Abstract
The explanation why only a subset of patients with heparin-induced thrombocytopenia (HIT) develop clinically apparent thromboses (HITT) remains uncertain. It has been proposed that platelet activation induced by cross-linking of FcγRIIA by anti-heparin/platelet factor 4 (PF4) antibodies is central to the pathogenesis of thrombosis. The observation that a common functional polymorphism of FcγRIIA, involving either an arginine (R) or histidine (H) at amino acid 131, may underlie disease susceptibility prompted us to investigate the prevalence of receptor isoforms in patients with HIT and HITT. Furthermore, because these isoforms reportedly differ in their avidity for immune complexes containing human IgG2 , we also analyzed sera from patients with HIT and HITT for the prevalence of various subclass-specific IgG anti-heparin/PF4 antibodies. No difference in the allele frequency of FcγRIIA-H131 or R131 was identified among 13 patients with HIT or 23 with HITT compared with 102 controls (χ2 = 1.21, P = .8). Furthermore, although most patients had IgG2 antibodies (62%), IgG1 was the predominant subclass in 30 of the 34 patients with IgG anti-heparin/PF4 antibodies and in 12 was the exclusive subclass found. Also, there was no association between the concordance of IgG2 anti-heparin/PF4 antibodies and the expression of FcγRIIA-H131 in patients with HITT compared with patients with thrombocytopenia alone. These results make it unlikely that the FcγRIIA-H131 isoform or IgG2 anti-heparin/PF4 antibodies are required to develop HITT, suggesting that factors in addition to cross-linking of FcγRIIA receptors contribute to the pathogenesis of thrombosis in patients with heparin-dependent antiplatelet antibodies.
HEPARIN IS THE most common cause of drug-induced thrombocytopenia.1,2 The major morbidity of heparin-induced immune thrombocytopenia (HIT) comes from the seemingly paradoxical development of thrombosis (HITT) in the 10% to 20% of patients in whom the disorder is not diagnosed promptly and heparin is continued.3 Although it is widely accepted that thrombosis occurs more commonly in patients with cardiovascular disease,4 no other predisposing factors have been identified to distinguish patients who develop isolated thrombocytopenia from those whose illness will be complicated by thrombosis.
The pathogenesis of thrombosis in patients with HITT is poorly understood. It has been proposed that platelet activation, initiated when platelet FcγRIIA receptors are cross-linked by HIT/HITT antibodies, may play an essential role in this process. This hypothesis is predicated primarily on the in vitro observation that platelet aggregation and secretion in response to HIT sera is abrogated by a monoclonal antibody to FcγRIIA that blocks the binding of IgG.5-7 However, it is unclear whether platelet activation by HIT/HITT antibodies differs in this respect from activation caused by other drug-dependent or autoreactive antiplatelet antibodies obtained from patients with bleeding disorders or even whether it distinguishes patients receiving heparin who develop thrombosis from those who present with asymptomatic thrombocytopenia.
Of interest, two functional isoforms of the FcγRIIA have been described in humans. A single-base substitution (G → A) in the codon for amino acid 131 results in an arginine (R) → histidine (H) change in the extracellular domain of the receptor, altering its binding affinity for human IgG2 .8-10 The FcγRIIA-H131 isoform binds human IgG2 with higher affinity than does FcγRIIA-R131, whereas each binds IgG1 and IgG3 with comparable affinities.8 Recently, it has been reported that platelets with the FcγRIIA-H/H131 genotype are unresponsive to HIT antibody in vitro.11 Yet, HIT has been reported to occur more commonly,11 if not exclusively,12 in donors who express at least one histidine allele at the 131 position. The explanation for this apparent discrepancy is not obvious, but may be attributable, in part, to differences in the specifics of the humoral response to heparin.
The antibodies that cause HIT recognize complexes composed of heparin or another glycosaminoglycan and either platelet factor 4 (PF4) or, on occasion, other heparin binding proteins.13-16 It has not been determined exactly how the mucopolysaccharide contributes to the formation of an antigenic epitope. However, of interest is the fact that the immune response to other polysaccharide antigens is often composed predominantly of antibodies of the IgG2 subclass.17 IgG1- and IgG3-containing antibodies typically activate complement, and cells coated with antibodies of these subclasses are rapidly cleared via FcγRI, FcγRII, and FcγRIII receptors expressed on splenic macrophages,17,18 whereas complexes containing predominantly IgG2 are handled less efficiently.19 Because platelets express ∼60% of the total cellular pool of FcγRII,20 differences in the responsiveness of platelets expressing FcγRIIA-H131 and R131 to IgG2 antiplatelet antibodies could influence the extent to which platelet aggregation occurs in vivo.
Based on this information, we tested the hypothesis that heparin-induced thrombocytopenia would be more likely to develop in those individuals whose immune response is composed predominantly of IgG1 and/or IgG3 antibodies directed at the PF4 component of the antigenic complex. Such antibodies would be predicted to bind equally well to platelets expressing either of the two FcγRIIA131 isoforms, sensitized platelets would be cleared rapidly by tissue macrophages, and the risk of thrombosis developing as a consequence of platelet activation would be lessened. In contrast, in those individuals in whom the immune response is composed predominantly of IgG2 antibodies, possibly directed towards the heparin component of the antigenic complex, platelet clearance by macrophages would be less efficient. The relatively prolonged survival of IgG2-sensitized platelets might permit sufficient time for antibody-mediated cross-linking of FcγRIIA and platelet activation to occur in susceptible individuals (H/H131 or H/R131), predisposing them to thrombosis. To test this hypothesis, we sought to correlate platelet FcγRIIA isoform and the subclass distributions of IgG anti-heparin/PF4 antibodies with the clinical presentation of patients with HIT/HITT.
MATERIALS AND METHODS
Patient inclusion and diagnostic criteria.Patients were identified upon referral to the Coagulation Laboratory of the Hospital of the University of Pennsylvania for laboratory testing for HIT/HITT. The diagnosis of HIT/HITT was made after review of each patient's medical records using published clinical criteria.21 The clinical histories of 62 patients referred to our Coagulation Laboratory between 1994 and 1996 were reviewed. Twenty-six patients were excluded from study, precluding an intention-to-treat analysis, because of our inability to either contact them due to death (n = 7), discharge (n = 2), lack of sufficient sample for antibody subclass determination (n = 6) or DNA extraction (n = 4), or a desire not to participate in the study on the part of the patient (n = 4) or attending physician (n = 3). The diagnosis was confirmed by measuring circulating heparin-dependent antiplatelet antibodies using the 14C-serotonin release assay (14C-SRA) as described.22 Informed consent was obtained from each patient and each patient's physician to permit chart review and to perform additional laboratory testing of residual blood samples. All studies were conducted according to the principles expressed in the Declaration of Helsinki.
Expression, isolation, and purification of recombinant human PF4 (r-PF4).r-PF4 was prepared in Escherichia coli BL21(DE3)pLys S (Novagen, Madison, WI) in a T7 promoter-driven vector and purified by a combination of heparin agarose and reverse phase chromatography as previously described.22 23 The purity of isolated r-PF4 was confirmed by sodium dodecyl sulfate (SDS)-gel electrophoresis and immunoblot using a polyclonal rabbit antihuman PF4 (Celsus Laboratories, Cincinnati, OH).
Heparin/PF4 enzyme-linked immunosorbent assay (ELISA) for determination of Ig isotypes and subclasses.Antibodies to heparin/PF4 complexes were detected using a modification of the heparin/PF4 ELISA previously described by us and others.13,22,24 25 Briefly, microtiter wells (Nunc, Roskilde, Denmark) were coated overnight at 4°C with 100 μL of a solution of phosphate-buffered saline (PBS) containing r-PF4 (15 μg/mL) and porcine heparin (0.4 U/mL; Elkins Sinn, Cherry Hill, NJ). Unreactive sites were blocked with 10% fetal bovine serum in PBS (10% FBS/PBS). Triplicate samples of patient (n = 36) or control plasma (n = 8), diluted 1:40 in PBS/10% goat serum (100 μL), were incubated for 1 hour at room temperature in wells containing heparin/PF4, and the unbound Ig was removed by washing with PBS/1% Tween-20 (PBST; BioRad, Richmond, CA).
To determine the Ig class of the bound antibody, alkaline phosphatase-conjugated goat antihuman IgG, IgA, or IgM (Sigma, St Louis, MO) diluted 1:5,000, 1:7,500, and 1:5000, respectively, in PBS/10% goat serum (100 μL) was added for 1 hour at 22°C. The alkaline phosphatase label was developed by adding 1 mg/mL p-Nitrophenyl phosphate (PNPP; Sigma) in substrate buffer (1 mol/L diethanolamine, 0.5 mmol/L MgCl2 , pH 9.8) and the optical density (OD) at 405 nm was measured. OD405 values greater than the mean + 3 standard deviations (SD) of control OD405 values were considered as positive.
To determine the IgG subclass distribution of the antibodies, wells were coated with heparin/PF4 complexes or with serial dilutions of subclass-specific human myeloma proteins diluted in PBS (from 10 μg/mL to 0.156 μg/mL of IgG1 , IgG2 , IgG3 , or IgG4 ). The heparin/PF4-coated wells were incubated with human serum as above, and myeloma protein-containing wells were incubated with 10% FBS/PBS. After 1 hour of incubation, wells were washed and mouse antihuman IgG subclass-specific antibodies were added for 1 hour at room temperature at the following dilutions: 1:15,000 for anti-IgG1 (Clone HP-6001; Sigma), 1:500 for anti-IgG2 (Clone SH-21; Sigma), 1:5,000 for anti-IgG3 (Clone HP-6050; Sigma), and 1:15,000 for anti-IgG4 (Clone HP-6025; Sigma). The wells were washed with PBST and goat antimouse antibody (Sigma) was added at optimal concentrations predetermined by measuring its binding to the isolated myeloma proteins (1:2,000 for anti-IgG1 , 1:200 for anti-IgG2 , 1:5,000 for anti-IgG3 , and 1:2,000 for anti-IgG4 ), and alkaline phosphatase activity was developed as described above. The optimal dilutions of anti–subclass-specific antibodies and goat antimouse antibodies were determined in initial experiments using myeloma proteins. No cross-reactivity among subclass-specific antibodies was noted when tested against nonmatching myeloma proteins. The concentration of IgG1-4 anti-heparin/PF4 was calculated using the Delta Soft II ELISA analysis software program (Biometallics, Princeton Junction, NJ) using concurrently measured myeloma standards and was expressed as a percentage of total bound IgG.
Genomic DNA isolation and FcR polymorphism analysis.Genomic DNA was isolated from residual blood cells as described26 after the 14C-SRA was performed. Briefly, the cells were washed free of plasma in 0.9% NaCl. The red blood cells were selectively lysed by adding 0.144 mol/L NH4Cl and 10 mmol/L NaHCO3 and the free hemoglobin was removed by centrifugation. The leukocyte pellet was washed with 0.9% NaCl and repelleted, and the cells were digested in Chambron's buffer A (10 mmol/L Tris-HCl, pH 8.0, 10 mmol/L EDTA, 10 mmol/L NaCl, 0.5% SDS) and proteinase K (10 mg/mL). High molecular weight genomic DNA was extracted using phenol:chloroform (1:1 vol:vol), precipitated in ethanol, and resuspended in 10 mmol/L Tris-HCl, 1 mmol/L EDTA, pH 7.5.
The FcγRIIA-H/R131 isoform was determined in samples from 36 patients with HIT/HITT and in 102 outpatient controls with no history of recent heparin exposure or past history of HIT. Polymorphism assignment was determined using a newly described allele-specific restriction enzyme digestion method developed in our laboratory that we have reported to show total concordance with the nucleotide sequence.27 Briefly, allele-specific enzyme restriction enzyme sites for Bst UI were introduced into an FcγRIIA PCR product as follows. A mutagenic sense primer (5′-GGAAAATCCCAGAAATTCTCGC-3′ ) was used to introduce a potential BstUI site on the 5′ end that terminates immediately adjacent to the polymorphic A/G nucleotide at codon 131. A second mutagenic antisense primer (5′-CAACAGCCTGACTACCTATTACGCGGG-3′ ) introduces a new BstUI site into every product on the 3′-end, serving as an internal control for the restriction digestion. The FcγRIIA genotype was determined by restriction enzyme digestion with BstUI followed by agarose gel electrophoresis. Expected lengths of fragments were as follows: uncut/undigested PCR product = 366 bp; H/H131 = 343 bp; R/R131 = 322 bp; and H/R131 = 343 bp and 322 bp. FcγRIIA genotype assignments were made only on completely digested products, ie, only those samples in which no 366-bp product was detected. Assignments were conducted in a blinded manner by two separate investigators who concurred in every analysis. Genotype assignments were confirmed by automated DNA sequencing in 11 HIT patients performed as previously described.27
Statistical analysis.Association of clinical characteristics, FcγRIIA genotypes, and presence of IgG2 subclass in patients with HIT or HITT was tested using χ2 analysis. The nonparametric Wilcoxon rank sum test was used to test for differences in the expression of IgG1 subclass antibodies between patients with HIT and HITT. Two-sided P values of ≤.05 were considered to be significant.
RESULTS
Patient characteristics.The salient clinical characteristics of the patient population studied are shown in Table 1. Twenty-three of the 36 patients (64%) experienced a clinically apparent thrombosis, of which 11 were arterial, 5 venous, and 7 both arterial and venous. The preponderance of cases of HITT compared with HIT in our study population likely reflects a referral bias in the samples submitted to our laboratory. There was no difference in the mean duration of heparin exposure in patients who developed thrombosis (mean = 7.6 ± 7.3 days) compared with those who did not (mean = 5.2 ± 3.3 days). There was no significant difference in the incidence of clinically overt cardiovascular disease (hypertension, diabetes mellitus, coronary artery, or peripheral vascular disease [19/23]) in patients who developed thrombosis and those who developed thrombocytopenia alone (10/13; χ2 = .17, P = .68).
Clinical Characteristics of 13 Patients With HIT and 23 With HITT
| Clinical Characteristics . | HIT (n = 13) . | HITT (n = 23) . |
|---|---|---|
| Sex | ||
| Female | 6 | 10 |
| Male | 7 | 13 |
| Mean age (yr) | 68 | 66 |
| Race | ||
| African-American | 1 | 3 |
| Caucasian | 12 | 20 |
| Mean heparin exposure (d) | 5.2 | 7.6 |
| History of cardiovascular disease | 10 | 19 |
| Type of thrombosis | ||
| Arterial | — | 11 |
| Venous | — | 5 |
| Both | — | 7 |
| Clinical Characteristics . | HIT (n = 13) . | HITT (n = 23) . |
|---|---|---|
| Sex | ||
| Female | 6 | 10 |
| Male | 7 | 13 |
| Mean age (yr) | 68 | 66 |
| Race | ||
| African-American | 1 | 3 |
| Caucasian | 12 | 20 |
| Mean heparin exposure (d) | 5.2 | 7.6 |
| History of cardiovascular disease | 10 | 19 |
| Type of thrombosis | ||
| Arterial | — | 11 |
| Venous | — | 5 |
| Both | — | 7 |
FcγRIIA genotype distribution in patients with HIT and HITT.Our first aim was to test the hypothesis that the prevalence of the FcRγIIA-His131 isoform would be greater in patients with HITT than in patients with HIT or among individuals in the general population. To do this, the gene encoding FcRγIIA was amplified from genomic DNA by polymerase chain reaction (PCR) using allele-specific primers into which a novel restriction site had been introduced.27 The resultant PCR products were digested with BstUI, and the migration pattern of the digest was analyzed by gel electrophoresis, allowing the H/R131 allotypes to be distinguished. This method correctly identified the H/R131 allotype in each of 50 normal patients in whom the nucleotide position 131 in the receptor was determined by direct sequencing. The alleles identified in the 36 patients with HIT/HITT and a total of 102 controls are shown in Table 2.
The FcγRIIA Genotype Distribution and Gene Frequency in Patients With HIT/HITT and Controls
| Clinical . | FcγRIIA Genotype . | Gene Frequency . | ||||
|---|---|---|---|---|---|---|
| . | N . | H/H131 . | H/R131 . | R/R131 . | H131 . | R131 . |
| HIT/HITT | 36 | 8 (22) | 19 (53) | 9 (25) | 0.486 | 0.513 |
| HIT | 13 | 2 (15) | 8 (62) | 3 (23) | 0.462 | 0.538 |
| HITT | 23 | 6 (26) | 11 (48) | 6 (26) | 0.5 | 0.5 |
| Controls | 102 | 20 (20) | 56 (55) | 26 (25) | 0.471 | 0.529 |
| Clinical . | FcγRIIA Genotype . | Gene Frequency . | ||||
|---|---|---|---|---|---|---|
| . | N . | H/H131 . | H/R131 . | R/R131 . | H131 . | R131 . |
| HIT/HITT | 36 | 8 (22) | 19 (53) | 9 (25) | 0.486 | 0.513 |
| HIT | 13 | 2 (15) | 8 (62) | 3 (23) | 0.462 | 0.538 |
| HITT | 23 | 6 (26) | 11 (48) | 6 (26) | 0.5 | 0.5 |
| Controls | 102 | 20 (20) | 56 (55) | 26 (25) | 0.471 | 0.529 |
χ2 analysis of HIT versus HITT versus controls or HIT versus HITT did not achieve statistical significance. Percentages are in parentheses.
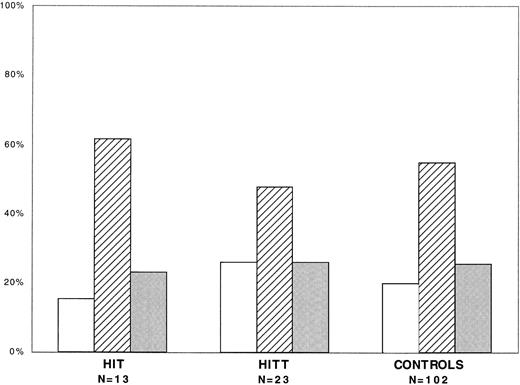
The distribution of FcγRIIA isoforms in patients with HIT and HITT and in healthy controls is shown in Fig 1. The H/H131, H/R131, and R/R131 isoforms were found in 22%, 53%, and 25%, respectively, of all patients with HIT/HITT compared, respectively, with 20%, 55%, and 25% of controls. Thus, there was no statistically significant difference in the prevalence of each isoform in patients with HIT/HITT and controls (χ2 = .11, P = .95) or between patients with HIT and those with HITT (χ2 = .75, P = .69).
FcγRIIA genotype distribution in HIT/HITT. FcγRIIA genotype distribution in patients with HIT (n = 13), HITT (n = 23), and controls (n = 102). Shown are percentages of each group expressing H/H131 (□), H/R131 (▨), or R/R131 ().
FcγRIIA genotype distribution in HIT/HITT. FcγRIIA genotype distribution in patients with HIT (n = 13), HITT (n = 23), and controls (n = 102). Shown are percentages of each group expressing H/H131 (□), H/R131 (▨), or R/R131 ().
Isotype distribution of anti-heparin/PF4 antibodies.Our second aim was to test the hypothesis that the prevalence of IgG2–anti-heparin/PF4 antibodies would be greater in patients with HITT than in patients with HIT. To do this, the class and subclass distribution of these antibodies were determined by ELISA. Neither IgG, IgA, nor IgM antibodies directed to heparin/PF4 were found in 2 patients' sera by ELISA; perhaps these antibodies recognized non-PF4–containing, heparin/protein complexes.16 In 13 of the 34 sera containing IgG antibodies, this was the only isotype detected, whereas IgG and IgM antibodies were found in combination in 6 patients (18%), IgG and IgA antibodies in 9 (26%), and all three isotypes were detected in the remaining 6 sera (18%). IgM plus IgG or IgA plus IgG antibodies were found in 10 (45%) patients with HITT, compared with 5 (42%) patients with thrombocytopenia alone. IgA antibodies in addition to IgG and/or IgM were noted in 11 patients with HITT (50%) when compared with 4 (33%) with HIT.
Optical Densities for IgG Subclass Assays for Control, HIT, and HITT Patients
| . | IgG1 . | IgG2 . | IgG3 . | IgG4 . |
|---|---|---|---|---|
| Controls (n = 8) | −0.002 ± 0.005 | 0.040 ± 0.057 | −0.006 ± 0.006 | 0.005 ± 0.006 |
| HIT (n = 12) | 0.706 ± 0.611 | 0.714 ± 0.735 | 0.455 ± 0.624 | 0.008 ± 0.013 |
| HITT (n = 22) | 0.685 ± 0.608 | 0.494 ± 0.805 | 0.05 ± 0.244 | 0.004 ± 0.008 |
| . | IgG1 . | IgG2 . | IgG3 . | IgG4 . |
|---|---|---|---|---|
| Controls (n = 8) | −0.002 ± 0.005 | 0.040 ± 0.057 | −0.006 ± 0.006 | 0.005 ± 0.006 |
| HIT (n = 12) | 0.706 ± 0.611 | 0.714 ± 0.735 | 0.455 ± 0.624 | 0.008 ± 0.013 |
| HITT (n = 22) | 0.685 ± 0.608 | 0.494 ± 0.805 | 0.05 ± 0.244 | 0.004 ± 0.008 |
The mean optical densities at 405 nm (OD405) and SD are shown for each subclass assay. OD405 values greater than mean + 3 SD are considered positive.
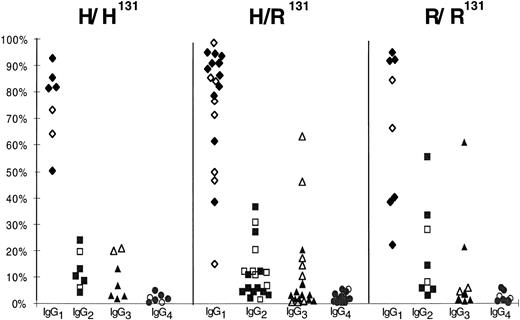
IgG subclass distribution in HIT/HITT. Distribution of IgG subclasses as a percentage of total anti-heparin/PF4 IgG in 34 HIT/HITT patients grouped by FcγRIIA genotype. Each panel represents the subclass distribution (IgG1-4 ) of patients with H/H131 (n = 7), H/R131 (n = 19), or R/R131 (n = 8). Open symbols refer to patients with HIT (n = 12), and solid symbols refer to patients with HITT (n = 22).
IgG subclass distribution in HIT/HITT. Distribution of IgG subclasses as a percentage of total anti-heparin/PF4 IgG in 34 HIT/HITT patients grouped by FcγRIIA genotype. Each panel represents the subclass distribution (IgG1-4 ) of patients with H/H131 (n = 7), H/R131 (n = 19), or R/R131 (n = 8). Open symbols refer to patients with HIT (n = 12), and solid symbols refer to patients with HITT (n = 22).
IgG subclass-specific anti-heparin/PF4 antibodies were measured by ELISA under conditions that had been optimized for each subclass individually using highly purified myeloma proteins. The ELISA method provides only an estimate of the amount of circulating antibody, because only antibodies with sufficient avidity to bind immobilized antigen and withstand the washing conditions are recognized. Sera were considered to contain significant amounts of subclass-specific antibody if the resultant OD405 was greater than or equal to the mean + 3 SD of that generated by 8 control sera studied concurrently. The mean OD405 values for controls and patients are shown in Table 3. Using these criteria, IgG subclass-specific antibodies were detected in 34 of the 36 HIT/HITT sera tested. IgG2 antibodies were detected in 21 of 34 (62%) of patient sera. However, a predominant IgG2 response was detected in only 2 patients. Rather, IgG1 antibodies were detected in 30 of 34 sera and in 12 cases was the only subclass found. IgG3 antibodies were detected in sera from 5 patients (only 1 of whom had HITT), whereas IgG4 antibodies were not detected in any sample. The subclass distribution of the 34 detectable IgG anti-heparin/PF4 antibodies expressed as the percentage of the total anti-heparin/PF4 IgG is shown in Fig 2. No statistically significant differences were found in the prevalence of IgG1 antibodies in patients with HITT compared with those with HIT as determined by the Wilcoxon Rank Sum test (P > .05), although a trend towards lower concentrations of IgG2 and IgG3 was noted in patients with the H/H131 phenotype (Fig 2).
Association between anti-heparin/PF4 isotype and FcγRIIA genotype.Our last goal was to determine whether IgG2 anti-heparin/PF4 antibodies and the H/H131 isoform occurred together more commonly in patients with HITT compared with HIT. The data shown in Fig 2 show that there were no differences in the prevalence of IgG2 anti-heparin/PF4 antibodies among patients with R/R131 (6/8), H/H131 (5/7), and H/R131 (10/19) isoforms (χ2 = 1.54, P = .46). Also, no differences were seen in the distribution of the three isoforms among the 13 patients with HITT and IgG2 antibodies (3 H/H131, 6 H/R131, and 4 R/R131) compared with the 8 patients with HIT and IgG2 antibodies (2 H/H131, 4 H/R131, and 2 R/R131), although too few patients fell into these various subcategories to perform statistical analysis of the data.
DISCUSSION
Antibodies to heparin/PF4 complexes have been detected in upwards of 90% of patients with HIT/HITT.13,14,22 The results of several recent studies have also shown an unexpectedly high frequency of such antibodies in all patients receiving heparin, especially among those who have had repetitive exposure to the drug.28,29 Yet, only approximately 1% of patients treated with heparin develop thrombocytopenia, and it has been estimated that only 10% to 20% of these individuals develop a clinically significant thrombotic complication.3 30 Thus, considerable gaps remain in our understanding of the relationship between anti-heparin/PF4 antibodies and the development of thrombosis.
Several groups of investigators have proposed that the untoward incidence of thrombosis in patients with heparin-induced thrombocytopenia may result from platelet activation that occurs when platelet FcγRIIA receptors are cross-linked by heparin-dependent antiplatelet antibodies.5,6 In support of this notion, it has been reported that a neutralizing anti-FcγRIIA antibody prevents platelet activation by HIT/HITT sera or plasma.5-7 Furthermore, Brandt et al11 recently reported that platelets containing at least one copy of His at position 131 of FcγRIIA were protected from activation by HIT sera in vitro. Yet, they and others have reported that patients whose platelets express FcγRIIA-H131 are more susceptible to develop HIT/HITT, leaving the exact relationship between receptor genotype, platelet activation, and clinical presentation open to further investigation.11 12
If platelet activation initiated by IgG anti-heparin/PF4 antibodies is sufficient to induce thrombosis, we reasoned that patients with HITT would indeed be more likely than patients with HIT or those in the general population to express the H131 isoform of FcγRIIA and to produce predominantly IgG2 heparin/PF4 antibodies. We based this hypothesis on the known increased affinity of FcγRIIA-H131 for IgG29,10 and the relative inefficiency of macrophage clearance of IgG2-containing immune complexes,19 a situation that together might be expected to favor platelet activation in vivo.
However, the results of the present study did not sustain this hypothesis. First, we found no differences in the distribution of the two FcγRIIA isoforms in 23 patients with HITT compared with 13 patients with HIT or when the entire HIT/HITT population was compared with 102 controls. Although our sample size would not preclude differences in the prevalence of the two FcγRIIA isoforms being detected when a larger cohort is analyzed, it is clear from our data that patients with the FcγRIIA-R/R131 isoform are not protected from developing thrombosis. Second, we found no apparent association between expression of the permissive FcγRIIA isoform, expression of IgG2 anti-heparin/PF4 antibodies, and thrombosis as predicted by our model. Although IgG2 anti-heparin/PF4 antibodies were indeed identified in a high proportion of patients (21/34), they were rarely found alone and were no more prevalent among patients with a history of thrombosis (13/22 [59%]) than among patients with thrombocytopenia alone (8/12 [67%]). Rather, IgG1 was the most prevalent subclass, occurring in 88% of HIT/HITT patients, and was the only isotype identified in 35%. However, our studies do not exclude the possibility that high-affinity IgG2 anti-heparin/PF4 antibodies that may bind to platelets in vivo are preferentially cleared from the circulation, as suggested by the trend towards a lower concentration of circulating IgG2 antibodies in patients with the H/H131 compared with those with R/R131 receptor isoform (Fig 2).
The present studies leave unresolved the issue of why only some patients exposed to heparin develop thrombocytopenia and thrombosis. In a few patients, IgM and/or IgA anti-heparin/PF4 antibodies may contribute to the process.31 In others, complement-mediated platelet activation or microparticle formation,32 antibody-mediated perturbation of endothelial cell function,24 33 or other, as yet uncharacterized, inherited or acquired vascular risk factors that predispose to a hypercoagulable state may all contribute to the risk of thrombosis in susceptible individuals. Additional studies are needed to determine the extent to which platelet activation precedes or results from such vascular injury.
Supported in part by National Institutes of Health Grants No. 40387 (D.B.C., M.P., and S.M.), HL 50790 (D.B.C.), HL 49517 (D.B.C.), HL 50827 (D.B.C.), HL 54749 (D.B.C., M.P., and S.M.), HL37419 (M.P.), and HL56265 (M.P.) and by a grant from Tobacco Research Council (No. 3152 to M.P.).
Address reprint requests to Douglas B. Cines, MD, Coagulation Laboratory, 7 Founders Pavilion, Hospital of the University of Pennsylvania, 3400 Spruce St, Philadelphia, PA 19104.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal