Abstract
The anti-CD25 immunotoxin (IT), RFT5-SMPT-dgA, was used in a phase I dose escalation trial in patients with refractory Hodgkin's lymphoma. The IT was constructed by linking the monoclonal antibody RFT5 via a sterically hindered disulfide linker to deglycosylated ricin-A. All patients in this trial were heavily pretreated with a mean of 5 (range, 2 to 8) different prior therapies, including autologous bone marrow transplantation in 8 of 15. The mean age was 29 years (range, 19 to 34 years). Thirteen of 15 patients had advanced disease (stage IV) with massive tumor burdens and 6 of 15 had B symptoms. The IT was administered intravenously over 4 hours on days 1, 3, 5, and 7 for total doses per cycle of 5, 10, 15, or 20 mg/m2. Patients received one to four cycles of treatment. The peak serum concentration of intact IT varied from 0.2 to 9.7 μg/mL. The serum half life (T1/2 ) of the IT ranged from 4.0 to 10.5 hours (mean, 6.1 hours). Side effects were related to vascular leak syndrome (VLS), ie, decreases in serum albumin, edema, weight gain, hypotension, tachycardia, myalgia, and weakness. Two patients had a National Cancer Institute (NCI) grade 2 allergic reaction with generalized urticaria and mild bronchospasm. At 15 mg/m2, 1 patient experienced a grade 3 myalgia. All 3 patients receiving 20 mg/m2 experienced NCI grade 3 toxicities (edema, nausea, dyspnea or tachycardia) and 1 patient had NCI grade 4 myalgia. Thus, the maximal tolerated dose was 15 mg/m2. Seven of 15 patients made human antiricin antibodies (≥1.0 μg/mL) and 6 of 15 developed human antimouse antibodies (≥1.0 μg/mL). Clinical response included 2 partial remissions, 1 minor response, 3 stable diseases, and 9 progressive diseases. As has been predicted from the preclinical tests, these data seem to indicate clinical effecicacy of this new IT in heavily pretreated Hodgkin's patients, thus warranting further clinical investigation.
HODGKIN'S LYMPHOMA (HL) is curable in 60% to 70% of patients with polychemotherapy regimens such as MOPP1 or ABVD2 and improved radiation techniques.3 However, less than 30% of those who relapse after initial chemotherapy attain durable disease-free remissions after second-line treatment.4 The outcome is even worse for those with primary refractory disease.5 Data from other malignant diseases such as colorectal cancer,6 myeloid leukemia,7 or non-Hodgkin's lymphoma (NHL)8 suggest that small numbers of residual tumor cells remaining after first-line treatment can cause late relapses. Thus, eliminating residual Hodgkin-Reed/Sternberg (H-RS) cells after first-line treatment might further improve the outcome of primary therapy in HL.
Immunotoxins (ITs) are ideally suited to eliminate residual tumor cells in HL for several reasons. (1) H-RS cells consistently express high levels of CD30 and IRac.9,10 (2) Human Hodgkin's lymphomas contain only a minority of malignant H-RS cells and are well vascularized.3 (3) Mechanisms of cell killing and side effects of ITs are completely different from those of conventional therapy. (4) ITs, unlike most chemotherapeutic agents, kill nondividing residual or dormant cells.11
The lymphoid activation markers CD25 and CD30 are present on nearly all H-RS cells but on only a small subset of activated lymphocytes.9 CD25 is the α-chain of the IL-2 receptor (IL-2R).12 This 55-kD glycoprotein is not expressed on resting lymphocytes and stem cells but is efficiently induced after T-cell activation. CD25 binds IL-2 with low affinity and has no signal transduction activity.13 IL-2Rs have been detected on cells at high levels in autoimmune disorders and on malignant cells in different hematopoietic malignancies.14 Strauchen and Breakstone15 were the first to show strong staining of H-RS cells using monoclonal antibodies (MoAbs) against CD25 in the majority of patients with HL.
We have evaluated available anti-CD25 and anti-CD30 MoAbs for their ability to make potent ITs against Hodgkin's-derived cell lines.16 The ITs were constructed by linking the antibody moiety via a sterically hindered linker (SMPT) to deglycosylated ricin-A (dgA). RFT5-SMPT-dgA (anti-CD25) was the most potent IT, inhibiting protein synthesis in L540 H-RS cells by 50% at a concentration (IC50 ) of 7 × 10−12 mol/L, which is identical to that of native ricin under the same experimental conditions. The binding of RFT5 to cryostat sections showed no major cross-reactivities with any normal human tissues other than lymphoid cells. Hence, a few large cells in tonsils and lymph nodes were stained. The antitumor activity of this IT was evaluated in nude mice with subcutaneous solid HL17 and in severe combined immunodeficiency (SCID) mice with disseminated human HL.18 In these mice, 40 μg of RFT5-SMPT-dgA destroyed more than 60% of solid H-RS tumors of 0.5-cm diameter and inhibited the growth of disseminated HL in the majority of the treated animals. RFT5-SMPT-dgA was at least seven times more effective than other ricin A-chain–based ITs, including those against CD30.19 In addition, RFT5 stained H-RS cells in fresh tissue sections from patients with HL nearly as intensely as the anti-CD30 MoAbs HRS-3 and Ber-H2.
Thus, RFT5-SMPT-dgA was selected for a Food and Drug Administration-approved clinical phase I trial in patients with refractory HL. We report here on the results of this phase I trial involving 15 patients with refractory HL. Responses in this group of heavily pretreated patients included 2 partial remissions (PR), 1 minor response (MR), 3 stable diseases (SD), and 9 progressive diseases (PD). The maximum tolerated dose (MTD) was 15 mg/m2.
PATIENTS AND METHODS
Patients.Fifteen patients with a histologically confirmed diagnosis of HL were entered into the trial and subsequently treated at the University of Cologne (Cologne, Germany). All patients were refractory to conventional therapy or had relapsed with evidence of progressive disease. To be eligible, patients older than 18 had to have measurable disease, a life expectancy of at least 3 months, a Karnofsky performance status of at least 50%, a creatinine level of less than 2.0 mg/100 mL, a creatinine clearance level of greater than 50 mL/min, a serum alanine aminotransferase (sALT) level less than three times the upper limit of normal, a total bilirubin level less than 1.5 mg/mL, an albumin level of greater than 75% of the lower limit of normal, and a cardiac ejection fraction of ≥35%. In addition, more than 30% of the H-RS cells had to stain with the anti-CD25 MoAb RFT5 if a tumor biopsy was feasible, although this was not mandatory. Exclusion criteria were uncontrolable infectious disease, collagen vascular disease, vasculitis, active second malignancy, chemotherapy or radiotherapy within 4 weeks, significant impairment of pulmonary function, a granulocyte count of less than 1,500/μL, a platelet count of less than 20,000/μL, any other major organ dysfunction unrelated to HL, pregnancy, and the presence of antibodies against mouse Ig or ricin greater than 1 μg/mL. Concomitant administration of steroids was permitted if patients had been treated with steroids for at least 4 weeks before enrollment. No change in steroid dose was allowed in patients evaluable for a clinical response.
IT.The IT, RFT5-SMPT-dgA, was prepared as described18 by linking dgA (Inland laboratories, Austin, TX) via the heterobifunctional crosslinker SMPT (Pierce, Rockford, IL) to the IgG1 murine MoAb RFT5 that recognizes the α-chain (CD25) of the IL-2R. The IT was formulated as a sterile solution at 0.5 mg/mL in 0.85% NaCl containing 5 mmol/L lysine. Lots of 5 mL to 20 mL were frozen and maintained at −70°C. A fraction of 0.5 mL of each lot was frozen to determine the concentration of the IT after thawing and filtering. Before use, the IT was filtered through a 0.22-μm filter.
Protocol design and regimen.RFT5-SMPT-dgA was administered intraveneously in 100 mL isotonic saline over 4 hours. The calculated total dose of the IT for one cycle of treatment was divided by four and administered on days 1, 3, 5, and 7. Cohorts with a minimum of 3 patients were treated with escalating doses of 5, 10, 15, and 20 mg/m2. Patients were not entered at the next higher level until all 3 patients in the previous cohort had completed study day 14. If 1 patient experienced grade III toxicity, 3 additional patients were enrolled at this dose level. If three grade III toxicities occurred at one dose level, then the previous dose level was regarded as the MTD. If 2 patients at one dose level experienced a grade III toxicity and 1 patient at the next dose level a grade IV toxicity, then the MTD was the dose at which the grade III toxicities occurred. If 1 patient experienced a grade III toxicity and another patient experienced a grade IV toxicity, then the previous dose level was defined as the MTD.
The study design was in accordance with the Declaration of Helsinki. This trial was approved by the Ethical Committees of the University of Cologne and the University of Texas, Southwestern Medical Center and performed under Food and Drug Administration Investigational New Drug application (IND No 3539). Before treatment, all patients gave written informed consent.
Pathology and eligibility.Biopsies of peripheral lymph nodes or bone marrow were used to analyze the expression of CD25 (RFT5) and CD30 (Ber-H2) on H-RS cells using the antistreptavidin-biotin complex (ABC) method. Because RFT5 did not stain the CD25 antigen on paraffin-embedded sections, the biopsy material was snap-frozen in liquid nitrogen and immunohistochemical analysis was performed using cryostatic sections. Patients in whom biopsies or fine-needle aspiration did not yield sufficient material for quantification of CD25 expression on H-RS cells were enrolled without knowledge of the antigen distribution if the diagnosis of HL had been histologically proven within the last 12 months.
Assessment of toxicity.Adverse events were graded according to the National Cancer Institute (NCI) common toxicity criteria as grade I (asymptomatic, easily tolerated), II (mild, tolerable), III (moderate, poorly tolerated), or IV (severe, life threatening). Vascular leak syndrome (VLS) was specifically graded as described elsewhere.20 In brief, grade I was defined as minimal ankle pitting edema; grade II as ankle pitting edema and weight gain of less than 15 lb; grade III as peripheral edema and weight gain of 15 to 25 lb or pleural effusion without pulmonary dysfunction; and grade IV as anasarca, pleural effusion, or ascites with respiratory deficit or edema greater than 25 lb; and grade V as pulmonary failure requiring mechanical ventilation assistance.
Evaluation of response.The staging procedure included clinical examination, routine blood analyses, chest x-ray, abdominal ultrasound, thoracal and abdominal computer tomography (CT) scans, skeleton scintigraphy, electrocardiogram, echocardiogram, body plethysmography, and bone marrow biopsy. Clinical staging was performed according to the Ann Arbor system. Clinical status and laboratory parameters were controlled before each cycle. Additional tumor evaluations were performed 28 to 35 days after the end of completion of treatment to document the duration of responses. Restaging, including CT scans of involved areas, was performed after each second cycle. World Health Organization criteria for response were used. A complete response (CR) was defined as the disappearance of all measurable disease for a minimum of 4 weeks without the appearence of new lesions. A partial remission (PR) was defined as a 50% or greater reduction in the sum of the products of the maximal and perpendicular diameters of all measurable lesions for at least 4 weeks. A minor response (MR) was defined as a decrease of 25% to 50% of the measurable tumor mass for at least 4 weeks without the appearence of new lesions. No change (NC) was defined when the criteria of CR, PR, MR, or PD were not met. Progressive disease (PD) was defined as the enlargement of measurable tumor volumes by more than 25% or the appearance of any new lesion. A minimum of 2 cycles was required to evaluate treatment efficacy, unless there was rapid progression. Responding patients were eligible for retreatment if they had less than 1 μg/mL human antimouse antibody and human anti-ricin antibody before the next cycle of treatment. Follow-up observation of patients after treatment was performed regularly.
Pharmacokinetics.To determine the levels of intact RFT5-SMPT-dgA in the peripheral blood of the patients, a newly developed enzyme-linked immunosorbent assay (ELISA) was used. Wells of a 96-well COSTAR microtiter plate (Greiner, Friedenhausen, Germany) were coated overnight at 4°C with 50 μL/well of 5 μg/mL affinity-purified rabbit-anti-ricin in coating buffer (0.2 mol/L Na2CO3 , 0.2 mol/L NaHCO3 ; Merck, Darmstadt, Germany). Plates were washed with buffer of TBS containing 0.2% Tween 20 (Merck) and 0.1% bovine serum albumin (BSA; Sigma, St Louis, MO) and were blocked for 1 hour at room temperature with 10% BSA and 10% horse serum in TBS. Thereafter, plates were washed three times again. Different concentrations (0.001 to 10 μg/mL) of IgG-dgA and patients sera diluted with 0.1% BSA and 10% horse serum in TBS were added to the plate and incubated at room temperature for 1 hour. After washing, 50 μL of (Fab′2 ) fragments of goat-antimouse IgG(H + L)-biotin (Medac, Hamburg, Germany; 1:10,000 in TBS containing 0.1% BSA and 0.2% Tween 20) were added and incubated for 1 hour at room temperature. Samples were then removed and plates were washed again. A streptavidine-horseradish peroxidase (HRP) conjugate (50 μL/well; Boehringer Mannheim, Mannheim, Germany) was added and incubated for 30 minutes. Bound IT was detected by addition of 100 μL of o-Phenylenediamine-dihydrochloride (Sigma). Extinction at 492 nm was measured with an ELISA reader (SLT Lab instruments, Crailsheim, Germany). Serum samples were obtained at −4, 0, 0.5, 1, 2, 4, 6, 8, 12, 24, 36, 48, 52, 72, 96, 100, 104, 120, 144, 148, 152, 168, 192, and 216 hours after the end of the first infusion. Elimination half-lifes (T1/2 ) and areas under the curve (AUC) were analyzed by using the PKCALC program (developed by Dr R.C. Shumaker, Merrel Dow Research Institute, Cincinnati, OH).21
Flow cytometry analyses.MoAbs against a variety of surface antigens, including CD3, CD4 CD8, CD16, CD19, CD25, CD45, CD56, and CD71, were purchased from Becton Dickinson (Mountain View, CA). Peripheral mononuclear blood cells (5 × 105 cells/test) were incubated with fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-labeled antibodies for 30 minutes in the dark. Cells were then washed and resuspended in ice-cold phosphate-buffered saline/0.01% NaN3 containing propidium iodide (PI; 2 μg/mL). A minimum of 1 × 104 cells was analyzed on a FACScan flow cytometer (Becton Dickinson) and the immunofluorescence data were displayed on a 4-decade log scale. PI emission was determined using linear amplification. All data were evaluated by Cellquest software (Becton Dickinson). To compare the alterations in the mean fluorescence intensity of the cells of each patient during therapy, the relative changes as compared with the values immediately before start of treatment were calculated. FACS analysis was performed 0, 4, 24, 168, and 216 hours after the initiation of the first IT infusion.
Cytokines and soluble antigens.The following cytokines and soluble antigens in the sera of the study patients were analyzed by standard ELISA methods: sCD25 (T-Cell Diagnostics, Cambridge, MA), sCD30 (Dako, Hamburg, Germany), sIL-1, sIL-2, sIL-6, and soluble tumor necrosis factor-α (sTNF-α; R & D Systems, Minneapolis, MN). Analyses were performed before the start of treatment and on study days 1, 3, 7, and 10 of each cycle.
Detection of human antimouse antibodies (HAMA) and human antiricin antibodies (HARA).HAMA and HARA were measured as previously described.22
RESULTS
Patients and tumor pathology.The demographics of the 15 patients treated are summarized in Table 1. Ten patients were men and 5 were women. The median age was 29 years (range, 19 to 34 years). Histopathology at first presentation was nodular sclerosis in most cases (10), followed by mixed cellularity (4) and lymphocyte predominance (1). Five of 15 patients had primary progressive disease. Most patients were heavily pretreated, with an average of 5 different chemotherapies (range, 2 to 8), including high-dose chemotherapy (HDCT) and autologous bone marrow transplantation (ABMT) in 8 of 15. In addition, all but 1 patient (no. 8) had received extensive radiotherapy.
Characteristics of Patients Treated With RFT5-SMPT-dgA
| Patient No. . | Age/Sex . | Histology . | Primary Resistant . | Prior Therapies . | ABMT/PSCT . | Karnofsky . | Stage . |
|---|---|---|---|---|---|---|---|
| 1 | 23/F | LP | N | 5 | N | 50 | IVB |
| 2 | 27/M | NS | N | 5 | Y | 70 | IVA |
| 3 | 34/M | NS | N | 8 | Y | 70 | IVB |
| 4 | 33/M | MC | N | 5 | Y | 50 | IVB |
| 5 | 31/F | NS | N | 4 | Y | 90 | IVA |
| 6 | 28/M | NS | Y | 6 | N | 60 | IVB |
| 7 | 32/F | NS | N | 5 | Y | 80 | IVA |
| 8 | 19/M | MC | Y | 2 | N | 80 | IVA |
| 9 | 34/M | NS | N | 6 | Y | 70 | IVA |
| 10 | 31/M | MC | Y | 5 | Y | 90 | IVA |
| 11 | 20/F | MC | N | 3 | N | 90 | IIA |
| 12 | 33/M | NS | N | 3 | Y | 80 | IVB |
| 13 | 28/M | NS | Y | 3 | N | 60 | IVB |
| 14 | 29/F | NS | Y | 6 | N | 90 | IVA |
| 15 | 33/M | NS | N | 3 | N | 90 | IIA |
| Patient No. . | Age/Sex . | Histology . | Primary Resistant . | Prior Therapies . | ABMT/PSCT . | Karnofsky . | Stage . |
|---|---|---|---|---|---|---|---|
| 1 | 23/F | LP | N | 5 | N | 50 | IVB |
| 2 | 27/M | NS | N | 5 | Y | 70 | IVA |
| 3 | 34/M | NS | N | 8 | Y | 70 | IVB |
| 4 | 33/M | MC | N | 5 | Y | 50 | IVB |
| 5 | 31/F | NS | N | 4 | Y | 90 | IVA |
| 6 | 28/M | NS | Y | 6 | N | 60 | IVB |
| 7 | 32/F | NS | N | 5 | Y | 80 | IVA |
| 8 | 19/M | MC | Y | 2 | N | 80 | IVA |
| 9 | 34/M | NS | N | 6 | Y | 70 | IVA |
| 10 | 31/M | MC | Y | 5 | Y | 90 | IVA |
| 11 | 20/F | MC | N | 3 | N | 90 | IIA |
| 12 | 33/M | NS | N | 3 | Y | 80 | IVB |
| 13 | 28/M | NS | Y | 3 | N | 60 | IVB |
| 14 | 29/F | NS | Y | 6 | N | 90 | IVA |
| 15 | 33/M | NS | N | 3 | N | 90 | IIA |
Abbreviations: NS, nodular sclerosis; MC, mixed cellularity; LP, lymphocyte predominance; N, no; Y, yes; PSCT, peripheral stem cell transplantation.
At study entry, the median performance status as measured by the Karnofsky index was 70 (range, 50 to 90). Most patients presented with advanced disease (stage IV: 13/15). Four patients were on steroids to control excessive fever or sweating. Organ involvement was as follows (Table 2): peripheral lymph nodes, 9 of 15; deep lymph nodes, 14 of 15; liver, 4 of 15; lung, 9 of 15; bone marrow, 7 of 13 (2 patients had not been evaluated within the last 12 months and refused biopsy); spleen, 1 of 10 (5 patients had been splenectomized); and other organs, 5 of 15 (muscle or meninx).
Organ Involvement of Patients Treated With RFT5-SMPT-dgA
| Patient. No. . | Peripheral Nodes . | Deep Nodes . | Liver . | Lung . | Bone Marrow . | Spleen . | Other* . |
|---|---|---|---|---|---|---|---|
| 1 | + | + | − | + | + | − | + |
| 2 | + | + | − | + | − | EX | + |
| 3 | + | + | − | − | − | − | + |
| 4 | − | + | + | − | + | EX | − |
| 5 | + | + | − | + | − | EX | − |
| 6 | + | + | − | + | + | EX | + |
| 7 | − | − | − | − | + | − | − |
| 8 | − | + | − | + | + | − | − |
| 9 | − | + | − | + | − | − | − |
| 10 | + | + | − | + | + | − | − |
| 11 | + | + | − | − | − | − | − |
| 12 | − | + | + | − | ND | EX | − |
| 13 | + | + | + | + | + | + | − |
| 14 | + | + | − | + | ND | − | + |
| 15 | − | + | − | − | ND | − | − |
| Patient. No. . | Peripheral Nodes . | Deep Nodes . | Liver . | Lung . | Bone Marrow . | Spleen . | Other* . |
|---|---|---|---|---|---|---|---|
| 1 | + | + | − | + | + | − | + |
| 2 | + | + | − | + | − | EX | + |
| 3 | + | + | − | − | − | − | + |
| 4 | − | + | + | − | + | EX | − |
| 5 | + | + | − | + | − | EX | − |
| 6 | + | + | − | + | + | EX | + |
| 7 | − | − | − | − | + | − | − |
| 8 | − | + | − | + | + | − | − |
| 9 | − | + | − | + | − | − | − |
| 10 | + | + | − | + | + | − | − |
| 11 | + | + | − | − | − | − | − |
| 12 | − | + | + | − | ND | EX | − |
| 13 | + | + | + | + | + | + | − |
| 14 | + | + | − | + | ND | − | + |
| 15 | − | + | − | − | ND | − | − |
Abbreviations: ND, not done; EX, previous splenectomy; −, no; +, yes.
Other organs involved were muscle and meninges.
The evaluation of CD25 expression on H-RS cells was hindered by the paucity of H-RS cells in most biopsies obtained; from a total of 5 lymph node biopsies performed, only 4 contained sufficient numbers of cells for a representative analysis (Table 3). In these 4 samples, more than 30% of the H-RS cells expressed the CD25 antigen. In 2 additional patients, material from lung (no. 9) or liver (no. 12) contained greater than 30% CD25+ H-RS cells. In contrast, the 4 bone marrow biopsies and the 1 fine needle biopsy did not yield sufficient amounts of material for a reliable analysis of the phenotype of H-RS cells. A general problem was the technical difficulty associated with reliable staining of bone marrow biopsies with the anti-CD25 MoAbs. CD30 expression was more pronounced than CD25 expression, except in patient no. 10. The percentage of CD25+ small lymphoid cells in the tissues analyzed was generally less than 1% (data not shown).
Staining of Biopsy Material of Patients Treated With RFT5-SMPT-dgA and Clinical Response
| Patient No. . | Biopsy . | H-RS Cells . | % CD25+ H-RS Cells . | % CD30+ H-RS Cells . | Response . |
|---|---|---|---|---|---|
| 1 | ND | ND | ND | ND | PD |
| 2 | Lymph node | +++ | ≥30% | ≥90% | MR |
| 3 | Lymph node | NE | NE | NE | SD |
| 4 | ND | ND | ND | ND | PD |
| 5 | ND | ND | ND | ND | SD |
| 6 | Lymph node | +++ | ≥30% | ≥90% | PD |
| 7 | ND | ND | ND | ND | SD |
| 8 | ND | ND | ND | ND | PD |
| 9 | Lung | +++ | ≥30% | ≥90% | PD |
| 10 | Lymph node | ++ | ≥40% | ≤10% | PR |
| 11 | Lymph node | ++ | ≥35% | ≥50% | PD |
| 12 | Liver | + | ≥30% | ≥50% | PR |
| 13 | ND | ND | ND | ND | PD |
| 14 | ND | ND | ND | ND | PD |
| 15 | ND | ND | ND | ND | PD |
| Patient No. . | Biopsy . | H-RS Cells . | % CD25+ H-RS Cells . | % CD30+ H-RS Cells . | Response . |
|---|---|---|---|---|---|
| 1 | ND | ND | ND | ND | PD |
| 2 | Lymph node | +++ | ≥30% | ≥90% | MR |
| 3 | Lymph node | NE | NE | NE | SD |
| 4 | ND | ND | ND | ND | PD |
| 5 | ND | ND | ND | ND | SD |
| 6 | Lymph node | +++ | ≥30% | ≥90% | PD |
| 7 | ND | ND | ND | ND | SD |
| 8 | ND | ND | ND | ND | PD |
| 9 | Lung | +++ | ≥30% | ≥90% | PD |
| 10 | Lymph node | ++ | ≥40% | ≤10% | PR |
| 11 | Lymph node | ++ | ≥35% | ≥50% | PD |
| 12 | Liver | + | ≥30% | ≥50% | PR |
| 13 | ND | ND | ND | ND | PD |
| 14 | ND | ND | ND | ND | PD |
| 15 | ND | ND | ND | ND | PD |
Abbreviations: ND, not done; NE, not evaluable; +, few; ++, moderate; +++, many.
Toxicity.All 3 patients at the first dose level of 5 mg/m2 total dose of RFT5-SMPT-dgA received 2 cycles of treatment. At the 10 mg/m2 dose level, 3 patients were treated, 1 receiving 2 cycles and 2 patients receiving 1 cycle. Because 2 grade III toxicities (myalgia; CKmax , 200 U/L; dyspnea) occured in patient no. 7 at the 15 mg/m2 dose level, 3 additional patients were treated at that level (total of 6). Patient no. 12 also achieved a grade III dyspnea. All 6 patients received 2 cycles, except for patient no. 9, who experienced a grade II allergic reaction during the first IT infusion of the second cycle that was subsequently stopped. In patient no. 10, 4 cycles were administered due to a substantial and continuing response. Three patients were treated at 20 mg/m2, with 2 receiving 2 cycles. Patient no. 15 received only 3 of the 4 planned IT infusions, ie, 75% of the calculated dose of 20 mg/m2. This was due to a grade IV myalgia on day 6 with a CKmax of 1,500 U/L. This reaction was rapidly reversed after the cessation of IT therapy and treatment with steroids (24 mg/d of dexamethasone for 4 days). All patients at this dose level experienced at least one grade III toxicity. Two patients had grade III nausea/vomiting requiring antiemetics, 2 patients had tachycardia (>140 bpm at rest) making the administration of beta-blocker necessary, and 1 patient experienced a VLS grade III with weight gain greater than 15 lb. Thus, the MTD of RFT5-SMPT-dgA is 15 mg/m2.
Table 4 lists all the adverse events according to standard NCI criteria. The most frequent side effects (≥NCI grade I) were myalgia (14/15), hypoalbuminemia (12/15), weight gain (15/15), tachycardia (9/15), dyspnea (8/15), hypotension (12/15), weakness/fatigue (15/15), and nausea/vomiting (9/15). All patients experienced VLS, with a trend towards more severe side effects at higher doses.
Toxicity in Patients Treated With RFT5-SMPT-dgA
| Patient No. . | Dose (mg/m2) . | Grade of Toxicity (WHO) . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Nausea/Vomiting . | Weakness . | Decrease in Albumin1 . | Weight Gain2 . | Hypotension3 . | Tachycardia . | Dyspnea . | Myalgia . | Joint Discomfort . | Thrombopenia . | Allergic Reaction . |
| 1 | 5 | 1 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 5 | 1 | 1 | 0 | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| 3 | 5 | 0 | 1 | 2 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| 4 | 10 | 0 | 1 | 2 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| 5 | 10 | 0 | 1 | 2 | 2 | 1 | 1 | 0 | 2 | 0 | 0 | 0 |
| 6 | 10 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 |
| 7 | 15 | 2 | 3 | 2 | 2 | 1 | 2 | 3 | 3 | 1 | 0 | 0 |
| 8 | 15 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 |
| 9 | 15 | 1 | 2 | 2 | 2 | 2 | 1 | 2 | 1 | 0 | 0 | 2 |
| 10 | 15 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| 11 | 15 | 2 | 2 | 2 | 2 | 1 | 1 | 2 | 2 | 1 | 2 | 0 |
| 12 | 15 | 1 | 2 | 1 | 1 | 2 | 2 | 3 | 2 | 0 | 0 | 2 |
| 13 | 20 | 0 | 2 | 1 | 3 | 2 | 3 | 2 | 1 | 0 | 0 | 0 |
| 14 | 20 | 3 | 3 | 2 | 2 | 1 | 3 | 3 | 2 | 1 | 0 | 0 |
| 15 | 20 | 3 | 3 | 2 | 2 | 2 | 2 | 3 | 4 | 2 | 0 | 0 |
| Patient No. . | Dose (mg/m2) . | Grade of Toxicity (WHO) . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Nausea/Vomiting . | Weakness . | Decrease in Albumin1 . | Weight Gain2 . | Hypotension3 . | Tachycardia . | Dyspnea . | Myalgia . | Joint Discomfort . | Thrombopenia . | Allergic Reaction . |
| 1 | 5 | 1 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 5 | 1 | 1 | 0 | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| 3 | 5 | 0 | 1 | 2 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| 4 | 10 | 0 | 1 | 2 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| 5 | 10 | 0 | 1 | 2 | 2 | 1 | 1 | 0 | 2 | 0 | 0 | 0 |
| 6 | 10 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 |
| 7 | 15 | 2 | 3 | 2 | 2 | 1 | 2 | 3 | 3 | 1 | 0 | 0 |
| 8 | 15 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 |
| 9 | 15 | 1 | 2 | 2 | 2 | 2 | 1 | 2 | 1 | 0 | 0 | 2 |
| 10 | 15 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| 11 | 15 | 2 | 2 | 2 | 2 | 1 | 1 | 2 | 2 | 1 | 2 | 0 |
| 12 | 15 | 1 | 2 | 1 | 1 | 2 | 2 | 3 | 2 | 0 | 0 | 2 |
| 13 | 20 | 0 | 2 | 1 | 3 | 2 | 3 | 2 | 1 | 0 | 0 | 0 |
| 14 | 20 | 3 | 3 | 2 | 2 | 1 | 3 | 3 | 2 | 1 | 0 | 0 |
| 15 | 20 | 3 | 3 | 2 | 2 | 2 | 2 | 3 | 4 | 2 | 0 | 0 |
Toxicity was evaluated in the first cycle.
Abbreviations: 1, percent maximum decrease in albumin (nadir) compared with the baseline pretreatment values (mean change: dose level 1, 25.7%; level 2, 31.2%; level 3, 25.4%; and level 4, 22.3%); 2, percent maximum increase over the starting body weight (mean change: dose level 1, 4.3%; level 2, 4.6%; level 3, 4.6%; and level 4, 7.7%); 3, Hypotension (systolic blood pressure <100 mmHg, percent maximum decrease in systolic blood pressure compared with baseline values; mean change: dose level 1, 22%; level 2, 19%; level 3, 20%; and level 4, 32%).
Clinical response.Overall, there were 2 PRs, 3 SDs, and 1 MR. Two patients at the 15 mg/m2 dose level achieved PRs lasting 4 and 18+ months, respectively. Patient no. 10 had involvement of mediastinal lymph nodes and lung. After 4 cycles of treatment, all lymph nodes were substantially reduced in size and number. The PR was maintained for 18+ months. Patient no. 12 had three histologically proven HD lesions in the liver measuring 6 cm × 3.5 cm, 4.5 cm × 4.5 cm, and 2 cm × 3 cm in diameter. Ten weeks after the first treatment with RFT5-SMPT-dgA, these lesions were not present. An additional parailiacal lymph node in this patient was not significantly affected by therapy. The responses are listed in Table 3.
Pharmacokinetics.The values for elimination half-life (T1/2 ), AUC, clearance (Cl), and maximum serum concentration (Cmax ) are listed in Table 5. In all patients, the Cmax was reached at the end of the IT infusion, returning to or close to baseline after 12 to 24 hours. The Cmax correlated only approximately with the administered dose. Possible reasons for the low coefficient of correlation are differences in tumor mass, variability of CD25 expression on tumor cells and peripheral blood cells, levels of soluble CD25 in the serum of the patients, and accessibility of the tumor. The Cmaxs achieved ranged from 0.2 to 9.7 μg/mL. The maximum T1/2s ranged from 3.97 to 10.53 hours, averaging 6.1 hours.
Pharmacokinetic Parameters of RFT5-SMPT-dgA
| Patient No. . | Dose (mg/m2) . | Cmax (μg/mL) . | T1/2max (h) . | AUC (mg × h/L)max . | Cl (mL/h)max . |
|---|---|---|---|---|---|
| 1 | 5 | 0.78 | 4.54 | 3.16 | 4,700 |
| 2 | 5 | 5.49 | 4.04 | 19.66 | 410 |
| 3 | 5 | 0.20 | 4.05 | 1.12 | 9,386 |
| 4 | 10 | 1.23 | 4.72 | 1.49 | 5,853 |
| 5 | 10 | 7.81 | 9.73 | 132.98 | 60 |
| 6 | 10 | 7.00 | 6.57 | 112.93 | 90 |
| 7 | 15 | 7.94 | 7.83 | 44.19 | 302 |
| 8 | 15 | 3.65 | 7.16 | 33.46 | 452 |
| 9 | 15 | 7.44 | 3.98 | 57.30 | 391 |
| 10 | 15 | 2.12 | 3.97 | 16.78 | 917 |
| 11 | 15 | 6.27 | 10.53 | 89.02 | 136 |
| 12 | 15 | 6.91 | 4.9 | 76.19 | 226 |
| 13 | 20 | 9.70 | 5.3 | 68.51 | 522 |
| 14 | 20 | 9.57 | 8.41 | 95.36 | 81 |
| 15 | 20 | 8.80 | 5.52 | 80.93 | 235 |
| Patient No. . | Dose (mg/m2) . | Cmax (μg/mL) . | T1/2max (h) . | AUC (mg × h/L)max . | Cl (mL/h)max . |
|---|---|---|---|---|---|
| 1 | 5 | 0.78 | 4.54 | 3.16 | 4,700 |
| 2 | 5 | 5.49 | 4.04 | 19.66 | 410 |
| 3 | 5 | 0.20 | 4.05 | 1.12 | 9,386 |
| 4 | 10 | 1.23 | 4.72 | 1.49 | 5,853 |
| 5 | 10 | 7.81 | 9.73 | 132.98 | 60 |
| 6 | 10 | 7.00 | 6.57 | 112.93 | 90 |
| 7 | 15 | 7.94 | 7.83 | 44.19 | 302 |
| 8 | 15 | 3.65 | 7.16 | 33.46 | 452 |
| 9 | 15 | 7.44 | 3.98 | 57.30 | 391 |
| 10 | 15 | 2.12 | 3.97 | 16.78 | 917 |
| 11 | 15 | 6.27 | 10.53 | 89.02 | 136 |
| 12 | 15 | 6.91 | 4.9 | 76.19 | 226 |
| 13 | 20 | 9.70 | 5.3 | 68.51 | 522 |
| 14 | 20 | 9.57 | 8.41 | 95.36 | 81 |
| 15 | 20 | 8.80 | 5.52 | 80.93 | 235 |
Maximum pharmacokinetic parameters of the first IT course consisting of 4 infusions over a 4-hour period every other day.
Abbreviations: Cmax , peak serum concentration; T1/2max , maximum elimination half-life; AUCmax , maximum area under the concentration versus time curve; Clmax , maximum clearance.
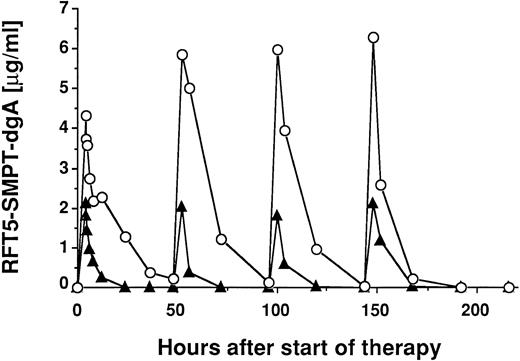
Figure 1 shows two plots of serum levels of the IT in 2 different patients treated with a total dose of 15 mg/m2.
Pharmacokinetics of RFT5-SMPT-dgA in 2 patients treated with 15 mg/m2 IT. Patient no. 10 (▴) received 4 infusions of 7.1 mg IT and patient no. 11 (○) received 4 infusions of 5.8 mg IT. Patient no. 10 had massive tumor burden with diffuse involvement of lung, liver, and bone marrow with high levels of sCD25 (6,497 U/mL), whereas patient no. 11 showed minimal tumor mass and significant lower sCD25 levels (523 U/mL). Both patients showed no significant differences in CD25 expression of peripheral mononuclear blood cells.
Pharmacokinetics of RFT5-SMPT-dgA in 2 patients treated with 15 mg/m2 IT. Patient no. 10 (▴) received 4 infusions of 7.1 mg IT and patient no. 11 (○) received 4 infusions of 5.8 mg IT. Patient no. 10 had massive tumor burden with diffuse involvement of lung, liver, and bone marrow with high levels of sCD25 (6,497 U/mL), whereas patient no. 11 showed minimal tumor mass and significant lower sCD25 levels (523 U/mL). Both patients showed no significant differences in CD25 expression of peripheral mononuclear blood cells.
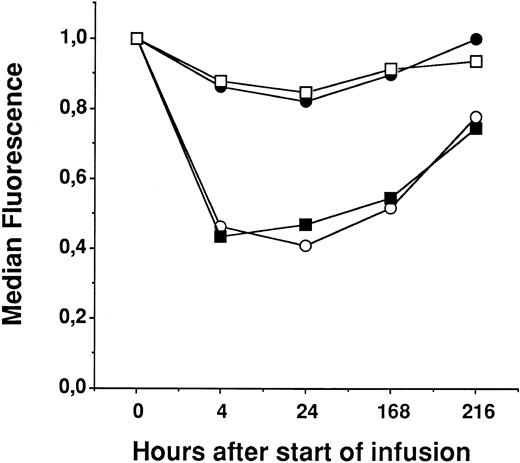
Flow cytometry analyses of peripheral cells.A variety of different antigens were analyzed on peripheral blood mononuclear cells (CD3, CD4, CD8, CD16, CD19, CD25, CD45RO, CD45RA, CD45RW, CD56, and CD71). Only the CD25+ cells showed significant changes during treatment with RFT5-SMPT-dgA. Figure 2 shows the median values of four CD25+ subpopulations of all 15 patients. CD25/CD3+ and CD25/CD4+ peripheral blood lymphocytes (PBLs) showed a significant decrease (P < .001) of median fluorescence intensity (MFI) immediately after the initiation of IT therapy, which persisted during therapy and recovered thereafter. The reduction of CD25+ cells probably reflects a destruction of CD25+ cells by the IT or, alternatively, a modulation of CD25, because the MoAb used for the FACS analysis of CD25 (anti-TAC) is cross-blocked by the MoAb used for construction of the IT (RFT5). CD25/CD8+ and CD25/CD19+ PBLs were much less effected. This might be due, in part, to the lower MFI of these cells as compared with that of the CD25/CD3+ and CD25/CD4+ cells.
FACS analysis of 4 subpopulations of CD25+ peripheral mononuclear blood cells in 15 patients treated with RFT5-SMPT-dgA. The median fluorescence before start of treatment was defined as relative value 1. Changes under therapy were calculated as compared with the value before IT therapy. CD25/CD3+ (○), CD25/CD4+ (▪), CD25/CD8+ (□), and CD25/CD19+ PBLs (•).
FACS analysis of 4 subpopulations of CD25+ peripheral mononuclear blood cells in 15 patients treated with RFT5-SMPT-dgA. The median fluorescence before start of treatment was defined as relative value 1. Changes under therapy were calculated as compared with the value before IT therapy. CD25/CD3+ (○), CD25/CD4+ (▪), CD25/CD8+ (□), and CD25/CD19+ PBLs (•).
Cytokines and soluble antigens.For a variety of cytokines (IL-2, IL-6, interferon-γ, and TNF-α) and soluble antigens (sCD25 and sCD30) in the sera of patients analyzed, there was no statistically significant correlation with treatment. However, there appeared to be a correlation between a decrease in sCD25 levels and clinical response. However, interpreting sCD25 levels in patients receiving MoAbs against CD25 can be misleading due to the interference of these MoAbs with the ELISA assay.
Development of HAMA and HARA.Table 6 summarizes the antibody response against the antibody and the toxin moiety of RFT5-SMPT-dgA. Six patients (40%) made HAMA greater than 1.0 μg/mL after the second to fourth cycle. In addition, 7 patients (47%) showed a HARA titer ≥1.0 μg/mL after the second to fourth cycle.
HAMA and HARA Titer in Patients Treated With RFT5-SMPT-dgA
| Patient No. . | Courses . | HAMA/HARA (μg/mL) . | Date . |
|---|---|---|---|
| Level 1 (5 mg/m2) | |||
| 1 | 2 | 0.2/0.7 | C2D10 |
| 2 | 2 | 10.2/37.9 | C2D10 |
| 3 | 2 | 0.26/1.0 | C2D9 |
| Level 2 (10 mg/m2) | |||
| 4 | 1 | 0.00/0.00 | C1D10 |
| 5 | 2 | 0.13/0.74 | C2D8 |
| 6 | 1 | 0.00/0.00 | C1D10 |
| Level 3 (15 mg/m2) | |||
| 7 | 2 | 7,689.6/1,323.7 | C2D9 |
| 8 | 2 | 522.3/165.9 | C2D9 |
| 9 | 2 | 0.00/0.00 | C1D10 |
| 10 | 4 | 0.8/0.00 | C4D8 |
| 11 | 2 | 1,375.0/570.0 | C2D10 |
| 12 | 2 | 129.0/876.8 | C2D9 |
| Level 4 (20 mg/m2) | |||
| 13 | 1 | 0.00/0.00 | C1D9 |
| 14 | 2 | 0.15/0.89 | C2D9 |
| 15 | 2 | 106.0/85.2 | C2D9 |
| Patient No. . | Courses . | HAMA/HARA (μg/mL) . | Date . |
|---|---|---|---|
| Level 1 (5 mg/m2) | |||
| 1 | 2 | 0.2/0.7 | C2D10 |
| 2 | 2 | 10.2/37.9 | C2D10 |
| 3 | 2 | 0.26/1.0 | C2D9 |
| Level 2 (10 mg/m2) | |||
| 4 | 1 | 0.00/0.00 | C1D10 |
| 5 | 2 | 0.13/0.74 | C2D8 |
| 6 | 1 | 0.00/0.00 | C1D10 |
| Level 3 (15 mg/m2) | |||
| 7 | 2 | 7,689.6/1,323.7 | C2D9 |
| 8 | 2 | 522.3/165.9 | C2D9 |
| 9 | 2 | 0.00/0.00 | C1D10 |
| 10 | 4 | 0.8/0.00 | C4D8 |
| 11 | 2 | 1,375.0/570.0 | C2D10 |
| 12 | 2 | 129.0/876.8 | C2D9 |
| Level 4 (20 mg/m2) | |||
| 13 | 1 | 0.00/0.00 | C1D9 |
| 14 | 2 | 0.15/0.89 | C2D9 |
| 15 | 2 | 106.0/85.2 | C2D9 |
The cycle (C) and day (D) on which the indicated concentrations of HAMA or HARA were detected is indicated under date.
DISCUSSION
In this phase I clinical trial, we evaluated the MTD and the dose-limiting toxicities (DLT) of an anti-CD25 ricin A-chain IT, RFT5-SMPT-dgA, in patients with refractory HD. The major findings are as follows. (1) The MTD of RFT5-SMPT-dgA is 15 mg/m2. (2) The DLTs are related to VLS, including myalgia, tachycardia, weakness, and fatigue. Other side effects were hypoalbuminemia, weight gain, dyspnea, nausea/vomiting, and hypotension. (3) Responses in this group of heavily preatreated patients (5 different chemotherapies; ABMT in 8/15) included 2 PRs, 1 MR, and 3 SDs. (4) The Cmaxs were roughly dose-dependent, varying from 0.2 to 9.7 μg/mL. The T1/2s of RFT5-SMPT-dgA ranged from 4.04 to 10.53 hours (mean, 6 hours). (5) Six of 15 patients made HAMA and 7 of 15 patients made HARA greater than 1.0 μg/mL.
Response, toxicity, and pharmacokinetics of the IT used in the present trial in patients with HL compare favorably to those observed with other IgG-SMPT-dgA–containing ITs against NHL. Amlot et al23 reported 5 PRs and 1 CR in 24 evaluable patients with relapsed NHL treated with the anti-CD22 IT, RFB4-SMPT-dgA. The IT was administered as 4-hour bolus infusion every 48 hours over 8 days. In a subsequent phase I trial, RFB4-SMPT-dgA was administered as a continuous infusion over 8 days with similiar clinical response (4 PRs) and toxicity.20 The MTD was 19.2 mg/m2/192 h. A comparison of bolus versus continuous infusion was reported recently using the anti-CD19 IT HD37-SMPT-dgA24 in patients with NHL. In this trial, there was 1 persisting CR of 23 evaluable patients in the bolus arm compared with 1 PR of 9 patients treated on the continuous infusion regimen. The MTD was 16 mg/m2/8 d in the intermittent bolus regimen and 19.2 mg/m2/8 d when the IT was administered via continuous infusion. Both regimens achieved comparable peak serum concentrations of the IT at the MTD. In addition, the toxicity profile was very similiar, suggesting no obvious advantage for a continuous infusion of IgG-based ricin A-chain ITs. In all these trials, VLS consisting of weight gain, edema, serum albumin decrease, and pulmonary edema was dose-limiting. The DLTs observed in the present trial were similiarly attributable to the VLS. However, we observed no major pulmonary edema or severe aphasia attributable to VLS. The major and limiting side effects were myalgia, tachycardia, weakness, and severe fatigue. One possible reason for the development of pulmonary edema is prior pulmonary involvement with lymphoma, as encountered in the first NHL trials.23 24 However, because 9 of 15 patients in our trial had proven pulmonary HL, other reasons must also be considered. The most obvious reason apart from the different histology is the younger average age of the Hodgkin's patients enrolled in the present trial (29 years) compared with an age range of 49 to 60 years in the NHL trials. Thus, different symptoms of the VLS might be more pronounced than others in different age groups.
The development of antibodies against the mouse IgG and against the toxin moiety of ITs has been observed in nearly all clinical trials with ITs.25 In general, the antibody response has been less pronounced in patients with lymphoma20,22,23 than in patients with solid tumors.26 In our trial, 6 patients made HAMA and 7 made HARA greater than 1.0 μg/mL. Both, HAMA and HARA are known to form complexes with the IT that are rapidly cleared.27 Another important factor that has been shown to substantially interfere with anti-CD25 MoAbs is the truncated soluble form (sCD25; sIL2Rα).28 These variables differed substantially between individual patients in this trial. An example of interindividual variability is shown in Fig 1 for 2 patients (nos. 10 and 11) both receiving the same IT dose (15 mg/m2). The higher IT concentrations in patient no. 11 can be explained in part by the greater than 10 times higher sCD25 level in patient no. 10. Other factors contributing to interindividual differences in IT leves are tumor mass, variability of CD25 expression on tumor cells and peripheral blood cells, and accessibility of the tumor.25
The IL-2R has been used as a target for immunotherapy in patients with chronic lymphocytic leukemia (CLL),29 adult T-cell leukemia,30 Szezary's syndrome,31 and other NHL.32 Patients with HD have been treated with recombinant fusion toxins such as DAB389IL-2 or DAB486IL-2, which are composed of a deletion mutant of diphtheria toxin (DT) and IL-2.32 LeMaistre et al33 used DAB486IL-2 in a phase I trial against CD25+ hematologic malignancies and reported no response in their 3 Hodgkin's patients. In contrast, one complete remission lasting more than 2 years was reported in 1 of 4 HL patients treated with DAB486IL-2.34 Foss et al35 recently reported the results of a phase I trial involving 73 lymphoma patients treated with DAB389 -IL-2. None of 17 patients with HL responded to the recombinant toxin. Although the clinical efficacy of ITs should be judged very carefully when based on phase I data, possible reasons contributing to these results are the shorter half-life of DAB389 -IL2 and DAB486IL-2 as compared with IgG-based ITs such as RFT5-SMPT-dgA and differences in antitumor efficacy, although no direct comparison of either DAB389 -IL-2 or DAB486IL-2 with RFT5-SMPT-dgA on Hodgkin cells is available. Another possible reason for ineffective DAB-IL-2 molecules is the possibility that Hodgkin cells express CD25 without the other subunits of the IL-2R.
CD30, another candidate antigen to target residual H-RS cells, has been used in labeling experiments in patients with end-stage HL. After the intravenous application of 20 to 30 mg of the anti-CD30 MoAb BerH2, Falini et al36 showed strong staining of nearly all H-RS cells on different tissue samples. They subsequently treated 12 HL patients with a chemically linked BerH2-Saporin-6 IT.37 Fever, malaise, anorexia, fatigue, mild myalgias, weight gain, and a fourfold to fivefold increase in liver enzymes were the main toxicities. Of the 12 patients treated, 4 achieved PRs and 3 had MRs, with a median duration of 2 months. The MTD of 0.8 mg/kg was established by reversible liver toxicity and VLS.
Ricin A-chain ITs constructed with BerH2 or other first generation anti-CD30 MoAbs gave weaker ITs when compared with the anti-CD25 IT RFT5-SMPT-dgA.38,39 Recently, new MoAbs against the CD30 antigen have become available40 that make fivefold to 7.5-fold more effective ricin A-chain ITs when compared with their predecessors.41 The most potent new anti-CD30 IT, Ki-4-SMPT-dgA, is currently being prepared for clinical trials. The rationale is to use the anti-CD25 IT RFT5-SMPT-dgA together with the new anti-CD30 IT. Experiments against H-RS cells in vitro and solid Hodgkin's tumors in mice showed that a mixture of two or more ITs (cocktail) against different antigens on H-RS cells are superior to the use of single ITs.42 In particular, relapses occured less frequently compared with animals after single IT treatment (5% v 33%). In NHL, IT cocktails have demonstrated superior effects in vitro43 and in SCID mice bearing disseminated Daudi lymphoma.44
In conclusion, RFT5-SMPT-dgA has shown encouraging efficacy at a dose level that is associated with tolerable toxicities. Multiple cycles of treatment could be administered without evidence of cumulative toxicities. RFT5-SMPT-dgA is currently being evaluated on a broader scale, including patients with smaller tumor masses and patients with NHL.
Supported by the Deutsche Krebshilfe Grant No. W 125/94/En2.
Address reprint requests to Andreas Engert, MD, Klinik I fuer Innere Medizin, Universitaet zu Koeln, Labor fuer Immuntherapie, LFI, EB 4, R 703, Joseph-Stelzmann-Straβe 9, D-50924 Koeln, Germany.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal