Abstract
Interleukin-12 (IL-12) and interferon-γ (IFN-γ) exert protective effects during experimental endotoxemia through upregulation of cellular immunity and phagocytic functions. They are part of a positive regulatory feedback loop that enhances the production of the other. Because critically ill patients show a marked suppression of T-cell and macrophage functions with a high susceptibility to infection, potential defects in the immunity/inflammation upregulating IL-12 IFN-γ pathway were studied. As an ex vivo model of endotoxemia, lipopolysaccharide (LPS) stimulated whole blood from 25 critically ill patients and 12 healthy individuals was incubated with either recombinant human (rh) IL-12 or rhIFN-γ, respectively. IFN-γ dose-dependently (P < .05) increased the release of IL-12 p40 and p70 into LPS-stimulated whole blood from healthy humans without effect in whole blood from critically ill patients. RhIL-12 p70 enhanced (P < .05) the secretion of IFN-γ in controls, while it was ineffective in LPS-stimulated whole blood from critically ill patients. The observed inhibition of the IL-12 IFN-γ pathway is not specific to LPS, since Staphylococcus aureus Cowan strain I (SAC)-stimulated whole blood from critically ill patients showed similar suppression. The secretion of IL-12 and IFN-γ was less reduced in critically ill patients when using isolated cultures of adherent cells or lymphocytes. Although preculture of whole blood from healthy humans with IL-10, but not with IL-4, mimicked suppression of the IL-12 IFN-γ pathway similar to that observed during critical illness, the release of antiinflammatory reacting cytokines (IL-4, IL-10, transforming growth factor [TGF]-β1 ) was decreased into LPS-stimulated whole blood from critically ill patients. These results indicate at least two mechanisms responsible for dramatic disturbances of the IL-12 IFN-γ pathway during critical illness: (1) deactivation of IL-12 and IFN-γ producing leukocytes in vivo early after the primary insult, and (2) presence of serum suppressive factors different from IL-4, IL-10, or TGF-β1 . Because IL-12 and IFN-γ upregulate essential immune functions, the marked inhibition of IL-12 and IFN-γ release may be pivotal for high susceptibility of critically ill patients to infection.
INTERLEUKIN-12 (IL-12) and interferon-γ (IFN-γ) represent both potent immunoreactive mediators, which upregulate immune functions pivotal for the protection against infectious pathogens (for review, see Trinchieri1 and Farrar and Schreiber2 ). IL-12 promotes TH1 type cytokine response, enhances specific and nonspecific cytolytic lymphocyte responses, and stimulates the proliferation of activated T lymphocytes and natural killer (NK) cells.1 INF-γ has potent microbicidal activities through upregulation of monocytes/macrophages with an increased secretion of proinflammatory cytokines.2 Moreover, IFN-γ promotes the antigen presenting process through enhanced expression of the major histocompatability complex (MHC) class II antigen.2 IL-12 induces T and NK cells to produce IFN-γ.3,4 On the other hand, IFN-γ upregulates the synthesis and release of IL-12 by phagocytic cells, B cells, and neutrophils.5-7 The interactions between IFN-γ and IL-12 appear to be involved in a positive feedback mechanism that results in the activation of phagocytic cells during bacterial and parasitic infection. In this light, most in vivo studies using murine sepsis models demonstrated that the IL-12 IFN-γ pathway plays an important role during acute infection with heightened resistance to pathogens and reduced mortality to a septic challenge.8-10 However, excessive doses of recombinant IL-12 appeared to decrease the resistance to a bacterial challenge after burn injury in mice.11
Critically ill patients are often anergic and show a high susceptibility to invading microorganisms through severe inhibition of the cellular, humoral, and phagocytic immune system.12-14 In this study, we investigated a potential downregulation of the IL-12 IFN-γ pathway in critically ill patients and the underlying mechanisms using lipopolysaccharide (LPS)-stimulated whole blood as an ex vivo model of endotoxemia.14 Although this system cannot completely depict cytokine interactions in the whole body, it has considerable relevance with respect to local compartmentalized cytokine interactions. Additionally, it excludes any influence of cell preparation techniques on cell receptors and preserves a natural environment.14
MATERIALS AND METHODS
Patient selection.Heparinized blood was obtained from 25 critically ill patients and 12 healthy volunteers. Whole blood from patients was taken either during the first 24 hours after severe injury (n = 17; injury severity score [ISS]15 39.8 ± 2.5 points; Acute Physiology and Chronic Health Evaluation [APACHE II] score16 20.2 ± 2.5 points) or after diagnosis of septic shock according to the criteria of Bone et al17 (n = 8; APACHE II score 20.3 ± 3.3 points). All injured patients enrolled showed either multiple life-threatening trauma or severe sepsis (Table 1). The group of healthy individuals was comparable to the group of critically ill patients with regard to age and sex. All patients were recruited into this study under informed consent guidelines approved by the Human Ethical Committee of the University of Zurich.
Demographic Data of Patients With Multiple Injuries or Severe Sepsis
| Patients after severe injury | |
| No. | 17 |
| Mean ISS15 | 39.8 ± 2.5 (range, 22-59 points) |
| Mean APACHE II16 | 20.2 ± 2.5 (range, 9-29 points) |
| Injury pattern | |
| Head | 77% |
| Thorax | 92% |
| Abdomen | 15% |
| Spine | 30% |
| Pelvis | 30% |
| Extremities | 54% |
| Death | 5/17 (29%)* |
| Patients with sepsis | |
| No. | 8 |
| Mean APACHE II16 | 20.3 ± 3.3 (9-31 points) |
| Sepsis origin | |
| Pneumonia (n = 6) | |
| Meningitis (n = 1) | |
| Wound infection (n = 1) | |
| Death | 2/8 (25%)† |
| Patients after severe injury | |
| No. | 17 |
| Mean ISS15 | 39.8 ± 2.5 (range, 22-59 points) |
| Mean APACHE II16 | 20.2 ± 2.5 (range, 9-29 points) |
| Injury pattern | |
| Head | 77% |
| Thorax | 92% |
| Abdomen | 15% |
| Spine | 30% |
| Pelvis | 30% |
| Extremities | 54% |
| Death | 5/17 (29%)* |
| Patients with sepsis | |
| No. | 8 |
| Mean APACHE II16 | 20.3 ± 3.3 (9-31 points) |
| Sepsis origin | |
| Pneumonia (n = 6) | |
| Meningitis (n = 1) | |
| Wound infection (n = 1) | |
| Death | 2/8 (25%)† |
Death due to brain edema (n = 4), hemorrhage (n = 1).
Death due to multiple-organ dysfunction syndrome (MODS) (n = 2).
Whole blood assay.The whole blood assay was performed as previously reported.14 Blood was drawn into heparinized syringes (20 U heparin/mL; heparin was tested for endotoxin: <5 pg/mL heparin). Aliquots of 5 mL blood were placed in sterile polypropylene tubes (Falcon; Becton Dickinson, Lincoln Park, NJ). For baseline values, one blood sample was immediately processed as described below. Baseline values reflect circulating levels of different cytokines in vivo at time of blood sampling. In addition, heparinized blood was incubated with or without 1 ng/mL LPS; Escherichia coli 055:B5; Sigma Chemical Co, St Louis, MO) in the presence of various concentrations of either recombinant human (rh) IL-12 (50, 5, 1, 0.1 ng/mL; biological activity 1.5 × 108 U/mg, endotoxin contamination 12 EU/mg in the Limulus assay; a gift from Dr M. Gately, Hoffmann-LaRoche, Nutley, NJ) or rhIFN-γ (100, 50, 10, 5, 1 ng/mL; biological activity 3 × 107 U/mg; endotoxin contamination <0.3 EU/mg; kindly provided by Dr G.R. Adolf, Bender, Wien, Austria). The blood containing tubes were placed on a rotator in a 5% CO2 atmosphere at 37°C. At 8 hours and 24 hours of incubation, the samples were removed. Plasma was prepared through centrifugation with 2,100 rpm at 4°C, aliquoted, and stored at −80°C until assayed for cytokines. In addition, whole blood from both groups was incubated with Staphylococcus aureus Cowan strain I (SAC; Pansorbin; 0.075% wt/vol; Calbiochem Corp, La Jolla, CA) for 8 hours and 24 hours, respectively. Plasma was prepared and stored as described above.
Secretion of IL-12 p40 and p70 Into Whole Blood
| . | . | IL-12 p40 (pg/mL) . | IL-12 p70 (pg/mL) . | ||
|---|---|---|---|---|---|
| . | . | LPS . | LPS + rhIFN-γ . | LPS . | LPS + rhIFN-γ . |
| 8 h | Control | 691.1 ± 122.7 | 1,518.8 ± 319.2* | 10.0 ± 4.5 | 236.6 ± 63.8* |
| Patient | 46.6 ± 6.2† | 159.0 ± 31.2*† | ND | ND† | |
| 24 h | Control | 964.9 ± 185.8 | 1,673.7 ± 254.5* | 9.0 ± 3.5 | 299.8 ± 105.1* |
| Patient | 52.7 ± 6.8† | 223.1 ± 47.0*† | ND | ND† | |
| . | . | IL-12 p40 (pg/mL) . | IL-12 p70 (pg/mL) . | ||
|---|---|---|---|---|---|
| . | . | LPS . | LPS + rhIFN-γ . | LPS . | LPS + rhIFN-γ . |
| 8 h | Control | 691.1 ± 122.7 | 1,518.8 ± 319.2* | 10.0 ± 4.5 | 236.6 ± 63.8* |
| Patient | 46.6 ± 6.2† | 159.0 ± 31.2*† | ND | ND† | |
| 24 h | Control | 964.9 ± 185.8 | 1,673.7 ± 254.5* | 9.0 ± 3.5 | 299.8 ± 105.1* |
| Patient | 52.7 ± 6.8† | 223.1 ± 47.0*† | ND | ND† | |
Whole blood obtained from healthy individuals (controls, n = 12) and critically ill patients (n = 25) was incubated with LPS (1 ng/mL) in the presence or absence of rhIFN-γ (10 ng/mL) for 8 hours and 24 hours, respectively. Plasma levels of IL-12 p40 (pg/mL) and p70 (pg/mL) were measured using specific ELISA. Data are presented as mean ± SEM.
Abbreviation: ND, not detectable.
P < .05 LPS versus LPS + rhIFN-γ.
P < .05 patient versus control.
The contamination of blood samples from the two groups with endotoxin through the added reagents was excluded using the Limulus amebocyte assay.
Isolation of adherent cells and lymphocytes.Peripheral blood mononuclear cells (PBMC) were separated from heparinized blood samples using density gradient centrifugation (Ficoll-Histopaque, Sigma Chemical Co, d = 1.077). After washing PBMC three times, viability was tested with trypan blue exclusion and cells counted for monocytes according to their cell configuration. After adjusting the cell suspension to 1 × 106/mL viable cells with typical monocytic cell configuration, cells were allowed to adhere onto 24-well plates (Costar, Cambridge, MA) at 37°C for 2 hours. The supernatants containing nonadherent cells were removed by repeated washing with Click's medium (Irvine Scientific, Irvine, CA). Thereafter, monolayers of adherent cells were stimulated with or without LPS (1 ng/mL; Escherichia coli 055:B5; Sigma Chemical Co) or SAC (Pansorbin; 0.075% wt/vol; Calbiochem) in the presence or absence of rhIFN-γ (10 ng/mL) for 24 hours. Supernatants of adherent cell cultures were harvested, filtered, aliquoted, and stored at −80°C until assayed for IL-12 p40 and p70.
The supernatants obtained after adherence of PBMC contained the nonadherent cell populations. After washing nonadherent cells three times, viability was tested using trypan blue exclusion and nonadherent cells adjusted to 1 × 106 cells/mL in RPMI 1640 medium (GIBCO-BRL, Life Technologies LTD, Paisley, Scotland) supplemented with 10% fetal calf serum (FCS; GIBCO-BRL). Since fluorescence-activated cell sorter (FACS; Profile Epics flow cytometer; Coulter, Hialeah, FL) analysis of nonadherent cells demonstrated the presence of >90% lymphocytes in both groups using fluorescein isothiocyanate (FITC)-conjugated anti-CD3 (T lymphocytes; clone UCHT1; Coulter) and phycoerythrin (PE)-conjugated anti-CD19 (B lymphocytes; clone 89B; Coulter) for detection, these cell suspensions are referred to as lymphocyte cultures throughout this report. A total of 1 × 106 lymphocytes/mL/well were cultured with or without LPS (1 ng/mL; Escherichia coli 055:B5; Sigma Chemical Co) or SAC (Pansorbin; 0.075% wt/vol; Calbiochem) in the presence or absence of rhIL-12 p70 (5 ng/mL) for 24 hours. Supernatants of lymphocyte cultures were harvested, filtered, aliquoted, and stored at −80°C until measured for IFN-γ.
Additionally, whole blood from healthy individuals was preincubated with either IL-4 (1,000 pg/mL; biological activity 1 × 107 U/mg; Genzyme Corp, Cambridge, MA) or IL-10 (2,500 pg/mL; biological activity 1.5 × 107 U/mg; endotoxin contamination <10 EU/mg; Schering-Plough Research Institute, Kenilworth, NJ) for 12 hours. Thereafter, PBMC were harvested using density gradient centrifugation as described above, washed three times, and incubated with LPS (1 ng/mL; Escherichia coli 055:B5; Sigma Chemical Co) for 24 hours. PBMC supernatants were harvested, filtered, aliquoted, and stored at −80°C until assayed for cytokines.
Cytokine measurements.Levels of IFN-γ in plasma and lymphocyte supernatants were measured with specific sandwich enzyme-linked immunosorbent assay (ELISA) using the antihuman IFN-γ monoclonal antibodies NIB42 for capture and 4S.B3 for detection (Pharmingen, San Diego, CA). Standards of rhIFN-γ (Pharmingen) were diluted in normal human plasma. The ELISA showed a sensitivity of >100 pg/mL. Concentrations of IL-12 p40 in plasma and supernatants of adherent cells were measured using the rat monoclonal anti-human IL-12 p40 antibody 2-4A1 for coating and the peroxidase-conjugated anti–IL-12 p40 antibody 4D6 for detection (antibodies and recombinant human IL-12 p40 kindly provided by Maurice K. Gately, Hoffman-LaRoche) (sensitivity >10 pg/mL).18 IL-12 p70 was determined with a commercially available ELISA kit (R & D Systems Inc, Minneapolis, MN) according to the manufacturer's guidelines. The ELISA uses a capture antibody that recognizes the IL-12 p70 heterodimer with a sensitivity >5 pg/mL.
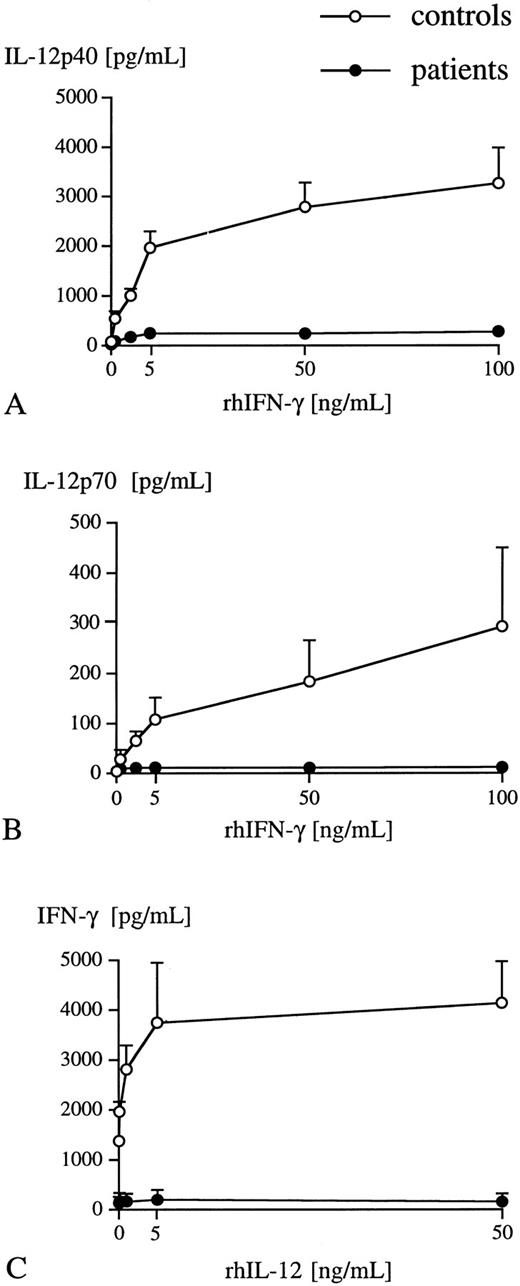
Dose-dependent effect of various concentrations of either rhIFN-γ or rhIL-12 p70 on IL-12 p40 (A), p70 (B), and IFN-γ (C) release into LPS-stimulated (1 ng/mL) whole blood obtained from either healthy individuals (○) (n = 3) or critically ill patients (•) (n = 3). Plasma levels of IL-12 p40, p70, and IFN-γ were measured after 24 hours of incubation using specific ELISA. Data are presented as mean ± SEM.
Dose-dependent effect of various concentrations of either rhIFN-γ or rhIL-12 p70 on IL-12 p40 (A), p70 (B), and IFN-γ (C) release into LPS-stimulated (1 ng/mL) whole blood obtained from either healthy individuals (○) (n = 3) or critically ill patients (•) (n = 3). Plasma levels of IL-12 p40, p70, and IFN-γ were measured after 24 hours of incubation using specific ELISA. Data are presented as mean ± SEM.
IL-4 and transforming growth factor (TGF )-β1 in plasma samples were measured using commercially available ELISA kits (Genzyme) with a sensitivity of >30 pg/mL for IL-4 and >50 pg/mL for TGF-β1 . For measurements of TGF-β1 , plasma samples were acidified as previously described.19 Without acidification, TGF-β1 was neither detectable in plasma from healthy humans, nor in critically ill patients. IL-10 was measured in plasma by ELISA with the rat antihuman IL-10 monoclonal antibody JES3-9D7 for capture and a rabbit antihuman IL-10 polyclonal antibody for detection (antibodies kindly provided by Satwant Narula, PhD, Schering-Plough Research Institute, Kenilworth, NJ).20 Recombinant hIL-10 (Schering-Plough) diluted in normal plasma was used for the standard curve. The ELISA showed a sensitivity of >20 pg/mL.
All samples were tested in duplicate.
Statistics.Results are demonstrated as mean ± standard error of mean (SEM). Statistical analysis was performed using nonparametric Mann-Whitney U-test with Bonferroni correction for multiple comparisons.
RESULTS
IFN-γ induced release of IL-12 p40 and p70 into whole blood.IL-12 p40 was not detectable at baseline (0 hours incubation time) in healthy individuals, while very low concentrations were found in plasma samples from critically ill patients (15.9 ± 2.4 pg/mL; 7 of 25 patients [28%]). In contrast to previous studies,21 rhIFN-γ slightly enhanced the release of IL-12 p40 in both groups, though these levels reflected only 10% of those detected in the presence of rhIFN-γ and LPS. Levels of IL-12 p40 in IFN-γ stimulated whole blood from critically ill patients (47.1 ± 11.5 pg/mL) were significantly decreased by 73% compared with the control group (172.7 ± 38.0 pg/mL). IL-12 p70 was not detectable at baseline and its secretion could not be induced through rhIFN-γ in any of the two groups during an incubation period of 24 hours, which is in line with previous studies.21 22
In the control group, LPS in a concentration of 1 ng/mL resulted in a significant increase of IL-12 p40 at 8 hours and 24 hours of incubation compared with baseline, while only minimal levels of IL-12 p70 (10.0 ± 4.5 pg/mL) were detected (Table 2). The amounts of produced IL-12 p40 largely exceeded those of IL-12 p70, which is in line with previous studies.23 Because high concentrations of 1 μg/mL LPS led to detectable IL-12 p70 levels in whole blood from healthy volunteers (41.3 ± 13.5 pg/mL), maximum release of IL-12 p40 and p70 seem to be dependent on the LPS dosage.23 In this study, LPS was used in a concentration of 1 ng/mL, since this LPS dosage reflects clinically relevant endotoxin concentrations that have been detected in patients with severe sepsis.24
The addition of rhIFN-γ and LPS to whole blood from healthy individuals led to a significant rise in IL-12 p40 and p70 secretion at 8 hours and 24 hours with a dose-dependent effect (Table 2, Fig 1A and B). Though rhIFN-γ increased the release of IL-12 p40 into whole blood from critically ill patients, total amounts of secreted IL-12 p40 were markedly suppressed by 89% at 8 hours and 87% at 24 hours compared with healthy individuals (Table 2). In addition, rhIFN-γ did not induce secretion of IL-12 p70 into LPS-stimulated whole blood obtained from critically ill patients at any time point or with any dosage of rhIFN-γ (Table 2, Fig 1B). Previous reports5 using isolated cell cultures suggested peak levels of IL-12 p70 release after 48 hours of incubation. To exclude the possibility that IL-12 p70 release into whole blood from critically ill patients occurs after 24 hours of incubation, control studies stimulating whole blood with LPS and rhIFN-γ for 36 hours were performed. These studies did not demonstrate a further increase of IL-12 p70 release between 24 hours and 36 hours of incubation in any of the two groups (data not shown).
Interleukin-12 p70 induced release of IFN-γ into whole blood.Baseline levels (0 hours incubation time) of IFN-γ were similar in both groups (controls, 263.3 ± 98.2 pg/mL; patients, 213.2 ± 52.5 pg/mL). rhIL-12 p70 in the absence of LPS resulted in a marked increase of IFN-γ by 102% at 8 hours and by 105% at 24 hours of incubation in the control group, while it did not enhance secretion of IFN-γ in critically ill patients (Table 3). LPS alone significantly enhanced the release of IFN-γ in controls, but did not affect secretion of IFN-γ in critically ill patients (Table 3). The addition of rhIL-12 p70 to whole blood from healthy humans in the presence of LPS resulted in a dose-dependent and significant increase of IFN-γ release at 8 hours (+338%) and 24 hours (+353%), while rhIL-12 p70 did not augment IFN-γ secretion into LPS-stimulated whole blood from critically ill patients (Table 3, Fig 1C).
Secretion of IFN-γ Into Whole Blood
| . | . | . | rhIL-12 p70 . | LPS . | LPS + rhIL-12 p70 . |
|---|---|---|---|---|---|
| 8 h | Control | 613.9 ± 155.0 | 1,241.3 ± 217.93-150 | 1,255.0 ± 179.83-150 | 2,690.8 ± 595.63-150 |
| Patient | 244.0 ± 51.83-151 | 442.7 ± 117.43-151 | 314.6 ± 67.93-151 | 333.7 ± 86.03-151 | |
| 24 h | Control | 712.4 ± 188.7 | 1,458.1 ± 217.63-150 | 1,301.8 ± 186.63-150 | 3,223.8 ± 527.83-150 |
| Patient | 327.3 ± 86.7 | 328.4 ± 87.73-151 | 278.2 ± 54.23-151 | 282.6 ± 70.03-151 |
| . | . | . | rhIL-12 p70 . | LPS . | LPS + rhIL-12 p70 . |
|---|---|---|---|---|---|
| 8 h | Control | 613.9 ± 155.0 | 1,241.3 ± 217.93-150 | 1,255.0 ± 179.83-150 | 2,690.8 ± 595.63-150 |
| Patient | 244.0 ± 51.83-151 | 442.7 ± 117.43-151 | 314.6 ± 67.93-151 | 333.7 ± 86.03-151 | |
| 24 h | Control | 712.4 ± 188.7 | 1,458.1 ± 217.63-150 | 1,301.8 ± 186.63-150 | 3,223.8 ± 527.83-150 |
| Patient | 327.3 ± 86.7 | 328.4 ± 87.73-151 | 278.2 ± 54.23-151 | 282.6 ± 70.03-151 |
Whole blood obtained from healthy individuals (controls, n = 12) and critically ill patients (n = 25) was incubated with or without LPS (1 ng/mL) in the presence or absence of rhIL-12 p70 (5 ng/mL) for 8 hours and 24 hours, respectively. Plasma levels of IFN-γ (pg/mL) were measured using specific ELISA. Data are presented as mean ± SEM.
P < .05 without agent versus rhIL-12 p70, LPS, or LPS + rhIL-12 p70.
P < .05 patient versus control.
The suppression of the IL-12 IFN-γ pathway in whole blood from critically ill patients was gradually higher in patients with severe sepsis compared with injured patients, though these differences were not statistically different (Table 4). In contrast, the degree of suppression was not different between survivors and nonsurvivors (data not shown).
Effect of Trauma and Sepsis on Release of IL-12 p40, p70, and IFN-γ Into Whole Blood
| . | Control (n = 12) . | Trauma (n = 17) . | Sepsis (n = 8) . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | LPS . | LPS + rhIFN-γ . | LPS + rhIL-12 p70 . | LPS . | LPS + rhIFN-γ . | LPS + rhIL-12 p70 . | LPS . | LPS + rhIFN-γ . | LPS + rhIL-12 p70 . |
| p40 | 964.9 ± 185.8 | 1,673.7 ± 254.54-150 | — | 48.1 ± 7.14-151 | 256.2 ± 58.4*† | — | 61.3 ± 14.54-151 | 161.4 ± 79.54-151 | — |
| p70 | 9.0 ± 3.5 | 299.8 ± 105.14-150 | — | ND4-151 | ND4-151 | — | ND4-151 | ND4-151 | — |
| IFN-γ | 1,301.8 ± 186.6 | — | 3,223.8 ± 527.84-150 | 320.0 ± 74.74-151 | — | 321.3 ± 98.84-151 | 187.7 ± 45.54-151 | — | 205.2 ± 72.74-151 |
| . | Control (n = 12) . | Trauma (n = 17) . | Sepsis (n = 8) . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | LPS . | LPS + rhIFN-γ . | LPS + rhIL-12 p70 . | LPS . | LPS + rhIFN-γ . | LPS + rhIL-12 p70 . | LPS . | LPS + rhIFN-γ . | LPS + rhIL-12 p70 . |
| p40 | 964.9 ± 185.8 | 1,673.7 ± 254.54-150 | — | 48.1 ± 7.14-151 | 256.2 ± 58.4*† | — | 61.3 ± 14.54-151 | 161.4 ± 79.54-151 | — |
| p70 | 9.0 ± 3.5 | 299.8 ± 105.14-150 | — | ND4-151 | ND4-151 | — | ND4-151 | ND4-151 | — |
| IFN-γ | 1,301.8 ± 186.6 | — | 3,223.8 ± 527.84-150 | 320.0 ± 74.74-151 | — | 321.3 ± 98.84-151 | 187.7 ± 45.54-151 | — | 205.2 ± 72.74-151 |
Whole blood obtained from healthy individuals (controls, n = 12) and critically ill patients (n = 25) was incubated with LPS (1 ng/mL) in the presence or absence of either rhIL-12 p70 (5 ng/mL) or rhIFN-γ (10 ng/mL) for 24 hours. The group of critically ill patients was divided according to the origin of the insult (trauma v sepsis). Plasma levels of IL-12 p40 (pg/mL), p70 (pg/mL), and IFN-γ (pg/mL) were measured using specific ELISA. Data are presented as mean ± SEM.
Abbreviation: ND, not detectable.
P < .05 LPS versus LPS + rhIFN-γ/LPS + rhIL-12 p70.
P < .05 patient versus control.
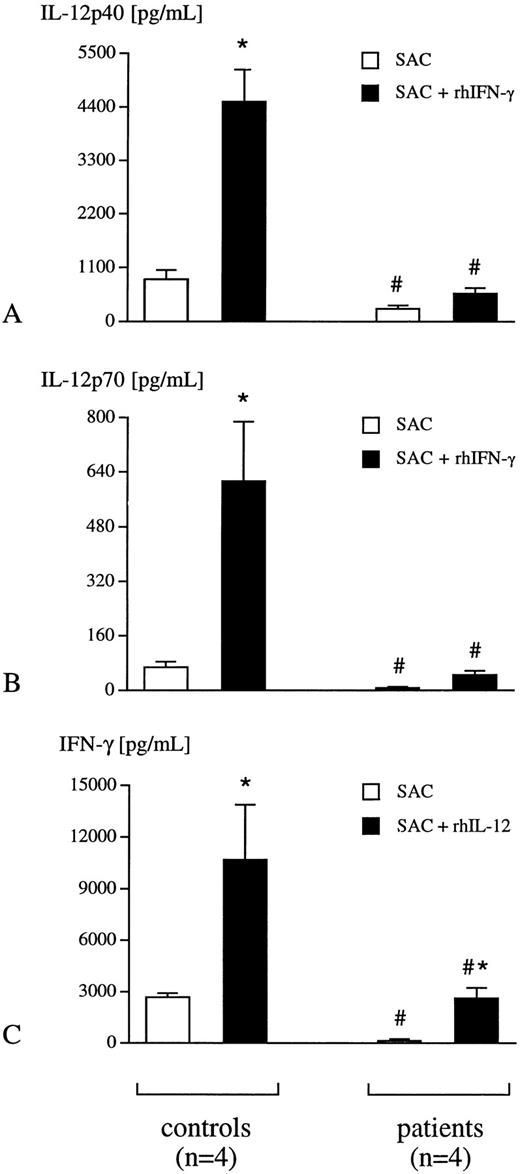
SAC induced release of IL-12 p40, p70, and IFN-γ into whole blood.To exclude a LPS-specific phenomenon, the release of IL-12 p40, p70, and IFN-γ was studied after the addition of SAC. In whole blood from healthy humans, the addition of SAC resulted in a significant secretion of IL-12 p40, p70, and IFN-γ (Fig 2A through C), which could be dramatically increased in the presence of either rhIFN-γ or rhIL-12 p70, respectively (Fig 2A through C). In contrast, SAC-induced secretion of these three cytokines was significantly suppressed in whole blood obtained from critically ill patients (Fig 2A through C).
Whole blood from healthy individuals (controls, n = 4) and critically ill patients (n = 4) was incubated with SAC (0.075% wt/vol) in the presence or absence of either rhIFN-γ (10 ng/mL) (A, B) or rh IL-12 p70 (5 ng/mL) (C) for 24 hours. Plasma levels of IL-12 p40 (A), p70 (B), and IFN-γ (C) were determined using specific ELISA. Data are presented as mean ± SEM; *P <. 05 SAC versus SAC + rhIFN-γ or SAC + rhIL-12 p70; #P < .05 patient versus control.
Whole blood from healthy individuals (controls, n = 4) and critically ill patients (n = 4) was incubated with SAC (0.075% wt/vol) in the presence or absence of either rhIFN-γ (10 ng/mL) (A, B) or rh IL-12 p70 (5 ng/mL) (C) for 24 hours. Plasma levels of IL-12 p40 (A), p70 (B), and IFN-γ (C) were determined using specific ELISA. Data are presented as mean ± SEM; *P <. 05 SAC versus SAC + rhIFN-γ or SAC + rhIL-12 p70; #P < .05 patient versus control.
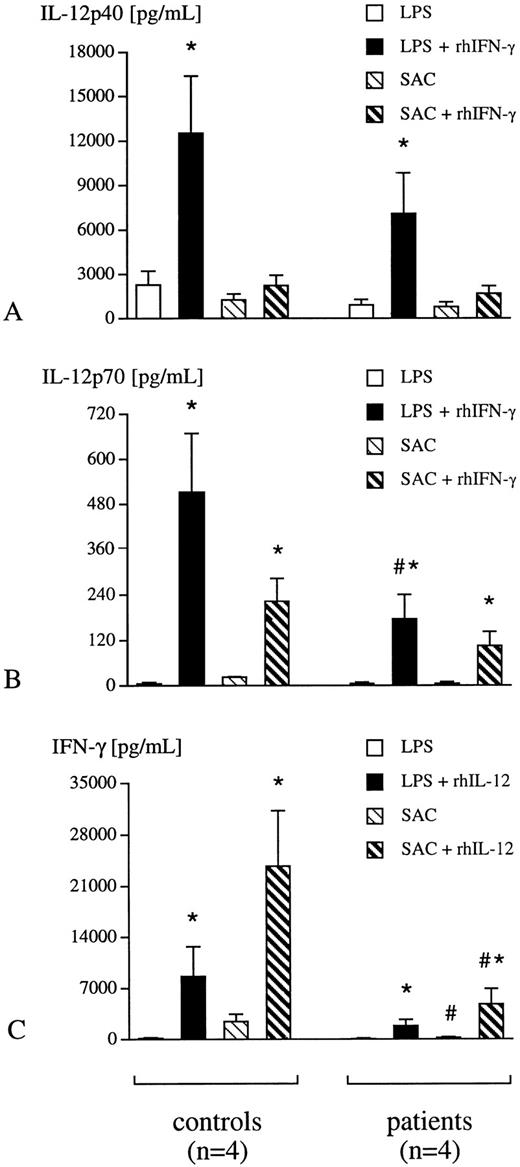
Secretion of IL-12 p40, p70, and IFN-γ by isolated adherent cells and lymphocytes.In addition, IL-12 p40 and p70 secretion by isolated adherent cells and IFN-γ release by purified lymphocytes was determined. These experiments give further information, whether differential leukocyte profiles on one hand and soluble suppressive factors in serum on the other hand, are responsible for suppression of the IL-12 IFN-γ pathway in whole blood. Isolated adherent cell and lymphocyte cultures, stimulated either with LPS or with SAC, demonstrated similar suppression of IL-12 p40, p70, and IFN-γ release in critically ill patients compared with healthy humans (Fig 3A through C). The addition of rhIFN-γ to adherent cell cultures in the presence of LPS did not only augment secretion of IL-12 p40, but also of IL-12 p70 in critically ill patients (Fig 3A and B). Moreover, rhIL-12 p70 increased LPS-induced release of IFN-γ by lymphocytes from critically ill patients (Fig 3C), which was not seen in the whole blood assay. Thus, the responsiveness to either rhIL-12 p70 or to rhIFN-γ in the presence of a bacterial challenge seems to be increased in isolated cell cultures from critically ill patients compared with the results obtained with whole blood, though the absolute secretory capacity of adherent cells and lymphocytes from critically ill patients is still reduced compared with healthy individuals (Fig 3A through C).
Adherent cells and lymphocytes were isolated from whole blood of healthy humans (controls, n = 4) and critically ill patients (n = 4). Adherent cells were incubated either with LPS (1 ng/mL) or SAC (0.075% wt/vol) in the presence or absence of rhIFN-γ (10 ng/mL) for 24 hours (A, B). In addition, lymphocytes from both experimental groups were incubated with LPS (1 ng/mL) or SAC (0.075% wt/vol) in the presence or absence of rhIL-12 p70 (5 ng/mL) for 24 hours (C). Concentrations of IL-12 p40 and p70 in adherent cell supernatants and of IFN-γ in lymphocyte supernatants were determined with specific ELISA. Data are presented as mean ± SEM; *P < .05 LPS/SAC versus LPS/SAC + rhIFN-γ/rhIL-12 p70; #P < .05 patient versus control.
Adherent cells and lymphocytes were isolated from whole blood of healthy humans (controls, n = 4) and critically ill patients (n = 4). Adherent cells were incubated either with LPS (1 ng/mL) or SAC (0.075% wt/vol) in the presence or absence of rhIFN-γ (10 ng/mL) for 24 hours (A, B). In addition, lymphocytes from both experimental groups were incubated with LPS (1 ng/mL) or SAC (0.075% wt/vol) in the presence or absence of rhIL-12 p70 (5 ng/mL) for 24 hours (C). Concentrations of IL-12 p40 and p70 in adherent cell supernatants and of IFN-γ in lymphocyte supernatants were determined with specific ELISA. Data are presented as mean ± SEM; *P < .05 LPS/SAC versus LPS/SAC + rhIFN-γ/rhIL-12 p70; #P < .05 patient versus control.
Release of antiinflammatory cytokines into whole blood.Since cytokines such as IL-4, IL-10, TGF-β1 have been found to inhibit synthesis and/or release of IL-12 and IFN-γ,6 25-28 levels of these cytokines have been determined in LPS-stimulated whole blood from both experimental groups (Table 5). Although IL-4 was only detected in minimal amounts in both groups, LPS-induced release of IL-10 (−92%) and of TGF-β1 (−54%) was significantly decreased into whole blood from critically ill patients compared with controls (Table 5).
Release of Antiinflammatory Cytokines Into Whole Blood
| . | Control (n = 6) . | Patient (n = 6) . | ||
|---|---|---|---|---|
| . | −LPS . | +LPS . | −LPS . | +LPS . |
| IL-4 (pg/mL) | ND | 7.8 ± 7.8 | 21.3 ± 11.2 | 22.7 ± 16.55-150 |
| IL-10 (pg/mL) | 68.0 ± 36.0 | 1,818.7 ± 394.9 | 123.8 ± 54.8 | 147.8 ± 41.75-150 |
| TGF-β1 (pg/mL) | 25,757 ± 1,256 | 28,771 ± 3,594 | 9,648 ± 2,2195-150 | 13,314 ± 2,6125-150 |
| . | Control (n = 6) . | Patient (n = 6) . | ||
|---|---|---|---|---|
| . | −LPS . | +LPS . | −LPS . | +LPS . |
| IL-4 (pg/mL) | ND | 7.8 ± 7.8 | 21.3 ± 11.2 | 22.7 ± 16.55-150 |
| IL-10 (pg/mL) | 68.0 ± 36.0 | 1,818.7 ± 394.9 | 123.8 ± 54.8 | 147.8 ± 41.75-150 |
| TGF-β1 (pg/mL) | 25,757 ± 1,256 | 28,771 ± 3,594 | 9,648 ± 2,2195-150 | 13,314 ± 2,6125-150 |
Heparinized whole blood obtained from healthy individuals (controls, n = 6) and critically ill patients (n = 6) was incubated with or without LPS (1 ng/mL) for 24 hours. Plasma levels of IL-4, 10, and TGF-β1 were measured using specific ELISA. Data are presented as mean ± SEM.
Abbreviation: ND, not detectable.
P < .05 patient versus control.
Effect of preincubation of whole blood with IL-4 or IL-10.To mimic a potential priming effect of IL-4 and IL-10 in vivo with consequent inhibition of secretory capacity of PBMC ex vivo, whole blood was preincubated with either rhIL-4 or rhIL-10 in concentrations observed in critically ill patients (Neidhardt et al, manuscript in preparation). Thereafter, PBMC were isolated and stimulated with LPS. The pretreatment with rhIL-10 markedly inhibited suppression of LPS-induced release of IL-12 p40 and IFN-γ, while rhIL-4 was ineffective (Table 6). Addition of rhIL-12 p70 to LPS-stimulated PBMC cultures could not attenuate IL-10-induced suppression of IFN-γ release and vice versa (data not shown).
Effect of rhIL-4 and rhIL-10 on Cytokine Secretion by PBMC
| . | . | — . | +rhIL-4 (1,000 pg/mL) . | +rhIL-10 (2,500 pg/mL) . | |||
|---|---|---|---|---|---|---|---|
| . | . | p40 (pg/mL) . | IFN-γ (pg/mL) . | p40 (pg/mL) . | IFN-γ (pg/mL) . | p40 (pg/mL) . | IFN-γ (pg/mL) . |
| Exp 1 | LPS | 106 | 404 | 114 | 430 | 15 | 0 |
| Exp 2 | LPS | 132 | 410 | 198 | 422 | 36 | 0 |
| Exp 1 | SAC | 572 | 694 | 618 | 572 | 151 | 378 |
| Exp 2 | SAC | 251 | 868 | 137 | 704 | 45 | 472 |
| . | . | — . | +rhIL-4 (1,000 pg/mL) . | +rhIL-10 (2,500 pg/mL) . | |||
|---|---|---|---|---|---|---|---|
| . | . | p40 (pg/mL) . | IFN-γ (pg/mL) . | p40 (pg/mL) . | IFN-γ (pg/mL) . | p40 (pg/mL) . | IFN-γ (pg/mL) . |
| Exp 1 | LPS | 106 | 404 | 114 | 430 | 15 | 0 |
| Exp 2 | LPS | 132 | 410 | 198 | 422 | 36 | 0 |
| Exp 1 | SAC | 572 | 694 | 618 | 572 | 151 | 378 |
| Exp 2 | SAC | 251 | 868 | 137 | 704 | 45 | 472 |
Whole blood obtained from healthy individuals (n = 2) was preincubated with either rhIL-4 (1000 pg/mL) or rhIL-10 (2500 pg/mL) for 12 hours. Thereafter, PBMC were isolated and stimulated with either LPS (1 ng/mL) or SAC (0.075% wt/vol) for 24 hours. Levels of IL-12 p40 and IFN-γ in supernatants from PBMC were determined with specific ELISA.
Abbreviation: Exp, experiment.
DISCUSSION
The results presented here indicate that the IL-12 IFN-γ pathway, which plays a key role in activation of phagocytic cells and of T-cell driven immunity, is significantly compromised in critically ill patients. These results are in line with previous animal experiments that demonstrated a reduced release of IFN-γ and IL-12 by splenocytes obtained from burned mice.29 In whole blood from healthy individuals, IFN-γ and IL-12 operate through a positive feedback loop through which IL-12 induces synthesis and release of IFN-γ and vice versa. This positive feedback mechanism is dramatically inhibited in whole blood obtained from critically ill patients. Monocytes as target cells of IFN-γ, as well as TH1-lymphocytes as target cells of IL-12 seem to be deactivated during critical illness with a reduced capacity to synthesize these inflammation upregulating cytokines. Though the pathogenesis of patients with severe injury is different from that of septic patients, the observed defects occur in both groups. The origin of critical illness does not seem to influence these disturbances in the IL-12 IFN-γ pathway.
The observed suppression of the IL-12 IFN-γ pathway is not specific to LPS, since parallel experiments using SAC as stimulus demonstrated a similar inhibition of IL-12 and IFN-γ secretion into whole blood from critically ill patients. Furthermore, experiments using isolated lymphocyte and adherent cell cultures were performed to exclude the possibility that suppression of IL-12 and IFN-γ release is due to alterations of differential leukocyte profiles, as they occur during critical illness.30 These experiments showed similar results than those obtained with whole blood. LPS-induced secretion of IL-12 p40 and p70 by adherent blood cells and IFN-γ release by lymphocytes were markedly inhibited in critically ill patients compared with healthy individuals. Thus, the suppression of LPS-induced release of IL-12 p40, p70, and IFN-γ release into whole blood from critically ill patients seems to be due to intracellular defects of PBMC rather than alterations of their absolute numbers. These observations are in line with our previous findings, which demonstrated that inhibition of LPS-induced proinflammatory cytokine release (TNF-α, IL-1β, IL-6) during severe sepsis (endotoxin tolerance) is related to reduced half-life of cytokine mRNA expression.14
With regard to the mechanisms of action of suppressed IL-12 IFN-γ pathway, two basic concepts were investigated in this study. The first concept involves a potentially increased release of antiinflammatory reacting cytokines such as IL-4, IL-10, and/or TGF-β1 , which are rapidly produced after shock, mechanical trauma, and during sepsis.31-33 These cytokines exert potent antiinflammatory and immunosuppressive abilities through deactivation of monocytes and suppression of T-helper and NK cell functions. Moreover, they markedly reduce proinflammatory cytokine release and inhibit IL-12, as well as IFN-γ synthesis and secretion.6,19,25-28,34,35 When measuring the release of IL-4, IL-10, and TGF-β1 into LPS-stimulated whole blood, the concentrations of these antiinflammatory reacting cytokines were significantly decreased in blood from critically ill patients. These results are in line with previous studies indicating reduced secretion of IL-10 by PBMC from injured patients and by splenocytes harvested from mice in a trauma-sepsis model.36 37
Though these antiinflammatory reacting mediators do not seem to cause suppression of the IL-12 IFN-γ pathway ex vivo in whole blood from critically ill patients, recent studies35 led us to hypothesize that an enhanced release of antiinflammatory mediators in the very early period after trauma or during sepsis may cause deactivation of monocytes and/or lymphocytes in vivo. This hypothesis was supported by measurements of IL-4 and IL-10 plasma levels in the first 10 hours after injury (Ertel et al, personal communication, July 1996, data not shown). In this early period after injury, plasma levels of IL-4 and IL-10 were markedly increased and demonstrated peak levels with decreasing concentrations close to the detection limit thereafter. Most of our whole blood assays of critically ill patients were performed 24 hours after injury or after diagnosis of sepsis. Deactivation of monocytes and lymphocytes may already have occurred in vivo during this time period. Moreover, because IL-10 self-limits its own and the synthesis of other antiinflammatory reacting cytokines as an autoregulatory feedback mechanism,34 35 the reduced release of IL-10 and TGF-β1 observed in LPS-stimulated whole blood from critically ill patients may already be caused by desensitization of monocytes in vivo in the first hours after the insult.
The hypothesis of early in vivo deactivation of PBMC is further supported by experiments in which whole blood from healthy humans was preincubated with either rhIL-4 or rhIL-10, thus mimicking the above described in vivo scenario during critical illness. Preincubation with rhIL-10 resulted in a significant suppression of LPS-induced secretion of IL-12 p40 and IFN-γ. In contrast to rhIL-10, rhIL-4 was ineffective. TGF-β1 was not tested, since recent studies clearly demonstrated a failure of TGF-β1 to induce hyporesponsiveness of monocytes to LPS.35 These data together with previous studies35 indicate that IL-10, but not IL-4 or TGF-β1 , plays a pivotal role in the downregulation of the proinflammatory reacting cascade including the IL-12 IFN-γ pathway.
The observation that isolated subpopulations of PBMC (adherent cells, lymphocytes) in contrast to whole blood can be activated through rhIL-12 p70 to release IFN-γ and vice versa implies that additional mechanisms of action may inhibit the IL-12 IFN-γ pathway. The second potential mechanism is based on previous animal experiments and clinical studies demonstrating the presence of circulating serum suppressive factors during experimental endotoxemia and after severe trauma.32,38 These suppressive proteins may explain two observations of our experiments: (1) the degree of suppressed IL-12 p40, p70, and IFN-γ release in critically ill patients was lower in isolated adherent cell and lymphocyte cultures than in whole blood, and (2) isolated adherent cells and lymphocytes responded to rhIFN-γ or rhIL-12 p70 with an increased release of IL-12 p70 and IFN-γ, which was not observed in whole blood from critically ill patients. These results suggest a potential involvement of circulating proteins, which may suppress secretory capacity of leukocyte subpopulations, especially in the later posttraumatic course. Thus, besides in vivo deactivation and desensitization of PBMC very early during critical illness through antiinflammatory reacting IL-10, the presence of serum suppressive peptides may contribute to the severe disturbances in the IL-12 IFN-γ pathway. Since IL-4, IL-10, and TGF-β1 are only responsible for 10% of total serum suppressive capacity during endotoxemia,32 additional, not yet identified, suppressive proteins may be involved. Furthermore, prostanoids such as prostaglandin E2 , which dramatically inhibits release of cytokine by monocytes and lymphocytes represent potential mediators involved in the suppression of the IFN-γ IL-12 pathway.39 40
The lack of proinflammatory reacting cells to adequately synthesize and secrete IL-12 and IFN-γ as potent stimulators of essential lymphocyte and phagocytic functions indicates a severe defect in the immune system of critically ill patients. The inhibition of this positive autoregulatory loop, which amplifies inflammatory responses to microbes, may represent a pivotal pathophysiologic mechanism that is responsible for critical immunosuppression and reduced responsiveness of monocytes/macrophages to bacterial challenges (endotoxin tolerance), as it was demonstrated in patients after severe injury and during sepsis.12-14
Previous reports1,41 42 emphasized a potential use of recombinant IFN-γ and/or IL-12 as therapeutic agents in the treatment of severe infection or in immunocompromised patients. However, our results make these suggestions questionable, since in critically ill patients, the target cells of IL-12 and IFN-γ do not seem to react to either one of these two mediators. Thus, further therapeutical strategies have to be directed towards neutralization of downregulatory mechanisms of the IL-12 IFN-γ pathway instead of infusion of recombinant IL-12 or IFN-γ.
ACKNOWLEDGMENT
We thank Dr Maurice Gately for revising the manuscript and his valuable comments.
Supported in part by a grant from the SBG, Zurich, and the AO/ASIF-Foundation, Bern, Switzerland.
Address reprint requests to Wolfgang Ertel, MD, Division of Trauma Surgery, Department of Surgery, University Hospital Zurich, Raemistrasse 100, CH-8091 Zurich, Switzerland.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal