hoedull marrow cells. These findings show that the transplanted cells are proliferating as well as differentiating.
The data suggest that an accessory, or facilitator cell interaction may be important for optimal stem cell engraftment in a nonablated syngeneic transplant. Previous studies have also indicated a requirement for a facilitator cell interaction. Kaufman et al31 identified a potential marrow accessory cell population, separate from hematopoietic stem cells, that facilitates engraftment of purified allogeneic marrow stem cells in a major histocompatibility complex-specific fashion, in the absence of graft-versus-host disease. The same group more recently showed the same phenomena of these marrow “facilitator cells” with the engraftment of fetal liver stem cells in allogeneic transplants.32 Following lethal irradiation, it was suggested that such “facilitator cells” were not required for syngeneic transplants. Our data further support the existence of a “non-stem cell facilitator population,” but suggest that these cells are also required in a nonablated syngeneic transplant setting.
Our data also suggest a spatial localization of stem cell “niches” to the endosteal surface, with all donor cells having a very high spatial affinity to this area. Even though the location of stem cell niches still remains a matter for speculation, mounting evidence does suggest that they may be in intimate association with bone. Histological examination of bones during recovery from hematopoietic depletion as a result of sublethal doses of irradiation shows that repopulation, as evidenced by the appearance of hematopoietic precursors, is first seen along the endosteal surface.4 Following the use of x-rays, this region has received the highest dose of irradiation, enhancing the significance that regeneration begins from an area with the least cell survival.
Previous studies have also indicated that BM cell populations conform to a well-defined spatial organization, with a relationship between location and the development of the various hematopoietic cell lineages. Lord et al33 showed an increase in progenitor spleen colony-forming cell (CFU-s) concentration in regions close to the inside of the femoral bone, in an association with a decrease in committed precursor cells of the granulocytic lineage. Although these progenitors are now known to be more mature than a stem cell, the data in combination with the observation that CFC, an even more mature subset of hematopoietic progenitor cells, increase toward the central marrow region34 suggest more primitive cells lie closest to the endosteal surface. Gong35 also showed that cells isolated from the endosteal bone surface were enriched for colony-forming units. These findings were independently confirmed using mainly histological procedures36,37 and reconfirmed more recently using a technique capable of fractionating the diaphyseal marrow cavity into distinct zones consisting of cells lying close to bone and those lying more centrally.34
Lord38 also noted a high spatial association of primitive hematopoietic progenitor cells (CFU-s) and endosteal surfaces, suggesting that hematopoietic stem cell differentiation occurs in direct proximity to endosteal osteoblasts within the marrow cavity. These observations have since been expanded in vitro using a long-term culture initiating primitive progenitor cell population (LTC-IC).39 This study suggests that not only do osteoblasts support primitive hematopoietic LTC-IC, but that the presence of these cells at the endosteal surface may also be due to a requirement for osteoblast-derived factors. Compared to the CFU-s, these cells are much more equivalent to the stem cell in their level of the hematopoietic hierarchy.40
Because of the technical impossibility of obtaining enough donor “stem cells” to be able to detect these cells in the marrow in situ at very short time intervals posttransplant, we had to rely on the engrafted cells proliferating to analyze their spatial distribution. As a consequence, analysis was done at 6 weeks posttransplant. At this time, donor cells, which consisted of progenitor cells, mature cells, and cells with the very primitive Rh/Hoedull stem cell characteristics, had a very high spatial association with the endosteal surface. However, it remains possible that these cells initially homed to another marrow region and migrated to this area postproliferation. A definitive answer will require further technical development so that very early analysis posttransplant is feasible.
Our data show the ability to engraft purified hematopoietic Rh/Hoedull stem cells in a nonablated, syngeneic murine model. Once engrafted, these cells had multilineage potential, as well as having the ability to maintain a significant population with the Rh/Hoedull phenotype. In addition, donor cells exhibited a high spatial association with the endosteal surface. However, the level of engrafted cells was lower than would be expected from transplanting whole marrow equivalents. This suggests the involvement of an accessory, or facilitator cell for optimal stem cell engraftment following a syngeneic transplant in a nonablated recipient.
ACKNOWLEDGMENT
The authors acknowledge Ruud Hulspas for his invaluable help and advice with the flow cytometric isolation of purified stem cells.
P.J.Q. was supported by grants from National Institutes of Diabetes and Digestive and Kidney Diseases (NIDDK, Rockville, MD) #49650-02 and #50222-01. H-U.W. was supported by a grant from the Office of Energy Research, Office of Environmental Research, US Department of Energy (Washington, DC) under contract DE-AC-03-76SF00098. S.K.N. is a CJ Martin Fellow, granted from the National Health and Medical Research Council (Canberra, ACT, Australia), and is supported by the Our Danny Cancer Fund, University of Massachusetts, Worcester.
Address reprint requests to Susan K. Nilsson, PhD, UMMC Cancer Center, 373 Plantation St, Suite 202, Worcester, MA 01605.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal