Abstract
Neutrophils are important effector cells of acute inflammation because of their potential capacity to synthesize various proinflammatory mediators, and inhibition of their production is expected to result in anti-inflammatory effects. In this study, we investigate the effects of the anti-inflammatory cytokines, interleukin-10 (IL-10) and IL-4, on prostanoid synthesis in human neutrophils. Neutrophils isolated from healthy donors constitutively produced a small amount of prostaglandin E2 (PGE2 ) without any stimulations, whereas they produced a large amount of PGE2 after lipopolysaccharide (LPS) stimulation. IL-10 and IL-4 selectively inhibited their LPS-induced PGE2 production. Inhibition by both cytokines occurred at an early stage of LPS stimulation. Anti–IL-10 treatment of LPS-stimulated neutrophils resulted in enhanced PGE2 production. LPS-induced PGE2 and thromboxane B2 (TXB2 ) production in aspirin-treated neutrophils was significantly inhibited by IL-10, IL-4, and NS-398. Moreover, IL-10 and IL-4 inhibited LPS-induced cyclooxygenase (COX) activity in neutrophils. Western blot and immunocytochemical analysis showed that COX-2 protein was clearly induced in LPS-stimulated neutrophils and that its induction was inhibited by both IL-10 and IL-4. Moreover, both of these cytokines inhibited COX-2 mRNA expression in LPS-stimulated neutrophils. These results raise the possibility that these two cytokines may both offer potent clinical utility as anti-inflammatory agents in the future.
IT HAS RECENTLY been understood that interleukin-10 (IL-10) and IL-4 are anti-inflammatory cytokines. This is due, at least in part, to their potent biologic effects on monocytes/macrophages. Both cytokines efficiently inhibit the production of proinflammatory cytokines such as tumor necrosis factor-α (TNF-α), IL-1α, IL-1β, IL-6, and IL-8 by monocytes/macrophages.1-5 Additionally, they significantly inhibit the production of other proinflammatory mediators, such as reactive oxygen intermediates, reactive nitrogen intermediates, and prostaglandins (PGs) in monocytes/macrophages.4,6-12 On the other hand, IL-10 and IL-4 enhance the production of IL-1 receptor antagonist (IL-1ra) possessing an effective anti-inflammatory property.1,13 14
Neutrophils are the first effector cells that migrate into tissue sites during various inflammatory reactions. The cells have long been considered to be terminally differentiated cells and are capable of performing little, if any, de novo protein synthesis. However, as recently reported,15 it is becoming evident that following appropriate stimuli, these cells can express substantial amounts of mRNA for cytokines, including TNF-α, IL-1β, IL-8, IL-12, IL-1ra, macrophage inflammatory protein-1α (MIP-1α), MIP-1β, IL-3, and granulocyte-macrophage colony-stimulating factor (GM-CSF ). Even though production of such cytokines in neutrophils is considerably less than that in monocytes on a single-cell basis, it must be noted that neutrophils quantitatively dominate over monocytes within inflamed tissues. In rheumatoid arthritis (RA) patients, an increase in the absolute number of neutrophils, together with their induced function, have both been shown to be closely related to the disease activity of arthritis.16 17 Thus, inhibition of the function of these neutrophils is expected to control such inflammatory diseases.
Cyclooxygenase (COX) is a key rate-limiting enzyme in the biosynthesis of PGs and thromboxane from arachidonic acid (AA). Recent evidence has shown that COX exists in at least two distinct isoforms, a constitutive form (COX-1) and an inducible form (COX-2). COX-1 is constitutively expressed in various cell types and is believed to maintain physiologic homeostatic conditions, whereas COX-2 can be inducibly expressed in several cell populations following extracellular stimuli such as cytokines, mitogens, and lipopolysaccharide (LPS)18-22; thus, this isoform appears to play more pivotal roles in inflammatory situations. We have previously shown that LPS-stimulated monocytes inducibly expressed COX-2 at both the mRNA and the protein levels, and that such expression was significantly inhibited by IL-10 and IL-4.23 It was recently shown that in the rat pleurisy model of acute inflammation, COX-2 protein existed mainly within the infiltrating neutrophils at inflamed lesions.24 Recent reports have shown that IL-10 and IL-4 not only inhibit the production of cytokines including TNF-α, IL-1α, IL-1β, IL-8, and IL-12, but also enhance IL-1ra production in neutrophils.25-31 However, there have been no reports that clearly demonstrate the neutrophil-derived COX-2 expression in the human system, or the regulation by IL-10 and IL-4 of neutrophil-derived prostanoid synthesis. In this study, we have investigated these aspects and offer further discussion on the mechanism of the anti-inflammatory functions of IL-10 and IL-4.
MATERIALS AND METHODS
Reagents.Fetal bovine serum (FBS) and RPMI 1640 were purchased from GIBCO (Grand Island, NY) and Nissui Chemical Co (Tokyo, Japan), respectively. RPMI 1640 supplemented with FBS (10%), glutamine (1 mmol/L), penicillin (100 U/mL), and kanamycin (80 μg/mL) was used for all experiments except for the instances noted. LPS from Escherichia coli 0111:B4, purified by the Westphal method, was obtained from Difco (Detroit, MI). AA and aspirin were obtained from Sigma (St Louis, MO). NS-398 was obtained from Biomol Research Laboratories, Inc (Plymouth Meeting, PA). Human recombinant (r) IL-4 was generously provided by Schering-Plough Research (Bloomfield, NJ). Human rIL-10 was kindly provided by Dr K.W. Moore (DNAX, Palo Alto, CA). The anti–IL-10 monoclonal antibody (MoAb) 19F1 and isotype-matched control antibody were kindly provided by Dr J. S. Abrams (DNAX). The anti–COX-2 was a mouse MoAb (Transduction Laboratories, Lexington, KY) that was found to be highly specific. The antibody did not cross-react with human COX-1 and it had no apparent cross-reactivity against other human cell proteins.
Isolation and culture of human neutrophils.To minimize monocyte and platelet contamination, highly purified neutrophils were prepared as recently described,27 with some modifications. Briefly, 30 mL of heparinized venous blood of healthy donors was mixed with 20 mL of 6% dextran/0.9% sodium chloride. After dextran sedimentation of erythrocytes, the plasma was layered onto Ficoll-Hypaque (Pharmacia LKB Biotechnology Inc, Piscataway, NJ), centrifuged, and the mononuclear cells at the interface were carefully removed with a Pasteur pipette. To further purify neutrophils, the remainder of the Ficoll-Hypaque phase (containing monocytes) was completely removed with a fresh Pasteur pipette. The tube wall was carefully washed with phosphate-buffered saline (PBS), and the cell pellet on the bottom of the tube was suspended in 5 mL of PBS. The suspension was transferred to a new tube and the residual contaminating erythrocytes were eliminated by hypotonic lysis. Subsequently, the cells were layered onto NycoPrep 1.063 (Nycomed Pharma, Oslo, Norway), centrifuged, and the NycoPrep phase (containing platelets) was completely removed with a fresh Pasteur pipette. The recovered neutrophils were washed three times and resuspended at a density of 1 × 107 cells/mL in RPMI 1640 media. The remaining cells contained more than 99.5% neutrophils according to morphology and were more than 98% viable as determined by trypan blue exclusion. In this neutrophil preparation, IL-6, a good marker for monocyte contamination,15 27 was undetectable even in the presence of LPS (data not shown). These cells were cultured in RPMI 1640 media with 10% FBS at 37°C in a humidified atmosphere with 5% CO2 at a cell density of 1 × 106 cells/mL, in all of the experiments.
Radioimmunoassay (RIA) for PGE2 and thromboxane B2 (TXB2 ).Analysis of PGE2 and TXB2 levels in the supernatants of neutrophils (1 × 106 cells) was performed with a commercially available RIA kit (New England Nuclear, Boston, MA), as described elsewhere.8
Analysis of COX activity.Neutrophils (1 × 106 cells) were incubated in RPMI 1640 containing 10% FBS with either IL-10 or IL-4 in the presence or absence of LPS for 18 hours. The medium was removed after the incubation, and cells were incubated in fresh medium containing AA (30 μmol/L) for 10 minutes to determine COX activity, as described by Fu et al.32 Levels of PGE2 were determined using RIA.
Western blot analysis.Neutrophils (1 × 107 cells) were incubated for 18 hours with either IL-10 or IL-4 in the presence or absence of LPS. After incubation, cells were obtained and lysed in 150 μL of solubilization buffer at 25°C (1% Tween 20, 10 mmol/L phenylmethylsulfonyl fluoride, and 50 mmol/L Tris-HCl, pH 8.0). Cell lysates were then sonicated for 15 seconds and centrifuged at 15,000g for 15 minutes. Supernatants were subsequently mixed 1:1 with sodium dodecyl sulfate (SDS) sample buffer. Equal amounts of protein (25 μg) were then separated on a 9% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto a polyvinylidene difluoride (PVDF ) membrane. The membrane was then blocked with PBS containing 5% skim milk and 0.1% Tween 20, and then incubated with 0.25 μg/mL of mouse anti–COX-2 MoAb in the blocking buffer at 25°C for 2 hours. The membrane was subsequently incubated with horseradish peroxidase–conjugated goat anti-mouse IgG (1:1,000 dilution) and analyzed using an Amersham enhanced chemiluminescence (ECL) system (Amersham, Arlington Heights, IL). Fuji X-omat AR film (Fuji Photo Film Co, Tokyo, Japan) with cassette closure times of 5 to 10 minutes resulted in adequate exposure to visualize the bands.
Immunocytochemistry.Neutrophils (1 × 107 cells) were incubated for 18 hours with either IL-10 or IL-4 in the presence or absence of LPS, and deposited on a glass slide by using a Cytospin II (Shandon Southern Instruments, Inc, Sewickley, PA). After air-drying, the slides were fixed for 30 minutes in ice-cold acetone. After rinsing in Tris-buffered saline (TBS), the slides were blocked with a 1:50 dilution of normal horse serum for 30 minutes at 37°C, then treated for 1 hour at 37°C with mouse anti–COX-2 MoAb or isotype-matched control antibody. After incubation, preparations were rinsed three times with TBS, overlaid with biotinylated horse anti-mouse IgG (1:200; Vector Laboratories Inc, Burlingame, CA), incubated for 30 minutes, and rinsed three times with TBS. The slides were then treated by streptavidin conjugated to alkaline phosphatase for 30 minutes at 37°C, rinsed three times with TBS, and overlaid with naphthol-AS-BI-phosphoric acid 100 mg/mL in N,N-dimethyl formamide (Sigma, St Louis, MO) for 15 minutes at 37°C to allow for color development. The slides were then fixed by 20% paraformaldehyde in TBS for 20 minutes, rinsed three times with TBS, and stained with Mayer's hematoxylin.
Northern blot analysis.Neutrophils (5 × 107 cells) were incubated for 5 hours with either IL-10 or IL-4 in the presence or absence of LPS, and total cellular RNA was isolated by the acid guanidium thiocyanate/phenol/chloroform extraction method as described by Chomczynski and Sacchi.33 Ten micrograms of total RNA was fractionated on 1.5% agarose gel containing 2.2 mol/L formaldehyde and was subsequently transferred to a zeta-Probe blotting membrane (BioRad, Richmond, CA). Hybridization was done (overnight at 42°C) in 5× SSPE containing 50% formamide, 5× Denhardt's solution, 1% SDS, and 0.2 mg/mL of denatured salmon sperm DNA. The filter was washed in 2× SSC, 0.1% SDS at 50°C (twice, 25 minutes each time) followed by 0.5× SSC, 0.1% SDS at 50°C (twice, 15 minutes each time). The cDNA probe used was the 1.5-kb fragment of human COX-2.34 The probe was labeled using a random primer labeling kit (Takara Shuzo, Kyoto, Japan) with [32P]dCTP. To estimate the levels of each mRNA, the filters were exposed to a Fuji imaging plate (BAS-III; Fuji Photo Film Co) for 12 hours. The hybridized bands were visualized with the BAS-2000 Bio-image analyzer (Fuji Photo Film Co).
Statistical analysis.Student's t-test was used to compare control and experimental groups. Values of P > .05 were considered not significant.
RESULTS
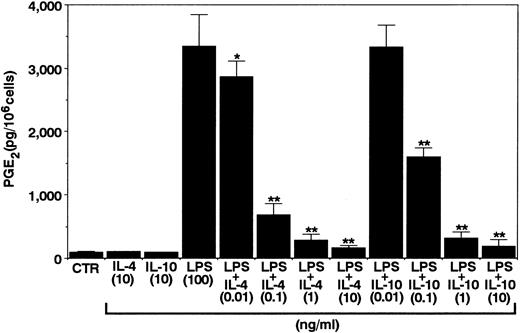
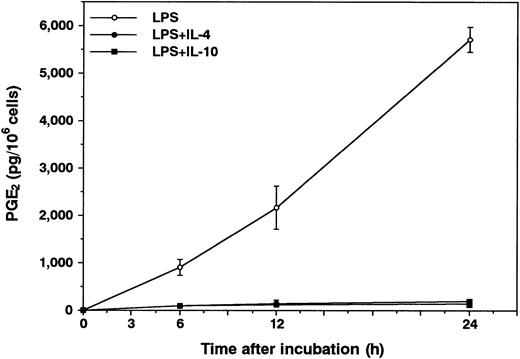
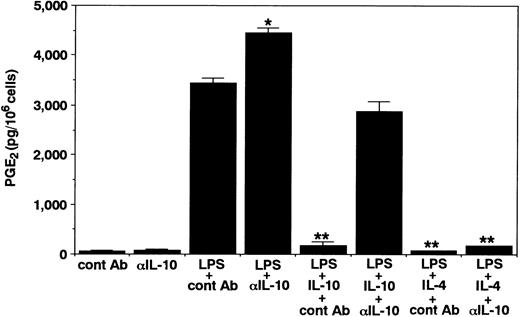
Effects of IL-10 and IL-4 on neutrophil-derived PGE2 production.To this end, we first determined the effects of IL-10 and IL-4 on PGE2 production in human neutrophils. As shown in Fig 1, in the absence of any stimuli, neutrophils constitutively produced small but detectable amounts of PGE2 in the culture supernatants. IL-10 and IL-4 at optimal concentrations did not affect the constitutive PGE2 production in neutrophils. Then, after activation by LPS, the cells inducibly produced high levels of PGE2 . Interestingly, both cytokines significantly inhibited LPS-induced PGE2 production in neutrophils. The inhibitory effects of both cytokines followed a dose-dependent fashion. We next determined the kinetics for the regulation by IL-10 and IL-4 of neutrophil-derived PGE2 production. As shown in Fig 2, PGE2 secretion could be detected 6 hours after LPS stimulation and this continued to increase up until 24 hours. Significant inhibition by both cytokines could already be observed 6 hours after LPS stimulation. Moreover, we determined the effect of neutralizing anti–IL-10 MoAb on neutrophil-derived PGE2 production. As shown in Fig 3, constitutive PGE2 production in neutrophils was not significantly affected by anti–IL-10 treatment. By contrast, LPS-induced PGE2 production in neutrophils was clearly enhanced by this treatment. In addition, the inhibition by IL-10 of LPS-induced PGE2 production in neutrophils was almost abrogated by anti–IL-10 treatment, whereas the inhibition by IL-4 was not affected by this treatment.
Effects of IL-10 and IL-4 on neutrophil-derived PGE2 production. Human neutrophils (1 × 106 cells) were cultured for 24 hours with the indicated concentrations of IL-10 and IL-4 in the presence or absence of LPS (100 ng/mL). RIA for PGE2 was performed as described in Materials and Methods. Results are expressed as the mean ± SEM of triplicate cultures. Similar results were obtained in three separate experiments. *P < .05, **P < .01 (compared with LPS only).
Effects of IL-10 and IL-4 on neutrophil-derived PGE2 production. Human neutrophils (1 × 106 cells) were cultured for 24 hours with the indicated concentrations of IL-10 and IL-4 in the presence or absence of LPS (100 ng/mL). RIA for PGE2 was performed as described in Materials and Methods. Results are expressed as the mean ± SEM of triplicate cultures. Similar results were obtained in three separate experiments. *P < .05, **P < .01 (compared with LPS only).
Kinetic studies for the inhibition by IL-10 and IL-4 of PGE2 production in LPS-stimulated neutrophils. Human neutrophils (1 × 106 cells) were cultured for 24 hours with or without 10 ng/mL of IL-10 or IL-4 in the presence of LPS (100 ng/mL). RIA for PGE2 was performed as described in Materials and Methods. Results are expressed as the mean ± SEM of triplicate cultures. Similar results were obtained in two separate experiments.
Kinetic studies for the inhibition by IL-10 and IL-4 of PGE2 production in LPS-stimulated neutrophils. Human neutrophils (1 × 106 cells) were cultured for 24 hours with or without 10 ng/mL of IL-10 or IL-4 in the presence of LPS (100 ng/mL). RIA for PGE2 was performed as described in Materials and Methods. Results are expressed as the mean ± SEM of triplicate cultures. Similar results were obtained in two separate experiments.
Effects of neutralizing anti–IL-10 MoAb on neutrophil-derived PGE2 production. Unstimulated or LPS-stimulated (100 ng/mL) neutrophils (1 × 106 cells) were cultured for 24 hours with neutralizing anti–IL-10 MoAb 19F1 or isotype-matched control antibody (10 μg/mL) in the presence or absence of 10 ng/mL of IL-10 or IL-4. RIA for PGE2 was performed as described in Materials and Methods. Results are expressed as the mean ± SEM of triplicate cultures. Similar results were obtained in two separate experiments. *P < .05, **P < .01 (compared with LPS only).
Effects of neutralizing anti–IL-10 MoAb on neutrophil-derived PGE2 production. Unstimulated or LPS-stimulated (100 ng/mL) neutrophils (1 × 106 cells) were cultured for 24 hours with neutralizing anti–IL-10 MoAb 19F1 or isotype-matched control antibody (10 μg/mL) in the presence or absence of 10 ng/mL of IL-10 or IL-4. RIA for PGE2 was performed as described in Materials and Methods. Results are expressed as the mean ± SEM of triplicate cultures. Similar results were obtained in two separate experiments. *P < .05, **P < .01 (compared with LPS only).
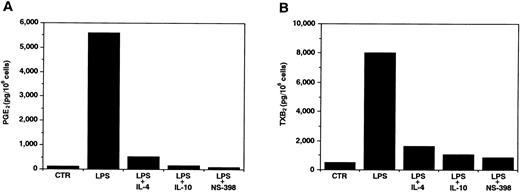
Effects of IL-10 and IL-4 on COX-2–derived PGE2 and TXB2 production in neutrophils.Recent studies have shown that in several cell types, LPS-induced prostanoid synthesis was mainly caused by the COX-2 expression. To inactivate endogenous COX-1, we treated neutrophils with aspirin for 2 hours,35 and determined their PGE2 and TXB2 production. As shown in Fig 4, after activation by LPS, the cells exhibited a remarkable PGE2 and TXB2 production, which was then significantly inhibited by IL-10 and IL-4. In addition, this LPS-induced prostanoid production was almost completely inhibited by a selective COX-2 inhibitor, NS-398.36 37 Taken together, these results indicate that prostanoid synthesis in LPS-stimulated neutrophils was regulated at the COX-2 level, and both IL-10 and IL-4 were able to act at this level.
Effects of IL-10 and IL-4 on COX-2–derived PGE2 (A) and TXB2 (B) production in neutrophils. Human neutrophils (1 × 106 cells) were treated for 2 hours in the presence of aspirin (500 μmol/L) to inactivate endogenous COX-1, washed three times with PBS, and then cultured for 24 hours with medium, LPS (100 ng/mL), LPS plus IL-4 (10 ng/mL), LPS plus IL-10 (10 ng/mL), or LPS plus NS-398 (1 μmol/L). RIA for PGE2 (A) and TXB2 (B) was performed as described in Materials and Methods. Similar results were obtained in two separate experiments.
Effects of IL-10 and IL-4 on COX-2–derived PGE2 (A) and TXB2 (B) production in neutrophils. Human neutrophils (1 × 106 cells) were treated for 2 hours in the presence of aspirin (500 μmol/L) to inactivate endogenous COX-1, washed three times with PBS, and then cultured for 24 hours with medium, LPS (100 ng/mL), LPS plus IL-4 (10 ng/mL), LPS plus IL-10 (10 ng/mL), or LPS plus NS-398 (1 μmol/L). RIA for PGE2 (A) and TXB2 (B) was performed as described in Materials and Methods. Similar results were obtained in two separate experiments.
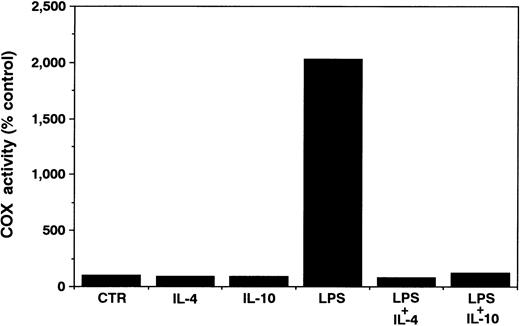
Effects of IL-10 and IL-4 on COX activity in neutrophils.We next determined the effects of both cytokines on neutrophil-derived COX activity. As shown in Fig 5, even in the absence of LPS, neutrophils constitutively showed a weak yet significant COX activity. Neither IL-10 nor IL-4 affected this activity. After activation by LPS, the cells exhibited a significant increase in COX activity. Then, both cytokines significantly inhibited this LPS-induced COX activity in neutrophils.
Effects of IL-10 and IL-4 on neutrophil-derived COX activity. Human neutrophils (1 × 106 cells) were cultured with or without 10 ng/mL of IL-10 or IL-4 in the presence or absence of LPS (100 ng/mL). After 18 hours, the culture medium was removed, and then fresh medium containing 30 μmol/L AA was added on top of the cell layer. After incubation for 10 minutes, the culture medium was assayed for PGE2 by RIA. Results are expressed as a percentage of COX activity of unstimulated neutrophils. Similar results were obtained in two separate experiments.
Effects of IL-10 and IL-4 on neutrophil-derived COX activity. Human neutrophils (1 × 106 cells) were cultured with or without 10 ng/mL of IL-10 or IL-4 in the presence or absence of LPS (100 ng/mL). After 18 hours, the culture medium was removed, and then fresh medium containing 30 μmol/L AA was added on top of the cell layer. After incubation for 10 minutes, the culture medium was assayed for PGE2 by RIA. Results are expressed as a percentage of COX activity of unstimulated neutrophils. Similar results were obtained in two separate experiments.
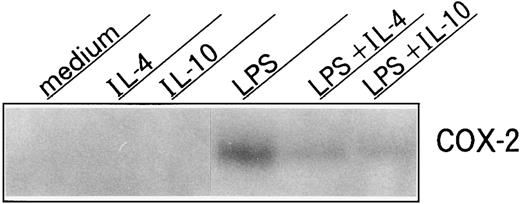
Effects of IL-10 and IL-4 on COX-2 protein expression in neutrophils.We next determined the effects of IL-10 and IL-4 on COX-2 protein expression in neutrophils by using Western blot analysis. As shown in Fig 6, unstimulated neutrophils exhibited no distinct COX-2 protein and neither of the cytokines affected this expression. By contrast, after stimulation by LPS, the expression of this protein was drastically induced and both cytokines significantly inhibited this expression.
Western blot of neutrophil-derived COX-2 protein expression. Human neutrophils (1 × 107 cells) were cultured with or without 10 ng/mL of IL-10 or IL-4 in the presence or absence of LPS (100 ng/mL). After 18 hours, cells were procured, cell lysates were centrifuged, and equal amounts of protein (25 μg) were then separated on a 9% SDS-PAGE and subjected to Western blot analysis with 0.25 μg/mL anti–COX-2 MoAb. Similar results were obtained in three separate experiments.
Western blot of neutrophil-derived COX-2 protein expression. Human neutrophils (1 × 107 cells) were cultured with or without 10 ng/mL of IL-10 or IL-4 in the presence or absence of LPS (100 ng/mL). After 18 hours, cells were procured, cell lysates were centrifuged, and equal amounts of protein (25 μg) were then separated on a 9% SDS-PAGE and subjected to Western blot analysis with 0.25 μg/mL anti–COX-2 MoAb. Similar results were obtained in three separate experiments.
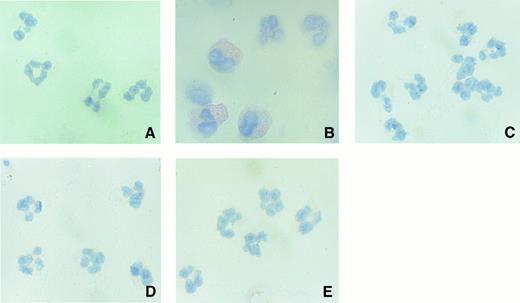
Immunocytochemical analysis of neutrophil-derived COX-2 protein.To further confirm the findings of Western blot studies, we next performed immunocytochemical analysis of neutrophil-derived COX-2 protein. Unstimulated neutrophils exhibited no specific staining of COX-2 protein (Fig 7A). After activation by LPS, neutrophils clearly demonstrated a significant staining for immunoreactive COX-2 protein (Fig 7B). However, as recently reported,24 it was noted that the intensity of staining in each cell varied to some extent. By contrast, LPS-stimulated neutrophils treated with IL-10 or IL-4 exhibited less immunoreactive COX-2 protein (Fig 7C and D). The level of isotype-matched control antibody binding was low, showing the specificity of the immunoreactive COX-2 localization (Fig 7E).
High-power (original magnification × 400) photomicrographs of immunolocalization of neutrophil-derived COX-2 protein. Human neutrophils (1 × 107 cells) were cultured for 18 hours with 10 ng/mL of IL-10 or IL-4 in the presence or absence of LPS (100 ng/mL). The cells were deposited on a glass slide and stained with anti–COX-2 MoAb (A, medium; B, LPS; C, LPS + IL-4; D, LPS + IL-10) or control antibody (E).
High-power (original magnification × 400) photomicrographs of immunolocalization of neutrophil-derived COX-2 protein. Human neutrophils (1 × 107 cells) were cultured for 18 hours with 10 ng/mL of IL-10 or IL-4 in the presence or absence of LPS (100 ng/mL). The cells were deposited on a glass slide and stained with anti–COX-2 MoAb (A, medium; B, LPS; C, LPS + IL-4; D, LPS + IL-10) or control antibody (E).
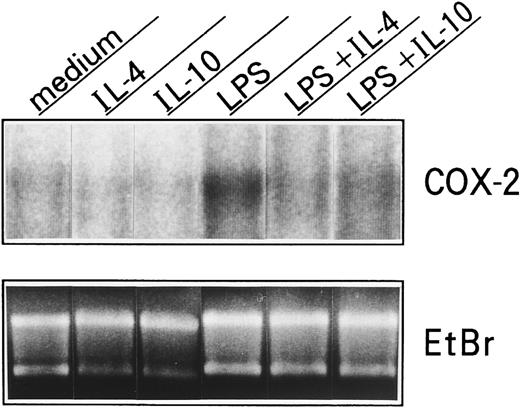
Effects of IL-10 and IL-4 on COX-2 mRNA expression in neutrophils.Because COX-2 protein has been shown to be also regulated at the RNA level in various cell types,19-23 we thus determined COX-2 mRNA expression in neutrophils. As shown in Fig 8, unstimulated neutrophils did not express COX-2 mRNA, and neither IL-10 nor IL-4 affected this expression. By contrast, after activation by LPS, COX-2 mRNA was drastically induced, and both cytokines significantly inhibited this expression.
Effects of IL-10 and IL-4 on neutrophil-derived COX-2 mRNA expression. Human neutrophils (5 × 107 cells) were cultured with or without 10 ng/mL of IL-10 or IL-4 in the presence or absence of LPS (100 ng/mL). After 5 hours, total RNA was isolated and analyzed by Northern blot using a COX-2 probe. Similar results were obtained in two separate experiments.
Effects of IL-10 and IL-4 on neutrophil-derived COX-2 mRNA expression. Human neutrophils (5 × 107 cells) were cultured with or without 10 ng/mL of IL-10 or IL-4 in the presence or absence of LPS (100 ng/mL). After 5 hours, total RNA was isolated and analyzed by Northern blot using a COX-2 probe. Similar results were obtained in two separate experiments.
DISCUSSION
In the present study, we investigated the mechanism of neutrophil-derived prostanoid synthesis and its regulation by IL-10 and IL-4. Consistent with previous reports,38,39 human neutrophils inducibly produced large amounts of PGE2 and TXB2 after activation by LPS, although the level of production on a cell basis in neutrophils was approximately 10-fold less than that in monocytes.8 IL-10 and IL-4 inhibited LPS-induced prostanoid production to the same degree. In addition to LPS, both TNFα and IL-1β were previously shown to activate neutrophil functions.25,40 Both cytokines, like LPS, stimulated neutrophils to produce a significant amount of PGE2 , which was again inhibited by IL-10 and IL-4 (data not shown). The inhibitory effects of IL-10 and IL-4 have also been reported regarding the production of cytokines such as TNF-α, IL-1α, IL-1β, IL-8, IL-12, MIP-1α, and MIP-1β in LPS-stimulated neutrophils.25-30 Compared with the inhibition of cytokine production, IL-10 and IL-4 seemed to be rather effective at inhibiting neutrophil-derived prostanoid production. On the other hand, the effects of both these cytokines on neutrophil function are not always inhibitory. IL-10 and IL-4 have been found to rather enhance IL-1ra production in LPS-stimulated neutrophils.29,31 Based on these findings, the effects of the two cytokines on neutrophil functions appear to be virtually identical to those on monocyte functions. However, that is not the case for superoxide anion production. In monocytes, IL-10 significantly inhibited the expression of NADPH oxidase subunit gp91-phox,41 whereas in neutrophils, IL-10 had no effect on gp91-phox expression.29
After activation by LPS, monocytes are able to produce endogenous IL-10, which in turn regulates their own production of various effector molecules.1,3,8 In the present study, the same is true for neutrophils. Anti–IL-10 treatment of LPS-stimulated neutrophils resulted in enhanced PGE2 production. After activation by LPS, our neutrophil preparation contained detectable amounts of IL-10, but not IL-6 (data not shown), suggesting that endogenous IL-10 was released from neutrophils, but not contaminating monocytes. Recently, in the mouse system, LPS-induced IL-10 production in neutrophils was clearly demonstrated.42 In our study, the inhibitory effect of IL-4 on LPS-induced PGE2 production in neutrophils was not explained by the secondary induction of endogenous IL-10. Thus, IL-10 and IL-4 independently regulate neutrophil-derived prostanoid production.
COX is a key rate-limiting enzyme in the synthesis of PGE2 and TXB2 from AA. Recent studies have shown that COX exists in at least two different isoforms, COX-1 and COX-2. Using recent sensitive approaches such as immunocytochemistry, it has been shown that in the rat pleurisy model of acute inflammation, the COX-2 protein clearly existed, mainly within infiltrating neutrophils at inflamed lesions.24 With regard to human systems, there have been few reports concerning neutrophil-derived COX-2 expression. COX-2 was shown to be significantly expressed in the synovial tissues of RA patients, especially within infiltrating mononuclear cells, endothelial cells of blood vessels, and subsynovial fibroblast-like cells.43 Considering the significance of neutrophil functions in RA disease activity,16 17 further investigations are essential for clarifying the pathogenesis of RA. In human neutrophils, immunoreactive COX-2 protein was clearly expressed within LPS-stimulated neutrophils, and treatment with IL-10 or IL-4 almost completely inhibited this expression. Similar findings were also obtained by Western blot analysis. On the other hand, in our system, COX-1 protein expression in neutrophils was hardly detected (data not shown). Considering that unstimulated neutrophils produced a small but detectable amount of PGE2 , it is probable that the cells express COX-1 protein very weakly. Thus, our findings that IL-10 and IL-4 bore no effect on constitutive PGE2 production in neutrophils suggested that perhaps neither of these cytokines has any effect on COX-1 protein expression. Together with the data of Fig 4, these findings indicate that IL-10 and IL-4 selectively inhibit COX-2 protein expression in neutrophils.
In our study, COX-2 mRNA was inducibly expressed in LPS-stimulated neutrophils, and this expression was almost completely inhibited by IL-10 and IL-4. The gene expression is thought to be regulated at the transcriptional and/or posttranscriptional level. We previously showed that in monocytes, the regulation by IL-10 and IL-4 of COX-2 mRNA expression occurred at both levels.23 However, in the present study we failed to determine whether a similar mechanism was involved in neutrophils for the following reasons. First, experiments with actinomycin D showed that significant inhibition by both cytokines of COX-2 mRNA expression occurred at early time points after LPS stimulation, and the rate of mRNA degradation could not be correctly calculated (data not shown). A similar situation was previously reported regarding the inhibition by IL-10 of TNF-α production in mouse macrophages.44 Second, as recently shown in neutrophil-derived cytokines,27 transcription of the COX-2 gene was hardly detected (data not shown). This is possibly because of low transcriptional rates in neutrophils, compared with those in monocytes. Recent reports have shown that IL-10 inhibited the production of MIP-1α, MIP-1β, and IL-8 in LPS-stimulated neutrophils by accelerating the degradation of mRNA.26 27 However, in those reports, data on the transcription were not provided. To our knowledge there have been no reports on the effect of IL-4 on gene transcription and mRNA stability in neutrophils. Based on these findings, we, at present, cannot rule out the possibility that IL-10 and IL-4 may regulate the LPS-induced COX-2 mRNA expression in neutrophils as well as in monocytes at both the transcriptional and posttranscriptional levels.
PGs are potent proinflammatory mediators that cause vasodilatation and hyperalgesia in vivo, these being the characteristic features of acute inflammation. It is now evident that neutrophils represent an important source of PGs in acute inflamed tissues. Recent studies have noted that those drugs showing selective inhibition of COX-2 expression exhibited potent anti-inflammatory effects in vivo, with fewer side effects.45 Therefore, our present findings that IL-10 and IL-4 significantly inhibited prostanoid production in LPS-stimulated neutrophils by selectively inhibiting COX-2 expression raise the possibility that both cytokines may offer clinical utility as anti-inflammatory agents in the future.
ACKNOWLEDGMENT
The authors thank Drs Toshihiro Miyamoto and Kei Ikeda (First Department of Internal Medicine, Kyushu University, Fukuoka, Japan) for helpful discussion, and K. Miller (Royal English Language Centre, Fukuoka, Japan) for proofreading the English used in this manuscript.
Supported in part by grants-in-aid for Scientific Research on Priority Areas from the Ministry of Education, Science and Culture of Japan and by the Special Coordination Funds for Science and Technology.
Address reprint requests to Takeshi Otsuka, MD, First Department of Internal Medicine, Faculty of Medicine, Kyushu University, 3-1-1 Maidashi, Higashi-ku, Fukuoka 812-82, Japan.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal