Abstract
The purpose of this report is to review the Fred Hutchinson Cancer Research Center experience of treating patients with venocclusive disease of the liver (VOD) after marrow transplantation using recombinant human tissue plasminogen activator (rh-tPA) and heparin. The charts of 42 patients who had received rh-tPA and heparin for the treatment of VOD between February 1991 and December 1995 were reviewed. Response to rh-tPA and heparin was defined as a reduction in total serum bilirubin by 50% within 10 days of starting treatment. Total serum bilirubin, percent weight gain, and serum creatinine before, after, and at the start of rh-tPA and heparin were examined to determine whether these laboratory values distinguished patients who responded to treatment from those who did not. We also evaluated whether evidence of multiorgan failure (requirement for supplemental oxygen, requirement for hemodialysis, requirement for mechanical ventilation) or whether the calculated probability of a fatal outcome from VOD could discriminate responders from nonresponders. In addition, the incidence and outcome of bleeding as a major complication of thrombolytic therapy was examined. Twelve patients responded to rhtPA and heparin and 30 patients did not. There were no statistically significant differences between responders and nonresponders in the day treatment was started, dose of rh-tPA, total serum bilirubin, and percent weight gain before, after, or at the start of treatment, or the calculated probability of dying from VOD on the day treatment with rh-tPA and heparin was begun. More nonresponding patients required dialysis or mechanical ventilation (11 of 30) before or at the start of rh-tPA and heparin than responding patients (0 of 12), P = .0183. Serum creatinine was greater at the start of treatment in nonresponding patients (1.9 ± 1.3 mg/dL) than in responding patients (1.1 ± 0.4 mg/dL), P = .0794. Ten patients had severe bleeding episodes, which resulted in death in three patients and may have contributed to death in an additional three patients. Treatment for VOD using rh-tPA and heparin was successful in 29% of patients but was associated with a significant risk of life-threatening hemorrhage. Requirement for supplemental oxygen, dialysis, or mechanical ventilation before the start of treatment were prognostic indicators of no response to thrombolytic therapy. We do not recommend treatment using tPA and heparin in patients with severe VOD who have already developed multiorgan dysfunction.
HEPATIC VENOCCLUSIVE disease (VOD) remains one of the most serious complications of high-dose cytoreductive therapy.1 The syndrome is the result of endothelial damage and hepatocyte injury in zone 3 of the liver acinus, which results in jaundice, painful hepatomegaly, and fluid retention. VOD has been reported to occur in more than half of marrow transplant patients,2,3 with a mortality rate of up to 39% in affected patients.2,4 The results of clinical trials aimed at prevention have been mixed.5-13 Even less well established is the appropriate treatment for patients with VOD.
Shulman et al14 reported that factor VIII and fibrinogen were deposited in subendothelial zones of terminal hepatic venules, leading to microthrombotic occlusion of sinusoidal blood flow. Based on this observation, many investigators have used thrombolytic agents to treat patients with VOD.15-24 These reports have been largely anecdotal; there are no controlled studies.
Eighty patients treated with recombinant human tissue plasminogen activator (rh-tPA) for VOD have been reported in the literature,15-24 with only one series including more than 10 patients.24 Treatment was reportedly successful in 28 of these patients. Life-threatening hemorrhage occurred in 16 patients, several of whom had fatal bleeding. Because of the great variability in the criteria used to institute thrombolytic therapy for VOD and the doses of rh-tPA employed, the efficacy of this agent remains unclear.
We reviewed the charts of the 42 patients who received rh-tPA and heparin at the Fred Hutchinson Cancer Research Center (FHCRC) from 1989 through 1995. We sought to determine the proportion of responding patients, to ascertain whether response to rh-tPA and heparin could be predicted, and to judge the safety of this agent in the setting of marrow transplantation.
MATERIALS AND METHODS
Marrow transplant preparation.Patients at the FHCRC were prepared for transplantation using one of the preparative regimens shown in Table 1 and, when appropriate, received graft-versus-host disease (GVHD) prophylaxis (Table 1).
Patient Characteristics
| No. of patients | 42 |
| Median age in yr (range) | 38.5 (1.6-62.7) |
| Diagnoses | |
| CML | 10 |
| ALL | 9 |
| AML/myelodysplasia | 8 |
| Solid tumors | 5 |
| Multiple myeloma | 6 |
| Non-Hodgkin's lymphoma | 1 |
| Other | 2 |
| Type of transplant | |
| Allogeneic | 31 |
| Autologous | 10 |
| Syngeneic | 1 |
| Preparative regimen | |
| Cy/TBI | 18 |
| Bu/Cy | 8 |
| Bu/Cy/TBI | 5 |
| Bu/Mel/TT | 7 |
| Total marrow radiation/Bu | 1 |
| ATG/Cy/TBI | 3 |
| HLA match (allogeneic recipients only) | |
| Matched sibling | 12 |
| Mismatched family member | 6 |
| Unrelated donor | 31 |
| GVHD prophylaxis (allogeneic recipients) | |
| Cyclosporine | 2 |
| Cyclosporine/MTX or TMTX | 25 |
| Cyclosporine + prednisone | 3 |
| FK506 + MTX 1 |
| No. of patients | 42 |
| Median age in yr (range) | 38.5 (1.6-62.7) |
| Diagnoses | |
| CML | 10 |
| ALL | 9 |
| AML/myelodysplasia | 8 |
| Solid tumors | 5 |
| Multiple myeloma | 6 |
| Non-Hodgkin's lymphoma | 1 |
| Other | 2 |
| Type of transplant | |
| Allogeneic | 31 |
| Autologous | 10 |
| Syngeneic | 1 |
| Preparative regimen | |
| Cy/TBI | 18 |
| Bu/Cy | 8 |
| Bu/Cy/TBI | 5 |
| Bu/Mel/TT | 7 |
| Total marrow radiation/Bu | 1 |
| ATG/Cy/TBI | 3 |
| HLA match (allogeneic recipients only) | |
| Matched sibling | 12 |
| Mismatched family member | 6 |
| Unrelated donor | 31 |
| GVHD prophylaxis (allogeneic recipients) | |
| Cyclosporine | 2 |
| Cyclosporine/MTX or TMTX | 25 |
| Cyclosporine + prednisone | 3 |
| FK506 + MTX 1 |
Abbreviations: Bu, busulfan; Cy, cyclophosphamide; TBI, total body irradiation; Mel, melphalan; TT, thiotepa; ATG, antithymocyte globulin; MTX, methotrexate; TMTX, trimetrexate; CML, chronic myeloid leukemia; ALL, acute lymphocytic leukemia; AML, acute myeloid leukemia.
Patient selection.VOD was defined clinically when two of the following features were present by day 20 posttransplant: jaundice plus painful hepatomegaly or unexplained weight gain.25 Forty-two patients who developed VOD after transplantation received rh-tPA and heparin. Ten patients with established severe VOD were enrolled on a protocol approved by the Institutional Review Board and were reported previously.16 Thirty-two additional patients gave informed consent and were treated at the discretion of the attending physician because of evidence that the patient in question was at risk of dying from VOD. Patients were excluded from treatment with rh-tPA because of ongoing bleeding, a history of central nervous system (CNS) lesions, or the inability to achieve platelet counts of >15,000/μL after transfusion. Abnormal mental status was evaluated by computed tomography or magnetic resonance scan of the head before thrombolytic therapy was initiated.
Treatment.rh-tPA was administered at total doses ranging from 5.4 mg (in an infant) to 120 mg, given over 2 to 4 days by 4-hour infusion. Heparin was administered as a bolus of 1,000 U at the start of rh-tPA and continued by constant infusion at a dose of 150 U/kg/d for 10 days.
Patient monitoring.Total serum bilirubin, serum creatinine, blood urea nitrogen (BUN), weight, complete blood count, fibrinogen, prothrombin time, activated partial thromboplastin time (aPTT), and fibrin degradation products were obtained before the start of therapy with rh-tPA and heparin and three times daily for 3 consecutive days. Thereafter, coagulation studies were performed daily until completion of the heparin infusion. Heparin dose was adjusted to maintain the aPTT to ≤1.2 times the upper limit of normal. Observation for any evidence of bleeding was made daily.
Definition of response.Response to rh-tPA and heparin was defined as a reduction in total serum bilirubin by 50% within 10 days of the start of treatment. Diuresis, weight reduction, and resolution of encephalopathy were not used in the evaluation of response because concurrent therapies (eg, diuretics, dialysis, and ventilatory support) might influence each of these parameters.
Evaluation of multiorgan failure and major bleeding.Renal insufficiency was defined by a doubling of the baseline creatinine (ie, the lowest serum level 1 to 3 days before marrow infusion). Renal failure was defined as a creatinine level of ≥3 mg/dL, a blood urea nitrogen ≥80 mg/dL, or the need for hemodialysis. The need for oxygen support was based on documentation of respiratory compromise as judged by arterial blood gas determination. Major bleeding was defined as any bleeding in the CNS or lungs, or requirement of ≥2 units of red blood cells per day for 2 consecutive days for bleeding from any site.
Statistical methods.A logistic regression model was used to estimate the probability of fatal VOD based on the patient's total serum bilirubin and percent weight gain on the day rh-tPA and heparin were begun, as previously described.26 The calculated probabilities of dying from VOD in the responding and nonresponding patients were compared using the Wilcoxon rank-sum test. Factors that may have influenced a response to therapy in responders and nonresponders were compared using the Fisher's exact test for categoric variables and the Mann-Whitney U test for continuous variables.
RESULTS
Patient characteristics.Forty-two patients were treated with rh-tPA and heparin. Demographic characteristics and transplant information are shown in Table 1.
Administration of tPA and heparin.Treatment with rh-tPA and heparin was begun a median of 12 days (range, 1 to 73 days) posttransplant. The median total dose was 60 mg (range, 5.4 to 120 mg) administered over 2 to 4 days. Heparin was begun at a dose of 1,000 U intravenously at the start of rh-tPA, followed by 150 U/kg/d by continuous infusion for 10 days.
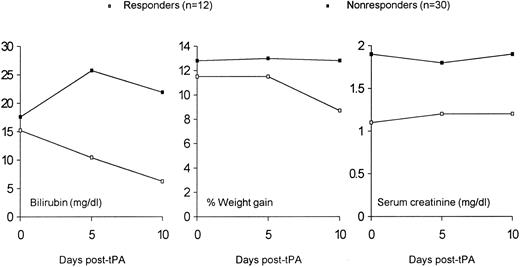
Efficacy of treatment.Twelve patients responded to treatment with rh-tPA and heparin. The total serum bilirubin in responding patients decreased from a mean of 15.2 mg/dL (range, 2.9 to 34.3 mg/dL) at the start of treatment to a mean of 6.2 mg/dL 10 days after the start of therapy. Mean total serum bilirubin was 17.6 mg/dL at the start of therapy in the 30 nonresponding patients. Only 12 nonresponders were alive 10 days after the start of treatment. Their mean total serum bilirubin was 22.1 mg/dL (range, 2.4 to 38.3 mg/dL). Mean total serum bilirubin prior to, at the start of, and after treatment with rh-tPA and heparin are shown in Fig 1.
Mean total serum bilirubin as a function of time before or after the start of rh-tPA and heparin. All responding patients were alive 5 and 10 days after the start of rh-tPA and heparin. Twenty-seven and 12 nonresponding patients were alive 5 days and 10 days, respectively, after the start of rh-tPA and heparin.
Mean total serum bilirubin as a function of time before or after the start of rh-tPA and heparin. All responding patients were alive 5 and 10 days after the start of rh-tPA and heparin. Twenty-seven and 12 nonresponding patients were alive 5 days and 10 days, respectively, after the start of rh-tPA and heparin.
Predictors of response.The presence of renal or pulmonary failure was a strong predictor of response, as no patient on dialysis or mechanical ventilation had a response to thrombolytic therapy (Table 2). Even less severe dysfunction of the kidneys had an influence on response, as evidenced by trends for higher serum creatinine and need for supplemental oxygen before the start of therapy in nonresponders compared with responders (P = .08 for both comparisons). No other factors were able to differentiate responders from nonresponders. Both groups were deeply jaundiced and had gained equivalent amounts of weight before the start of treatment.
Characteristics of Responding and Nonresponding Patients (mean ± SD)
| Parameter . | All Patients (n = 42) . | Responding Patients . | Nonresponding Patients . | P Value* . |
|---|---|---|---|---|
| . | . | (n = 12) . | (n = 30) . | . |
| Day posttransplant of start of tPA | 17.5 ± 16.7 | 15.2 ± 15 | 18.4 ± 17.5 | .6163 |
| tPA dose, mg | 54.7 ± 28.5 | 58.3 ± 24.8 | 53.2 ± 30.1 | .5402 |
| % Weight gain 10 d before tPA | 2.8 ± 4.5 | 2.2 ± 3.4 | 3.0 ± 4.9 | .5588 |
| % Weight gain 5 d before tPA | 6.5 ± 5.2 | 6.2 ± 4.8 | 6.6 ± 5.4 | .5968 |
| % Weight gain at start of tPA | 12.5 ± 7.2 | 11.5 ± 6.9 | 12.8 ± 7.3 | .5776 |
| Total serum bilirubin 10 d before tPA (mg/dL) | 3.5 ± 4.1 | 3.1 ± 3.6 | 3.6 ± 4.3 | .7174 |
| Total serum bilirubin 5 d before tPA (mg/dL) | 6.6 ± 6.3 | 6.5 ± 6.1 | 6.6 ± 6.5 | .7488 |
| Total serum bilirubin at start of tPA (mg/dL) | 16.9 ± 8.5 | 15.2 ± 7.9 | 17.6 ± 8.7 | .3582 |
| Serum creatinine 10 d before tPA | 0.8 ± 0.3 | 0.9 ± 0.2 | 0.8 ± 0.3 | .7070 |
| Serum creatinine 5 d before tPA (mg/dL) | 1.0 ± 0.5 | 0.8 ± 0.2 | 1.0 ± 0.6 | .3278 |
| Serum creatinine at start of tPA (mg/dL) | 1.7 ± 1.2 | 1.1 ± 0.4 | 1.9 ± 1.3 | .0794 |
| No. of patients on dialysis before tPA | 6/42 | 0/12 | 6/30 | .1589 |
| No. of patients requiring O2 before tPA | 21/42 | 3/12 | 18/30 | .0855 |
| No. of patients on mechanical ventilation before start of tPA | 8/42 | 0/12 | 8/30 | .0804 |
| No. of patients on either dialysis or mechanical ventilation before start of tPA | 11/42 | 0/12 | 11/30 | .0183 |
| Parameter . | All Patients (n = 42) . | Responding Patients . | Nonresponding Patients . | P Value* . |
|---|---|---|---|---|
| . | . | (n = 12) . | (n = 30) . | . |
| Day posttransplant of start of tPA | 17.5 ± 16.7 | 15.2 ± 15 | 18.4 ± 17.5 | .6163 |
| tPA dose, mg | 54.7 ± 28.5 | 58.3 ± 24.8 | 53.2 ± 30.1 | .5402 |
| % Weight gain 10 d before tPA | 2.8 ± 4.5 | 2.2 ± 3.4 | 3.0 ± 4.9 | .5588 |
| % Weight gain 5 d before tPA | 6.5 ± 5.2 | 6.2 ± 4.8 | 6.6 ± 5.4 | .5968 |
| % Weight gain at start of tPA | 12.5 ± 7.2 | 11.5 ± 6.9 | 12.8 ± 7.3 | .5776 |
| Total serum bilirubin 10 d before tPA (mg/dL) | 3.5 ± 4.1 | 3.1 ± 3.6 | 3.6 ± 4.3 | .7174 |
| Total serum bilirubin 5 d before tPA (mg/dL) | 6.6 ± 6.3 | 6.5 ± 6.1 | 6.6 ± 6.5 | .7488 |
| Total serum bilirubin at start of tPA (mg/dL) | 16.9 ± 8.5 | 15.2 ± 7.9 | 17.6 ± 8.7 | .3582 |
| Serum creatinine 10 d before tPA | 0.8 ± 0.3 | 0.9 ± 0.2 | 0.8 ± 0.3 | .7070 |
| Serum creatinine 5 d before tPA (mg/dL) | 1.0 ± 0.5 | 0.8 ± 0.2 | 1.0 ± 0.6 | .3278 |
| Serum creatinine at start of tPA (mg/dL) | 1.7 ± 1.2 | 1.1 ± 0.4 | 1.9 ± 1.3 | .0794 |
| No. of patients on dialysis before tPA | 6/42 | 0/12 | 6/30 | .1589 |
| No. of patients requiring O2 before tPA | 21/42 | 3/12 | 18/30 | .0855 |
| No. of patients on mechanical ventilation before start of tPA | 8/42 | 0/12 | 8/30 | .0804 |
| No. of patients on either dialysis or mechanical ventilation before start of tPA | 11/42 | 0/12 | 11/30 | .0183 |
P values compare responding with nonresponding patients.
The probability of dying as a result of VOD was estimated using a previously described method26 for the 9 responding patients and 19 nonresponding patients who began treatment on or before day 16 posttransplant (the model's coefficients were calculated up to day 16 and not beyond). The mean probabilities of a fatal outcome were calculated based on total serum bilirubin and percent weight gain on the day rh-tPA and heparin were begun and were 59% for responders and 64% for nonresponders. These probabilities were not significantly different (P = .56).
Bleeding as a major complication.Thirty-seven patients (88%) bled during or after treatment with rh-tPA and heparin, which was severe in 10 patients (28%). Bleeding occurred a median of 3 days (range, 0 to 13 days) after the start of thrombolytic therapy. The median platelet count at the start of bleeding was 28,000/μL (range, 9,000 to 88,000/μL) in the 10 patients with major bleeding complications. Nine were on heparin at the time of bleeding. Two had laboratory evidence of disseminated intravascular coagulation. Two patients had fatal intracranial hemorrhages and 1 had a massive and fatal pulmonary hemorrhage. Three other patients had major hemorrhages that may have contributed to death. One bled in the lungs and brain while 2 had pulmonary hemorrhages. Four other patients had serious bleeding episodes which did not contribute to death. The characteristics of the 10 patients who had major bleeding complications are shown in Table 3.
Sites of Major Bleeding
| Bleeding Site(s) . | No. of Patients . | Bleeding Caused Death . | |
|---|---|---|---|
| . | . | Yes . | Possibly . |
| Brain | 2 | 2 | |
| GI tract | 3 | 1 | 2 |
| Lung | 3 | 1 | 2 |
| Lung, brain | 1 | 1 | |
| GI tract, venipuncture, vagina, ETT | 1 | ||
| Bleeding Site(s) . | No. of Patients . | Bleeding Caused Death . | |
|---|---|---|---|
| . | . | Yes . | Possibly . |
| Brain | 2 | 2 | |
| GI tract | 3 | 1 | 2 |
| Lung | 3 | 1 | 2 |
| Lung, brain | 1 | 1 | |
| GI tract, venipuncture, vagina, ETT | 1 | ||
Abbreviations: GI, gastrointestinal; ETT, endotracheal tube.
Survival.Two nonresponding patients and 8 responding patients survived beyond than 100 days posttransplant. The 2 nonresponding patients remain alive greater than 332 and 933 days posttransplant. Two of the 8 responding patients remain alive greater than 126 and 1,412 days posttransplant. Causes of death are shown in Table 4.
Causes of Death
| Major Causes of Death . | Responding Patients (n = 12) . | Nonresponding Patients (n = 30) . |
|---|---|---|
| Relapse | 2 | 1 |
| Infection | 6 | 5 |
| Organ failure | 1 | 12 |
| GVHD | 1 | 1 |
| Hemorrhage | 0 | 3 |
| Major Causes of Death . | Responding Patients (n = 12) . | Nonresponding Patients (n = 30) . |
|---|---|---|
| Relapse | 2 | 1 |
| Infection | 6 | 5 |
| Organ failure | 1 | 12 |
| GVHD | 1 | 1 |
| Hemorrhage | 0 | 3 |
DISCUSSION
rh-tPA and heparin were used to treat 42 patients with VOD after marrow transplantation. The response rate was 29%. There were no significant differences in laboratory or physical findings between responders and nonresponders, with the exception of the serum creatinine at the start of treatment with rh-tPA and heparin. The mean probabilities of a fatal outcome from VOD, based on a previously described logistic regression model,26 were calculated using total serum bilirubin and percent weight gain on the day rh-tPA and heparin were started and were not different. Multiorgan failure before the start of thrombolytic therapy was the major prognostic factor for a poor response. Major bleeding occurred in 24% of patients and was clearly fatal in 7% of patients. Although the small numbers of patients do not allow for complete confidence that similar patients will not respond, thrombolytic therapy which is initiated after development of multiorgan dysfunction is unlikely to be successful.
Two reports have suggested that the success of thrombolytic therapy for VOD depends on early treatment. Goyal et al27 treated 8 patients with rh-tPA. Four were treated within 7 days of onset of disease and all responded. Two were treated late (18 to 24 days after the onset) and did not respond. Two patients had multiorgan failure at the start of treatment with rh-tPA and did not respond either. Schriber et al24 treated 45 patients with established VOD or suspected VOD. Patients with established VOD met the criteria of either McDonald et al25 or Jones et al28 while patients with suspected VOD had unexplained hyperbilirubinemia. Eighteen of 35 patients with suspected VOD treated with rh-tPA did not progress to clinical VOD; 13 of these patients survived more than 100 days. Seventeen patients with suspected VOD progressed to clinical VOD. Of these patients, 10 had resolution of VOD and 8 survived more than 100 days. Of the 7 patients with clinical VOD at the start of treatment with tPA, none had complete resolution of VOD (although 2 improved on treatment) and none survived to day 100. It is unknown what proportion of patients treated for suspected VOD would ever have developed clinical VOD. Nonetheless, these data and our own suggest that the success of treatment with rh-tPA is improved with earlier application. In fact, this may explain the initially encouraging results in our original group of 10 patients.16 Because of our concern regarding catastrophic hemorrhage, patients on dialysis or mechanical ventilation were not eligible for the pilot study. Thus, we may have selected patients who were likely to respond.
The incidence of life-threatening or fatal bleeding in our study was significant. Three patients died of bleeding as a direct result of treatment and the deaths of three additional patients might be attributable to treatment with rh-tPA and heparin. Nine of the 10 patients who developed serious hemorrhagic complications were on heparin at the time they began to bleed. No patient with severe bleeding responded to treatment. Although it is true that patients who had serious bleeding were desperately ill, it was disconcerting to families, physicians, and nurses to have death occur so precipitously from bleeding.
These data have dampened our original enthusiasm for treatment of established VOD with rh-tPA and heparin. Even though most of the patients in this series were treated before day 20, the seriousness of their VOD was obvious in the week before treatment was started. The reason for the delay from diagnosis of VOD to treatment with thrombolytic agents may be related to a fear that bleeding complications would worsen the outcome — a fear that proved to be well-founded. All patients who developed serious bleeding were nonresponders. However, we do not know if earlier institution of thrombolytic therapy would be associated with fewer hemorrhagic complications.
Other approaches to the treatment of VOD are needed. Eight patients have been described in the literature who received liver transplants for severe liver dysfunction (usually VOD) after marrow transplantation,23,29-34 including 1 who received a related donor transplant.35 Seven of the eight patients had received allogeneic marrow and one had received autologous peripheral blood progenitor cells. Five patients died soon after liver transplantation of pneumonitis (2 patients) or rejection of the grafted liver (3 patients). Three patients were reported to be long-term survivors with normally functioning donor livers. One died of leukemic relapse. Two were alive greater than 10 and 36 months after transplantation. The patient who is alive more than 36 months after liver transplant developed mild skin GVHD in which lymphocytes from the donor liver were found in the skin biopsy specimen. In addition, a bone marrow biopsy obtained 2 years after the liver transplant showed cells expressing HLA class I antigens of the liver donor. Potential problems with this approach include finding a suitable donor liver, management of multiorgan dysfunction and coagulopathy, and prevention of donor graft rejection. It is an approach, however, which merits further investigation.
Surgical approaches to the treatment of VOD after marrow transplantation have also included porto-systemic,35 spleno-renal,36 and Leveen shunting.37 Although these techniques have been reported to be surgically successful, no controlled studies were performed.
Smith et al38 reported on a patient with severe VOD after marrow transplantation treated using transjugular intrahepatic portosystemic shunting (TIPS). This is a technique where a channel is created between the hepatic vein and the portal vein using a percutaneously inserted tranjugular catheter. The channel is kept patent with a metal stent. This method has been used in patients with bleeding esophageal varices, intractable ascites, and the Budd-Chiari syndrome.39 It has the advantage of being minimally invasive and, if bleeding should occur, it would likely be intravascular. Potential risks of TIPS is a worsening in hepatic encephalopathy caused by shunting blood away from the hepatic circulation and local problems caused by the catheter or stent. A controlled trial of TIPS for VOD has not been performed.
A potential new medical approach is the use of defibrotide. Defibrotide is a novel polydeoxyribonucleotide derived from mammalian tissue (typically porcine) with several activities relevant to the treatment of VOD: stimulation of thrombomodulin synthesis, increase in endogenous tissue plasminogen activator, and decrease in plasminogen activator inhibitor type 1.40-44 It also has little, if any, anticoagulant activity. Richardson et al45 treated eight patients with severe VOD using defibrotide (Paul Richardson, personal communication, May 1996). Complete responses (ie, bilirubin ≤2 mg/dL) were achieved in three patients, none of whom had responded to rh-tPA. Further study of this agent is necessary.
In conclusion, thrombolysis using rh-tPA and heparin remains unproven for the treatment of hepatic venocclusive disease. The risk of bleeding in VOD patients treated with rh-tPA and heparin is high and the response rate suboptimal. It is our current practice to offer early thrombolytic therapy to patients with progressive VOD whose probability of a fatal outcome is ≥20%, provided that renal and pulmonary function are preserved. Additional studies are needed to determine whom to treat, when to treat them, and how much to treat them with.
ACKNOWLEDGMENT
We thank Christy Elsey and Julie Maloy for their assistance with the statistical analysis.
Supported in part by Grants No. CA 18029, CA 47748, and CA 15704 from the National Cancer Institute, National Institutes of Health, Department of Health and Human Services.
Address reprint requests to Scott I. Bearman, MD, Bone Marrow Transplant Program, University of Colorado Health Sciences Center, Box B-190, 4200 E Ninth Ave, Denver, CO 80262.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal