Abstract
To develop an in vivo model wherein human hematopoiesis occurs, we transplanted severe combined immunodeficiency (SCID) mice with either human fetal bone marrow (HFBM) or human fetal liver (HFL). After transplantation of SCID mice with cultured HFBM (BM-SCID-hu mice) or HFL cells (Liv-SCID-hu mice), significant engraftment of the mouse bone marrow (BM) and population of the peripheral blood with human leukocytes was detected. Human colony-forming unit–granulocyte macrophage and burst forming unit-erythroid were detected in the BM of the BM-SCID-hu and Liv-SCID-hu mice up to 8 months after transplantation. When the HFBM or HFL cells were transduced with a retroviral vector before transplantation, integrated retroviral sequences were detected in human precursor cells present in the SCID mouse BM and in leukocytes circulating in the peripheral blood (PB) up to 7 months after transplantation. The PB of the BM-SCID-hu mice also became populated with human T cells after implantation with human thymic tissue, which provided a human microenvironment wherein human pre-T cells from the BM could mature. When the HFBM was retrovirally transduced before transplantation, integrated retrovirus was detected in sorted CD4+CD8+ double positive and CD4+ single positive cells from the thymic implant and CD4+ cells from the PB. Taken together, these data indicated that the BM of our BM-SCID-hu and Liv-SCID-hu mice became engrafted with retrovirally transduced human hematopoietic precursors that undergo the normal human hematopoietic program and populate the mouse PB with human cells containing integrated retroviral sequences. In addition to being a model for studying in vivo human hematopoiesis, these mice should also prove to be a useful model for investigating in vivo gene therapy using human stem/precursor cells.
HUMAN PRECURSOR cells can engraft the bone marrow (BM) of immunodeficient mice,1,2 but significant BM engraftment with human myeloid and erythroid precursors only occurred if these mice were treated with human cytokines after transplantation.3 Furthermore, the human hematopoiesis observed by some investigators in the BM of cytokine-treated, transplanted C.B-17 scid/scid severe combined immunodeficiency (SCID) mice was only associated with minimal peripheral engraftment by human myeloid and lymphoid cells.2,3 We postulated that the degree of engraftment of the murine BM and population of the peripheral lymphoid compartment with human cells would be markedly increased if the SCID mice were transplanted with human fetal BM (HFBM), which contains a greater proportion of human precursor cells than does pediatric or adult BM.4,5 We showed that irradiated SCID mice transplanted with cultured HFBM cells (BM-SCID-hu mice) displayed a high degree of engraftment of the mouse BM and peripheral lymphoid tissue with human lymphoid and myeloid cells without the requirement for exogenous human cytokine therapy.6
The potential to introduce genes into cells capable of self-renewal and maturation into different lineages has made hematopoietic stem/precursor cells an important target for gene therapy.7 However, the efficacy of gene therapy has been limited by several factors including inefficient vectors and the low expression of the transfected gene by the transduced cells.8 9 Therefore, research in the development of techniques to improve the effectiveness of human gene therapy would be greatly facilitated by the development of an in vivo model wherein human stem/precursor cells could be transduced with retroviral vectors, transplanted into mice, undergo hematopoietic maturation in the murine BM and then populate the peripheral lymphoid tissue with mature transduced human cells. In the present study we showed that our BM-SCID-hu mice should provide a useful model for studying in vivo gene therapy using human stem/precursor cells. Using clonogenic assays, we showed that the BM of the BM-SCID-hu mice exhibited long-term engraftment with human precursor cells. When HFBM cells were transduced with a retroviral vector before transplantation, integrated retroviral sequences were detected in human precursor cells present in the BM of the BM-SCID-hu mice. Furthermore, integrated retroviral sequences were also detected in the peripheral blood mononuclear cells (PBMC) of these mice. Similar results were also observed in irradiated SCID mice transplanted with human fetal liver (HFL) cells (Liv-SCID-hu mice). We also showed that human T-cell maturation occurred in BM-SCID-hu mice implanted with human thymus, which populated the peripheral blood (PB) of the SCID mice with human T cells containing integrated retroviral sequences.
MATERIALS AND METHODS
Animals.A colony of SCID mice was established from founder C.B-17 scid/scid mice delivered by cesarean section (Taconic Farms, Germantown, NY) and was maintained in pathogen-free sterile isolators (Standard Safety, Palatine, IL) without prophylactic antibiotic treatment at the Albert Einstein College of Medicine Animal Institute (Bronx, NY) as described.10 After transplantation, the mice were housed in a Maxi-Miser Caging System (Thoren Caging Systems, Inc, Hazelton, PA), which provides positive pressure individual ventilation of class 100 HEPA-filtered to each cage and exhausts air from each individual cage. All of the mice were cared for according to institutional guidelines and all food, water, caging, and bedding were autoclaved before use. Our experience has been that maintaining the SCID mice in an ultraclean environment is crucial for getting reproducible engraftment with human cells.
Construction of BM-SCID-hu and Liv-SCID-hu mice.After the elective termination of pregnancy, HFBM cells were obtained from fetuses (18 to 23 gestational week) as described.11 HFL was sectioned, gently teased apart, filtered through a stainless steel mesh, and mononuclear cells were isolated by Ficoll-Hypaque density centrifugation. The HFBM cells and HFL cells were washed twice in phosphate-buffered saline (PBS), counted and cultured in RPMI 1640 with added fetal calf serum (FCS; 10% vol/vol), penicillin/streptomycin (100 U/mL), and Gentamicin (GIBCO-BRL, Grand Island, NY) (50 μg/mL) at 4 × 106 cells/cm2 at 37°C for 4 days. The cultured HFBM and HFL cells were obtained, washed twice in PBS, counted, and resuspended at 8 × 107 cells/ mL. SCID mice (6 to 8 weeks old) were irradiated (375 cGy), anesthetized with Pentobarbital (40 to 80 mg/kg; Anpro Pharmaceutical, Arcadia, CA), and then injected intravenously (IV) in a tail vein with 4 × 107 cultured HFBM cells or cultured HFL cells in a total volume of 500 μL. The procedures used in this study were reviewed and approved by the Albert Einstein College of Medicine Committee on Clinical Investigation.
Antibodies and reagents.FCS, PBS, Iscove's modified Dulbecco's medium (IMDM), Gentamicin, L-glutamine, and penicillin/streptomycin were obtained from GIBCO-BRL, methylcellulose, and β-mercaptoethanol were obtained from Sigma (St Louis, MO), stem cell factor (SCF ) and interleukin-6 (IL-6) were kind gifts of Dr S. Gillis (Immunex Corp, Seattle, WA), Procrit (Epoietin alfa) was obtained from Ortho Biotech (Raritan, NJ), and recombinant human granulocyte colony-stimulating factor (rhGM-CSF) was obtained from Genetics Institute (Cambridge, MA). Peridinin chlorophyll protein (PerCP)-conjugated mouse monoclonal antibody (MoAb) to human CD45, fluorescein isothiocyanate (FITC)-conjugated MoAbs to human CD4, CD13, CD14, or CD20 and phycoerythrin (PE)-conjugated MoAbs to human CD8, CD11b, CD19, CD33, or CD34 were obtained from Becton Dickinson (Mountain View, CA).
Flow cytometric analysis and sorting.At the indicated times, BM-SCID-hu and Liv-SCID-hu mice were bled and mononuclear cells were isolated from the PB as described.6 BM cells were obtained by killing the mice and then lavaging the mouse femurs with ice-cold PBS. Single cell suspensions were obtained from the implanted human thymus tissue by gently teasing it apart, and then filtering the suspension through a sterile stainless steel mesh. All cell suspensions were washed twice, counted, and resuspended at 1 × 106 cells/mL in PBS. For three-color flow cytometric analysis, cells were stained with the indicated PerCP-, PE-, and FITC-conjugated mouse MoAb and 10,000 events were analyzed by flow cytometry as described.6 Control HFBM or HFL cells were used to set gates for lymphoid (gate R1) or myeloid (gate R2) cells as described.12 Cut-off values for the quadrants were set after compensation for PE versus FITC versus PerCP emission based on the analysis of single, double, and triple staining of positive and negative control samples (human fetal BM and C.B-17 mouse BM, respectively) as well as appropriate FITC, PE, or PerCP labeled isotype controls. All the antibodies used were human-specific and minimally cross-reactive as determined by performing the appropriate control experiments.6 10
Sorting of human CD4+ cells from the mouse PB and CD4+ and CD4+CD8+ thymocytes from the thymic implant was performed using a modification of a previously described technique.13 After incubating the cells with PerCP-conjugated mouse MoAb to human CD45 and FITC-conjugated MoAb to human CD4, or with PerCP-conjugated mouse MoAb to human CD45, FITC-conjugated MoAb to human CD4 and PE-conjugated antibody to human CD8, the cells were washed and sorted using the FACStar Plus cell sorter (Becton Dickinson). The region specific for human CD45+CD4+CD8− cells or human CD45+CD4+CD8+ cells was identified and these cells were sorted into a sterile 12 × 75 mm test tube containing 1 mL of PBS. At the end of the assay, a small aliquot of sorted cells was reanalyzed on the FACStar to evaluate sorting purity. Sorted cells were spun down, cellular DNA was extracted and neo-specific polymerase chain reaction (PCR) was performed as described below.
Hematopoietic precursor cell assay.Hematopoietic precursor cells from human fetal BM and liver were assayed using a variation of a previously described method.14 Mononuclear cells obtained from the indicated tissue were isolated by Hypaque/Ficoll density centrifugation and 5 × 104 cells/mL were cultured for 2 weeks in 35 mm Petri dishes (Nunc Co, Naperville, IL) in 1 mL of IMDM containing 1.2% methylcellulose, 30% FCS, 1% L-glutamine, and β-mercaptoethanol (5 × 10−5 mol/L) with added rh-Epo (2 U/mL), rhIL-3 (50 ng/mL), rhIL-6 (10 ng/mL), and rh-SCF (20 ng/mL). Human hematopoietic precursor cells obtained from murine BM were assayed using a modification of a previously described method.15 Murine BM cells (1 to 2 × 105 cells) obtained from the murine femur were plated in 35 mm Petri dishes in 1 mL of IMDM containing 1.2% methylcellulose, 15% FCS, 15% human serum, 1% L-glutamine, and β-mercaptoethanol (5 × 10−5 mol/L). Two weeks of culture in the presence of human serum and the combination of recombinant human cytokines, Epo (2 U/mL), IL-3 (50 ng/mL), GM-CSF (10 U/mL), and SCF (20 ng/mL) resulted in the selective growth of human precursors. Murine BM cells were used as a negative control in each experiment and by 2 weeks of culture, no significant murine precursor cell growth was observed. To confirm the human origin of the colonies, single colonies of different lineages were individually obtained from cultures with a Pasteur pipette and analyzed for human β2-microglobulin DNA with human-specific PCR primers as described.6 All cultures were performed in triplicate, incubated at 37°C in a humidified atmosphere with 5% CO2 for 14 days and the mean number of colony-forming unit granulocyte-macrophage (CFU-GM), burst-forming unit-erythroid (BFU-E) and colony-forming unit mixed lineage (CFU-mix) colonies counted using an inverted microscope are presented. Colonies were defined as having greater than 50 cells and were characterized by their morphology and appearance. Colonies with cells having brick-red hemaglobinized color were classified as BFU-E, colonies consisting of granulocytes and macrophages were scored as CFU-GM and colonies made up of a mixture of erythroid and myeloid cells were considered CFU-mix. To normalize the number of human hematopoietic progenitors present in each mouse, the percentage of human CD45+ cells in the BM of each transplanted SCID mouse was determined by flow cytometric analysis, multiplied by the initial plating cell concentration and is reported as colonies/5 × 104 human mononuclear cells.
Retroviral transduction of HFBM and HFL cells.HFBM and HFL cells were transduced with the neo gene-containing LN retroviral vector, which was derived from the LNL6 vector by deletion of the env and some noncoding regions of neo to prevent the generation of replication-competent retrovirus.16 High-titered retrovirus was produced by PG13/LN, a hybrid retrovirus-producing cell line that expresses the gibbon ape leukemia virus env and the Moloney murine leukemia virus gag-pol proteins16 that produced the LN retrovirus vector in culture supernatant at over 106 CFU/mL. Alternatively, HFBM and HFL cells were transduced with the neo gene-containing MY-2 vector17 and produced in high titer (>106 CFU/mL) from the supernatant of a PG-13 transfected cell line. After being cultured for 72 hours, HFBM cells were incubated with retrovirus-containing supernatant with polybrene (4 to 8 μg/mL) for 24 to 48 hours. Growth factors (IL-3 [50 ng/mL], IL-6 [20 ng/mL], and SCF [20 ng/mL]) were added during the transduction in some experiments. After retroviral transduction, the cells were obtained and some cells were assayed for the presence of CFU in methylcellulose culture whereas other cells were injected IV into irradiated SCID-mice.
PCR analysis.Leukocytes were isolated from blood by lysis of red blood cells with 0.16 mol/L NH4Cl buffer followed by 2 washes with PBS. Individual colonies were obtained from methylcellulose plates and then washed twice with PBS. Genomic DNA was isolated from the cells by resuspending them in lysis buffer (1% Triton X-100 [Pierce, Rockford, IL]/10 mmol/L Tris HCl, pH 7.0/1 mmol/L EDTA) and boiling for 15 minutes. The supernatant was procured and 5 μL was amplified by PCR with primers specific for the neo gene for 60 cycles (94°C for 1 minute, 65°C for 1 minute, and 72°C for 1 minute). The nucleotide sequences for the 5′ and 3′ primer pairs were GGAAGCCGGTCTTGTCGATC and CGAAATCTCGTGATGGCAGG and generate a 420-base pair product. After PCR, 17 μL of the reaction mixture was electrophoresed through a 1.5% NuSieve/ 0.5% SeaKem agarose (FMC, Rockland, ME) gel containing ethidium bromide, and the amplified products were visualized under ultraviolet light. The identity of the amplified product was confirmed by Southern blotting with a biotin-labeled probe specific for an intervening sequence, CTCGTCGTGACCCATGGCGATGCCTGC as described.18 The specificity of the PCR reaction was confirmed by including positive and negative controls in each run.
Statistical analysis of the data.The Wilcoxon's rank sum test was used for all statistical comparisons made.
RESULTS
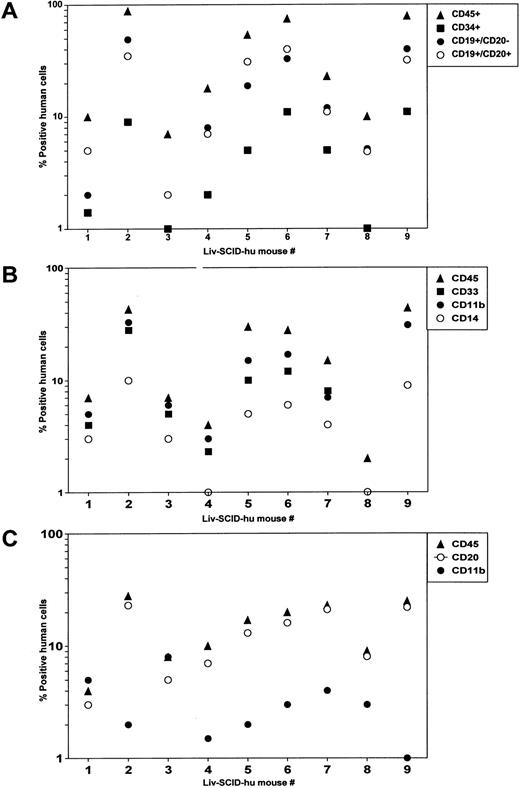
Reconstitution of SCID mice with HFL mononuclear cells.Significant hematopoiesis occurs in the HFL, which contains a large number of primitive precursor cells that have a greater proliferative and differentiative capacity than do precursor cells present in adult BM.5 Therefore, we investigated whether precultured HFL cells could successfully engraft irradiated SCID mice in the absence of treatment with human cytokines as we had shown for HFBM cells.6 After HFL was disassociated, mononuclear cells were isolated by Hypaque/Ficoll density centrifugation and placed in culture. After 4 days, the cells were procured and were IV injected (4 × 107 cells/mouse) into irradiated SCID mice (Liv-SCID-hu mice). Four months later, the mice were killed and the population of human cells present in the murine BM and PB were evaluated by flow cytometry. Human lymphoid cells were present in the Liv-SCID-hu BM including CD34+ precursor cells and B cells whose lineage ranged from immature CD19+CD20− B cells to more mature CD19+CD20+ B cells (Fig 1A). Because differentiation of myeloid cells in the human BM is characterized by the sequential expression of CD33, CD11b, and CD14,19 the cells present in the myeloid gate of the Liv-SCID-hu BM were evaluated for the expression of these markers. As shown in Fig 1B, the BM from the Liv-SCID-hu mice contained myeloid cells expressing human CD33, CD11b, and CD14. To determine whether engraftment of the Liv-SCID-hu mice was associated with population of the PB with human B cells and monocytes, mononuclear cells in the PB of the Liv-SCID-hu mice were assessed for expression of CD20 and CD11b. As shown in Fig 1C, human CD20+ B cells and CD11b+ monocytes were detected in the Liv-SCID-hu mouse PB. Thus, as reported for HFBM cells,6 cultured HFL cells could engraft SCID mice without the provision of exogenous human cytokines.
Analysis of SCID mice transplanted with HFL. Four months after transplantation of irradiated SCID mice (n = 9 mice) with HFL cells: (A) mononuclear cells isolated from the BM and cells in the lymphoid gate were analyzed by flow cytometry for the expression of human CD45, CD34, CD19, and CD20. (B) Mononuclear cells isolated from the BM and cells in the myeloid gate were analyzed by flow cytometry for the expression of human CD45, CD33, CD11b, and CD14. (C) Mononuclear cells isolated from the PB were analyzed by flow cytometry for the expression of human CD45, CD20, and CD11b.
Analysis of SCID mice transplanted with HFL. Four months after transplantation of irradiated SCID mice (n = 9 mice) with HFL cells: (A) mononuclear cells isolated from the BM and cells in the lymphoid gate were analyzed by flow cytometry for the expression of human CD45, CD34, CD19, and CD20. (B) Mononuclear cells isolated from the BM and cells in the myeloid gate were analyzed by flow cytometry for the expression of human CD45, CD33, CD11b, and CD14. (C) Mononuclear cells isolated from the PB were analyzed by flow cytometry for the expression of human CD45, CD20, and CD11b.
Detection of human hematopoietic precursors in the BM of BM-SCID-hu and Liv-SCID-hu mice by clonogenic assays.To determine whether human hematopoietic precursor cells were present in the BM of the BM-SCID-hu and Liv-SCID-hu mice, BM cells obtained from the mice at least 3 months after transplantation were assessed for the presence of human CFU-GM, BFU-E, and CFU-mix precursors in clonogenic assays. Because variable numbers of human cells were present in the BM of different transplanted SCID mice, the number of progenitors was normalized based on the percentage of human CD45+ cells present in the BM. The number of human precursor cells present in fetal BM (n = 4 fetuses) and fetal liver (n = 5 fetuses) were compared to the number of human precursor cells present in the BM of BM-SCID-hu mice (n = 4 mice) and Liv-SCID-hu mice (n = 7 mice) (Table 1). CFU-GM precursors were present in the BM of BM-SCID-hu and Liv-SCID-hu mice at levels that were comparable to those present in the fetal BM (P = .309) and fetal liver (P = .625). BFU-E precursors were also present in the BM of the BM-SCID-hu and Liv-SCID-hu mice, but at levels that were less than those detected in the fetal BM (P = .0294) and fetal liver (P = .1038) before transplantation. CFU-mix precursors were detectable in the BM of some of the BM-SCID-hu and Liv-SCID-hu mice (range from 0− to 2 colonies/5 × 104 cells) at levels that were less than those detected in fetal BM (P = .0497) and fetal liver (P = .0304). Taken together, these data indicate that 3 months after transplantation, significant numbers of hematopoietic precursor cells were present in the BM of the BM-SCID-hu and Liv-SCID-hu mice.
Analysis of Human Precursor Cells Present in the BM of BM-SCID-hu Mice and Liv-SCID-hu Mice
| . | CFU-GM . | BFU-E . | CFU-Mix . |
|---|---|---|---|
| BM-SCID-hu mouse | |||
| 144a5 | 47 | 4 | 0 |
| 144c1 | 43 | 11 | 0 |
| 149c1 | 62 | 14 | 2 |
| 149c2 | 45 | 16 | 0 |
| Mean (± SEM) | 49.2 ± 4.3 | 11.3 ± 2.6 | 0.5 ± 0.5 |
| Liv-SCID-hu mouse | |||
| 144c2 | 3 | 2 | 0 |
| 144c3 | 31 | 2 | 0 |
| 144c6 | 104 | 60 | 0 |
| 141a3 | 94 | 19 | 0 |
| 141a5 | 45 | 8 | 2 |
| 149c3 | 79 | 49 | 0 |
| 149c4 | 82 | 38 | 1 |
| Mean (± SEM) | 62.6 ± 13.9 | 25.4 ± 8.9 | 0.4 ± 0.3 |
| Control HFBM No. | |||
| 1 | 54 | 87 | 2 |
| 2 | 61 | 22 | 2 |
| 3 | 48 | 108 | 5 |
| 4 | 61 | 87 | 12 |
| Mean (± SEM) | 56.0 ± 3.1 | 76.0 ± 18.7 | 5.3 ± 2.4 |
| Control HFL No. | |||
| 1 | 67 | 62 | 10 |
| 2 | 96 | 60 | 6 |
| 3 | 65 | 22 | 14 |
| 4 | 53 | 8 | 6 |
| 5 | 96 | 60 | 6 |
| Mean (± SEM) | 75.4 ± 19.6 | 42.4 ± 25.5 | 8.3 ± 3.5 |
| . | CFU-GM . | BFU-E . | CFU-Mix . |
|---|---|---|---|
| BM-SCID-hu mouse | |||
| 144a5 | 47 | 4 | 0 |
| 144c1 | 43 | 11 | 0 |
| 149c1 | 62 | 14 | 2 |
| 149c2 | 45 | 16 | 0 |
| Mean (± SEM) | 49.2 ± 4.3 | 11.3 ± 2.6 | 0.5 ± 0.5 |
| Liv-SCID-hu mouse | |||
| 144c2 | 3 | 2 | 0 |
| 144c3 | 31 | 2 | 0 |
| 144c6 | 104 | 60 | 0 |
| 141a3 | 94 | 19 | 0 |
| 141a5 | 45 | 8 | 2 |
| 149c3 | 79 | 49 | 0 |
| 149c4 | 82 | 38 | 1 |
| Mean (± SEM) | 62.6 ± 13.9 | 25.4 ± 8.9 | 0.4 ± 0.3 |
| Control HFBM No. | |||
| 1 | 54 | 87 | 2 |
| 2 | 61 | 22 | 2 |
| 3 | 48 | 108 | 5 |
| 4 | 61 | 87 | 12 |
| Mean (± SEM) | 56.0 ± 3.1 | 76.0 ± 18.7 | 5.3 ± 2.4 |
| Control HFL No. | |||
| 1 | 67 | 62 | 10 |
| 2 | 96 | 60 | 6 |
| 3 | 65 | 22 | 14 |
| 4 | 53 | 8 | 6 |
| 5 | 96 | 60 | 6 |
| Mean (± SEM) | 75.4 ± 19.6 | 42.4 ± 25.5 | 8.3 ± 3.5 |
The numbers of myeloid precursors (CFU-GM) and erythroid precursors (BFU-E) present in the BM of BM-SCID-hu mice (n = 4 mice) and Liv-SCID-hu mice (n = 7 mice) and fetal BM (n = 4 fetuses) and fetal liver (n = 5 fetuses) were assessed as described in Materials and Methods. The mean number of human precursor cells/5 × 104 human cells in duplicate clonogenic assays is shown.
Long-term reconstitution of BM-SCID-hu and Liv-SCID-hu mice.To evaluate the SCID mice for long-term engraftment by cultured HFBM, the PB of 16 mice transplanted with HFBM was examined at 3 months and then again at either 6 or 9 months after transplantation (Table 2). Three months after transplantation, human CD45+ cells (>1%) were present in the PB of all 16 of the mice examined. Six months later, human CD45+ cells (>1%) were detected in the PB of 9 of 12 BM-SCID-hu mice. The other 4 BM-SCID-hu mice were assessed 9 months after transplantation and human CD45+ cells (>1%) were detected in the PB of all 4 of these mice. Similar results were observed with SCID mice transplanted with HFL where human CD45+ cells were detected in the PB of 5 of 5 mice examined 9 months after transplantation (Table 3).
Long-Term Reconstitution of the PB of SCID Mice With Human Cells After Transplantation With HFBM
| BM-SCID-hu . | % Human CD45+ Cells . | ||
|---|---|---|---|
| Mouse . | 3 Mo. . | 6 Mo. . | 9 Mo. . |
| 119c2 | 44 | 69 | ND |
| 122c1 | 23 | 18 | ND |
| 122c2 | 11 | 13 | ND |
| 122c3 | 4 | 0.5 | ND |
| 125-2 | 14 | 0 | ND |
| 125-3 | 23 | 2 | ND |
| 125-4 | 4 | 7 | ND |
| 125-5 | 19 | 61 | ND |
| 126-2 | 35 | 26 | ND |
| 126-3 | 4 | 0 | ND |
| 126-4 | 4 | 2 | ND |
| 126-5 | 29 | 16 | ND |
| 131b1 | 80 | ND | 16 |
| 131b2 | 4 | ND | 4 |
| 132b3 | 80 | ND | 75 |
| 132b4 | 16 | ND | 8 |
| BM-SCID-hu . | % Human CD45+ Cells . | ||
|---|---|---|---|
| Mouse . | 3 Mo. . | 6 Mo. . | 9 Mo. . |
| 119c2 | 44 | 69 | ND |
| 122c1 | 23 | 18 | ND |
| 122c2 | 11 | 13 | ND |
| 122c3 | 4 | 0.5 | ND |
| 125-2 | 14 | 0 | ND |
| 125-3 | 23 | 2 | ND |
| 125-4 | 4 | 7 | ND |
| 125-5 | 19 | 61 | ND |
| 126-2 | 35 | 26 | ND |
| 126-3 | 4 | 0 | ND |
| 126-4 | 4 | 2 | ND |
| 126-5 | 29 | 16 | ND |
| 131b1 | 80 | ND | 16 |
| 131b2 | 4 | ND | 4 |
| 132b3 | 80 | ND | 75 |
| 132b4 | 16 | ND | 8 |
Irradiated SCID mice were transplanted with cultured HFBM cells and then the PB was analyzed for the presence of human CD45+ cells at 3 months and then at either 6 months or at 9 months after transplantation.
Abbreviation: ND, not done.
Long-Term Reconstitution of the PB of SCID Mice With Human Cells After Transplantation With Cultured HFL
| Liv-SCID-hu Mouse . | % Human CD45+ Cells . | |
|---|---|---|
| . | 3 Mo. . | 9 Mo. . |
| 132c1 | 14 | 7 |
| 132c2 | 5 | 5 |
| 132c3 | 65 | 40 |
| 132c4 | 29 | 7 |
| 132c5 | 11 | 10 |
| Liv-SCID-hu Mouse . | % Human CD45+ Cells . | |
|---|---|---|
| . | 3 Mo. . | 9 Mo. . |
| 132c1 | 14 | 7 |
| 132c2 | 5 | 5 |
| 132c3 | 65 | 40 |
| 132c4 | 29 | 7 |
| 132c5 | 11 | 10 |
Irradiated SCID mice were transplanted with cultured HFL cells and then the PB was analyzed for the presence of human CD45+ cells at 3 months and then at 9 months after transplantation.
To determine if long-term engraftment was associated with continued human hematopoiesis, the BM of 6 BM-SCID-hu mice and 1 Liv-SCID-hu mouse was evaluated at 7 or 8 months after transplantation for the presence of human progenitor cells (Table 4). The BM of all of the mice contained a distinct population of human CD34+ cells, and a large population of immature human CD19+/CD20− B cells. Engraftment of the mice with functional human precursor cells was indicated by the detection of human CFU-GM, BFU-E, and CFU-mix in the BM of these mice. Thus, taken together these data show the long-term engraftment of the BM of BM-SCID-hu mice and Liv-SCID-hu mice with human precursor cells capable of populating the PB of the mice with human leukocytes.
Long-Term Population of Mouse Bone Marrow With Human Precursor Cells
| Mouse No. . | Time After Transplant (Mo.) . | % Cells Positive for Human CD . | No. Human Colonies . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | 45+ . | 34+/10− . | 34+/10+ . | 19+/20− . | 19+/20+ . | 13+/33+ . | CFU-GM . | BFU-e . | CFU-mix . |
| 189c6 | 7 | 89 | 2 | 19 | 45 | 43 | 50 | 89 | 11 | 3 |
| 192c3 | 7 | 25 | 2 | 3 | 10 | 10 | 7 | 242 | 3 | 0 |
| 193b1 | 7 | 66 | 2 | 9 | 25 | 19 | 63 | 112 | 50 | 5 |
| 187c5 | 8 | 86 | 1 | 15 | 51 | 29 | 44 | 72 | 23 | 2 |
| 187c6 | 8 | 18 | 1 | 1 | 7 | 6 | 6 | 136 | 7 | 0 |
| 187c7 | 8 | 23 | 1 | 3 | 12 | 8 | 1 | 134 | 5 | 0 |
| 188a2 | 8 | 77 | 4 | 17 | 45 | 29 | 43 | 122 | 27 | 3 |
| Mouse No. . | Time After Transplant (Mo.) . | % Cells Positive for Human CD . | No. Human Colonies . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | 45+ . | 34+/10− . | 34+/10+ . | 19+/20− . | 19+/20+ . | 13+/33+ . | CFU-GM . | BFU-e . | CFU-mix . |
| 189c6 | 7 | 89 | 2 | 19 | 45 | 43 | 50 | 89 | 11 | 3 |
| 192c3 | 7 | 25 | 2 | 3 | 10 | 10 | 7 | 242 | 3 | 0 |
| 193b1 | 7 | 66 | 2 | 9 | 25 | 19 | 63 | 112 | 50 | 5 |
| 187c5 | 8 | 86 | 1 | 15 | 51 | 29 | 44 | 72 | 23 | 2 |
| 187c6 | 8 | 18 | 1 | 1 | 7 | 6 | 6 | 136 | 7 | 0 |
| 187c7 | 8 | 23 | 1 | 3 | 12 | 8 | 1 | 134 | 5 | 0 |
| 188a2 | 8 | 77 | 4 | 17 | 45 | 29 | 43 | 122 | 27 | 3 |
The numbers of myeloid precursors (CFU-GM) and erythroid precursors (BFU-E) present in the BM of BM-SCID-hu mice (192c3, 187c5, 187c6, 188a2, 189c6, and 193b1) and a Liv-SCID-hu mouse (187c7) were assessed using clonogenic assays as described in Materials and Methods. The mean percentage of human precursors/5 × 104 human cells is shown. Expression of the indicated human CD was determine by flow cytometry.
Hematopoiesis by HFBM and HFL transduced with retroviral vectors.A mouse exhibiting in vivo maturation in the BM of human hematopoietic precursor cells that mediates population of the PB with mature human PBMC would be an ideal model for studying in vivo gene therapy using human precursor cells. To determine if our BM-SCID-hu mice and Liv-SCID-hu mice would be useful in these studies, BM cells isolated from 8 fetuses and liver cells obtained from 6 fetuses were transduced with a retroviral vector, LN, by culturing for 3 days in media with human IL-3, IL-6, and SCF and then for 1 to 2 days with the retroviral vector with a multiplicity of infection of about 1. After being washed, an aliquot of cells was placed into clonogenic assays and the rest of the cells were injected IV into irradiated SCID mice. After 2 weeks of culture, ten clones from each HFBM donor (n = 8 fetuses) and HFL donor (n = 6 fetuses) were picked from the plate and assayed for integrated retrovirus by PCR (Table 5). Integrated retrovirus was detected in a mean of 22.5 ± 5.6% of the colonies (range 10 to 60%) obtained from the transduced HFBM cells with a representative gel shown in Fig 2. Similar results were obtained for HFL cells transduced with LN with integrated retrovirus detected in a mean of 15 ± 3.7% of the colonies (range 0 to 30%). One month after transplantation with the transduced HFBM or HFL cells, the mice were bled and the PBMC were evaluated for the presence of integrated retrovirus by PCR. As shown in Fig 3, retroviral sequences were detected in the PBMC of 4 of 7 BM-SCID-hu mice and 4 of 7 Liv-SCID-hu mice examined.
Clonogenic Assays of Fetal BM and Liver Cells Transduced With a Retroviral Vector
| . | CFU-GM . | BFU-E . | % neo Positive Colonies . |
|---|---|---|---|
| Fetal bone marrow no. | |||
| 1 | 56 | 49 | 10 |
| 2 | 53 | 52 | 20 |
| 3 | 47 | 64 | 20 |
| 4 | 74 | 49 | 60 |
| 5 | 106 | 41 | 10 |
| 6 | 35 | 47 | 20 |
| 7 | 35 | 42 | 20 |
| 8 | 27 | 67 | 20 |
| Fetal liver no. | |||
| 1 | 143 | 37 | 30 |
| 2 | 177 | 28 | 10 |
| 3 | 109 | 122 | 0 |
| 4 | 110 | 126 | 20 |
| 5 | 54 | 106 | 20 |
| 6 | 41 | 93 | 10 |
| . | CFU-GM . | BFU-E . | % neo Positive Colonies . |
|---|---|---|---|
| Fetal bone marrow no. | |||
| 1 | 56 | 49 | 10 |
| 2 | 53 | 52 | 20 |
| 3 | 47 | 64 | 20 |
| 4 | 74 | 49 | 60 |
| 5 | 106 | 41 | 10 |
| 6 | 35 | 47 | 20 |
| 7 | 35 | 42 | 20 |
| 8 | 27 | 67 | 20 |
| Fetal liver no. | |||
| 1 | 143 | 37 | 30 |
| 2 | 177 | 28 | 10 |
| 3 | 109 | 122 | 0 |
| 4 | 110 | 126 | 20 |
| 5 | 54 | 106 | 20 |
| 6 | 41 | 93 | 10 |
HFBM or HFL was transduced with a neo containing vector (LN) and then the number of human CFU-GM and BFU-E was measured by clonogenic assays. The % of neo-positive colonies was based on the analysis of 10 randomly picked colonies for incorporated neo gene by PCR analysis with neo-specific primers followed by a Southern blot with an intervening sequence-specific probe.
Detection of neo gene by PCR analysis of individual primary colonies after clonogenic assays. HFBM was transduced with LN, a retroviral vector containing the neo gene as described in Materials and Methods. The cells were then plated in clonogenic assays and after 2 weeks, random colonies were picked and assessed for incorporated neo gene by PCR. A representative ethidium bromide gel showing the presence of the neo primer pair-amplified product in randomly picked colonies is shown.
Detection of neo gene by PCR analysis of individual primary colonies after clonogenic assays. HFBM was transduced with LN, a retroviral vector containing the neo gene as described in Materials and Methods. The cells were then plated in clonogenic assays and after 2 weeks, random colonies were picked and assessed for incorporated neo gene by PCR. A representative ethidium bromide gel showing the presence of the neo primer pair-amplified product in randomly picked colonies is shown.
Detection of neo gene by PCR analysis of PB from SCID mice transplanted with HFBM or HFL cells transduced with a retroviral vector. HFBM and HFL cells were transduced with LN, a retroviral vector containing the neo gene as described in the Materials and Methods section and then transplanted into irradiated SCID mice. One month later the mice were bled and the PBMC were assessed for incorporated neo gene by PCR. An ethidium bromide-stained gel showing the presence of the neo primer pair-amplified product in the PBMC of BM-SCID-hu mice (n = 7 mice) and Liv-SCID-hu mice (n = 7 mice) is shown.
Detection of neo gene by PCR analysis of PB from SCID mice transplanted with HFBM or HFL cells transduced with a retroviral vector. HFBM and HFL cells were transduced with LN, a retroviral vector containing the neo gene as described in the Materials and Methods section and then transplanted into irradiated SCID mice. One month later the mice were bled and the PBMC were assessed for incorporated neo gene by PCR. An ethidium bromide-stained gel showing the presence of the neo primer pair-amplified product in the PBMC of BM-SCID-hu mice (n = 7 mice) and Liv-SCID-hu mice (n = 7 mice) is shown.
To determine whether the mouse BM was engrafted with transduced human hematopoietic cells, the BM from BM-SCID-hu mice (n = 6 mice) and Liv-SCID-hu mice (n = 5 mice) was obtained between 3 months and 7 months after transplantation and the number of human CFU-GM and BFU-E precursors was determined by clonogenic assays. Integration of retrovirus in precursor cells was evaluated by assessing 10 colonies randomly selected from the plate for incorporated retrovirus by PCR. A representative Southern blot of a transferred gel probed with an intervening sequence probe is shown in Fig 4. As shown in Table 6, human precursor cells containing integrated retrovirus were detected in an average of 30% and 22% of the human precursor cells present in the BM of BM-SCID-hu mice and Liv-SCID-hu mice, respectively. This correlated with continued population of the PB of these mice with human CD45+ cells and detection of retrovirally transduced PBMC in 3 of 6 BM-SCID-hu mice and 2 of 5 Liv-SCID-hu mice (Table 6). Taken together, the presence of human precursor cells containing integrated retroviral vector in the BM of BM-SCID-hu mice and Liv-SCID-hu mice 6 months or greater after transplantation provides evidence for long-term engraftment with transduced human hematopoietic cells.
Detection of neo gene by PCR analysis of primary colonies isolated from the BM of SCID mice transplanted with HFBM transduced with a retroviral vector. HFBM was transduced with LN, a retroviral vector containing the neo gene as described in the Materials and Methods section and then transplanted into irradiated SCID mice. Two months later the BM of the mice was procured, placed in clonogenic assays, and the colonies were assessed for incorporated neo gene by PCR. A representative Southern blot using an intervening sequence probe specific for the neo primer pair-amplified product in randomly picked colonies is shown.
Detection of neo gene by PCR analysis of primary colonies isolated from the BM of SCID mice transplanted with HFBM transduced with a retroviral vector. HFBM was transduced with LN, a retroviral vector containing the neo gene as described in the Materials and Methods section and then transplanted into irradiated SCID mice. Two months later the BM of the mice was procured, placed in clonogenic assays, and the colonies were assessed for incorporated neo gene by PCR. A representative Southern blot using an intervening sequence probe specific for the neo primer pair-amplified product in randomly picked colonies is shown.
Population of the BM and PB of BM-SCID-hu Mice and Liv-SCID-hu Mice With Human Cells Transduced With Retroviral Vectors
| Mouse No. . | Tissue . | Vector . | Time After Transplant (mo) . | BM . | PB . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | %CD45+ . | %CD34+ . | CFU-GM . | BFU-E . | % Colonies neo Positive . | %CD45+ . | neo Positive . |
| 184c2 | HFBM | MY2 | 3 | 61 | 13 | 92 | 3 | 20 | 2 | neg |
| 184c4 | HFBM | MY2 | 3 | 47 | 16 | 172 | 18 | 50 | 23 | pos |
| 182d3 | HFBM | MY2 | 3 | 85 | 14 | 155 | 20 | 30 | 20 | pos |
| 182d5 | HFL | MY2 | 3 | 17 | 1 | 51 | 0 | 30 | 8 | neg |
| 181c4 | HFBM | LN | 4 | 25 | 3 | 272 | 35 | 40 | 9 | neg |
| 179b1 | HFL | LN | 4 | 58 | 10 | 140 | 7 | 30 | 30 | neg |
| 203-5 | HFL | MY2 | 6 | 14 | 1 | 17 | 0 | 10 | 5 | pos |
| 204-2 | HFL | MY2 | 6 | 20 | 5 | 270 | 0 | 10 | 1 | neg |
| 204-3 | HFL | MY2 | 6 | 68 | 13 | 179 | 15 | 30 | 31 | pos |
| 197a2 | HFBM | MY2 | 7 | 74 | 18 | 135 | 4 | 0 | 38 | pos |
| 197a5 | HFBM | MY2 | 7 | 30 | 7 | 110 | 0 | 40 | 6 | neg |
| Mouse No. . | Tissue . | Vector . | Time After Transplant (mo) . | BM . | PB . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | %CD45+ . | %CD34+ . | CFU-GM . | BFU-E . | % Colonies neo Positive . | %CD45+ . | neo Positive . |
| 184c2 | HFBM | MY2 | 3 | 61 | 13 | 92 | 3 | 20 | 2 | neg |
| 184c4 | HFBM | MY2 | 3 | 47 | 16 | 172 | 18 | 50 | 23 | pos |
| 182d3 | HFBM | MY2 | 3 | 85 | 14 | 155 | 20 | 30 | 20 | pos |
| 182d5 | HFL | MY2 | 3 | 17 | 1 | 51 | 0 | 30 | 8 | neg |
| 181c4 | HFBM | LN | 4 | 25 | 3 | 272 | 35 | 40 | 9 | neg |
| 179b1 | HFL | LN | 4 | 58 | 10 | 140 | 7 | 30 | 30 | neg |
| 203-5 | HFL | MY2 | 6 | 14 | 1 | 17 | 0 | 10 | 5 | pos |
| 204-2 | HFL | MY2 | 6 | 20 | 5 | 270 | 0 | 10 | 1 | neg |
| 204-3 | HFL | MY2 | 6 | 68 | 13 | 179 | 15 | 30 | 31 | pos |
| 197a2 | HFBM | MY2 | 7 | 74 | 18 | 135 | 4 | 0 | 38 | pos |
| 197a5 | HFBM | MY2 | 7 | 30 | 7 | 110 | 0 | 40 | 6 | neg |
HFBM or HFL was transduced with a neo containing vector (either LN or MY2) and then transplanted into SCID mice. At the indicated time, the mouse BM and PB were analyzed. The percentage of human CD45+ and CD34+ cells was determined by flow cytometry and the number of human CFU-GM and BFU-E (presented as number of colonies/5 × 104 human cells plated) was measured by clonogenic assays. Incorporated neo gene was detected in colonies and the peripheral blood by PCR analysis with neo-specific primers followed by a Southern blot with an intervening sequence-specific probe. The data for the BM are presented as % of neo-positive colonies based on the analysis of 10 randomly picked colonies and the data for the PB are presented as detection of neo sequences in lysed PBMC (pos) or as no detection of neo sequences in lysed PBMC (neg).
Effect of implanting thymus in BM-SCID-hu mice.Although significant numbers of human B cells and monocytes were detected in the PB of the BM-SCID-hu mice, no human T cells were detected.6 We postulated that this was due to the absence of a human thymic environment wherein the human pre-T cells migrating from the BM could differentiate into mature human T cells. Previously, we showed that murine pre-T cells from the mouse BM could home to and differentiate in human thymic tissue implanted under the kidney capsule and populate the periphery with mature murine T cells.10 Continued human thymopoiesis in SCID-hu mice depends on the co-implantation with the thymus of a source of precursor cells such as fetal liver, as evidenced by the involution by 3 months of >90% of human thymic tissue implanted alone into SCID mice.20 21 We postulated that human pre-T cells present in the BM of BM-SCID-hu mice could migrate into the human thymic implant, differentiate into T cells and populate the PB of the mice with mature human T cells. Therefore, we implanted human thymic tissue into either 4 BM-SCID-hu mice, 2 Liv-SCID-hu mice, or 5 SCID mice, and then 3 months later determined the population of human cells present in the PB by flow cytometry. Whereas very few human cells were detected in the PB of SCID mice implanted with human fetal thymus, human CD19+ B cells and human CD4+ and CD8+ T cells were detected in the PB of BM-SCID-hu mice implanted with human thymic tissue (Table 7). Thus, long-term population of the PB of SCID mice with human B cells and T cells occurred after transplantation with HFBM and implantation of human fetal thymus.
Human T Cells Are Present in the PB of BM-SCID-hu Mice Implanted With Human Thymus
| SCID Mouse No. . | Transplanted Tissue . | % Positive Human Cells . | |||
|---|---|---|---|---|---|
| . | . | CD45 . | CD4 . | CD8 . | CD19 . |
| 1 | HFBM + thy | 33 | 14 | 5 | 4 |
| 2 | HFBM + thy | 25 | 8 | 3 | 8 |
| 3 | HFBM + thy | 7 | 2 | 1 | 5 |
| 4 | HFBM + thy | 31 | 12 | 3 | ND |
| 5 | HFL + thy | 22 | 18 | 4 | ND |
| 6 | HFL + thy | 10 | 8 | 2 | ND |
| 1 | thy | 0 | 0 | 0 | 0 |
| 2 | thy | 0.3 | 0 | 0 | 0 |
| 3 | thy | 0.3 | 0 | 0 | 0 |
| 4 | thy | 2 | 0.6 | 0.6 | 0 |
| 5 | thy | 3 | 0.6 | 0 | 0 |
| SCID Mouse No. . | Transplanted Tissue . | % Positive Human Cells . | |||
|---|---|---|---|---|---|
| . | . | CD45 . | CD4 . | CD8 . | CD19 . |
| 1 | HFBM + thy | 33 | 14 | 5 | 4 |
| 2 | HFBM + thy | 25 | 8 | 3 | 8 |
| 3 | HFBM + thy | 7 | 2 | 1 | 5 |
| 4 | HFBM + thy | 31 | 12 | 3 | ND |
| 5 | HFL + thy | 22 | 18 | 4 | ND |
| 6 | HFL + thy | 10 | 8 | 2 | ND |
| 1 | thy | 0 | 0 | 0 | 0 |
| 2 | thy | 0.3 | 0 | 0 | 0 |
| 3 | thy | 0.3 | 0 | 0 | 0 |
| 4 | thy | 2 | 0.6 | 0.6 | 0 |
| 5 | thy | 3 | 0.6 | 0 | 0 |
SCID mice were transplanted with HFBM (n = 4 mice) or HFL (n = 2 mice) and then implanted with human thymus (HFBM + thy or HFL + thy) or SCID mice (n = 5 mice) were implanted with human thymus alone (thy). Three months later the PB was assessed for the presence of human cells expressing CD45, CD4, CD8, and CD19. The mean percentage (± SEM) of cells expressing the indicated human surface marker present in the PB of the indicated SCID mouse is shown.
Abbreviation: ND, not done.
To confirm that precursors from the BM migrated into the thymus where they could differentiate into mature T cells and populate the PB, SCID mice transplanted with HFBM transfected with a retroviral vector containing the neo gene were implanted with human fetal thymus (BM-thy-SCID-hu mice). Three months after implantation, two BM-thy-SCID-hu mice were killed and the implanted thymus and the BM cells were procured. Thymocytes obtained from the implant were incubated with antibodies directed to human CD4, CD8, and CD45 and the immature CD4+CD8+ human thymocytes were isolated by sorting on a FACStar Plus cell sorter (Becton Dickinson Immunosystems, San Jose, CA). DNA was extracted from the BM cells and the sorted CD4+CD8+ human thymocytes and analyzed for the presence of incorporated neo gene by PCR. As shown in Fig 5A, the neo gene was detected in the BM cells and the immature human CD4+CD8+ human thymocytes from both mice. To show that the PB of BM-thy-SCID-hu mice become populated with mature T cells containing integrated neo sequences, human CD45+CD4+ cells were sorted from the PB and the thymic implant of another BM-thy-SCID-hu mouse whose PB contained 21% human CD45+ cells, of which 12% were CD4+ and 5% were CD8+. DNA was isolated from the sorted CD45+CD4+CD8− cells and incorporated neo gene was detected by PCR in human CD45+CD4+CD8− cells present in the PB and the thymic implant (Fig 5B). Taken together, these data indicated that retrovirally transduced precursor cells present in the BM migrated to the implanted thymus, matured and populated the PB with human T cells.
Detection of neo gene by PCR analysis of sorted human cells. (A) Human CD45+CD4+CD8+ cells were sorted from the thymic implants of 2 BM-thy-SCID-hu mice transplanted with HFBM transduced with the neo-containing MY-2 vector. The cellular DNA from the sorted human CD45+CD4+CD8+ thymocytes and from the mouse BM was extracted and assessed for integrated retrovirus by PCR with a neo-specific primer pair. (B) Human CD45+CD4+CD8− cells were sorted from the thymic implant and PB of a BM-thy-SCID-hu mouse transplanted with HFBM transduced with the neo-containing MY-2 vector and the DNA was extracted and assessed for integrated retrovirus by PCR with a neo-specific primer pair. Southern blots using an intervening sequence probe specific for the neo primer pair-amplified product are shown.
Detection of neo gene by PCR analysis of sorted human cells. (A) Human CD45+CD4+CD8+ cells were sorted from the thymic implants of 2 BM-thy-SCID-hu mice transplanted with HFBM transduced with the neo-containing MY-2 vector. The cellular DNA from the sorted human CD45+CD4+CD8+ thymocytes and from the mouse BM was extracted and assessed for integrated retrovirus by PCR with a neo-specific primer pair. (B) Human CD45+CD4+CD8− cells were sorted from the thymic implant and PB of a BM-thy-SCID-hu mouse transplanted with HFBM transduced with the neo-containing MY-2 vector and the DNA was extracted and assessed for integrated retrovirus by PCR with a neo-specific primer pair. Southern blots using an intervening sequence probe specific for the neo primer pair-amplified product are shown.
DISCUSSION
Although the BM of irradiated SCID mice can be engrafted with human precursor cells after infusion with human pediatric BM cells, significant engraftment of the mouse BM with human cells only occurred if the mice were continuously treated with human cytokines and, despite cytokine treatment, only minimal engraftment of the murine peripheral compartment with human cells was observed.3 We postulated that HFBM, which contains more human precursor cells than pediatric and adult BM and exhibit a greater proliferative and differentiative potential than precursor cells obtained later in ontogeny,4,5 would provide a better source of precursor cells for the reconstitution of irradiated SCID-mice with human cells. We showed that after irradiated SCID mice were transplanted with cultured HFBM, their BM became significantly engrafted with human precursor cells and their periphery was populated with human B cells and monocytes independent of the provision of extraneous human cytokines.6 In the present study, we have expanded on our previous observations regarding cultured HFBM by showing that transplantation with cultured HFL cells could significantly engraft the BM of SCID mice with human precursor cells and populate the SCID mouse PB with human B cells and monocytes. HFL is a rich source of hematopoietic precursor cells containing from 107 to 109 mononuclear cells of which 1% to 4% are CD34+CD45RAloCD71lo stem/precursor cells making it extremely amenable to in vitro cellular expansion and potential clinical use.5,22,23 Because hematopoietic cells from fetal liver are not contaminated by donor T cells, in utero or postnatal transplantation with fetal liver cells has proved to be an effective modality for treating several congenital immune and hematological disorders.24,25 In addition, for these reasons fetal liver cells may also prove to be an excellent source of hematopoietic cells that can be used for stem cell-mediated gene therapy.26 Our Liv-SCID-hu model should prove useful in studying the in vivo engraftment and effects of different interventions on the maturation of human precursor cells obtained from the HFL.
Analysis of the BM of the BM-SCID-hu mice and Liv-SCID-hu mice by flow cytometry showed human CD34+ cells and by clonogenic assays showed human hematopoietic precursor cells including CFU-GM, BFU-E, and CFU-mix up to 8 months after transplantation. This suggested that long-term, active human hematopoiesis was occurring in the BM-SCID and Liv-SCID-hu mouse BM and was mediating population of the mouse PB with human mononuclear cells. Several observations indicate that it is unlikely that the human PBMC detected in the BM-SCID-hu mice and the Liv-SCID-hu mice were residual circulating cells from the initial IV injected human cells. First, no circulating human mononuclear cells were detected in SCID mice that received uncultured HFBM and HFL cells that were not engrafted with human cells in the BM.6 Second, irradiation of SCID mice is required for their successful BM engraftment27 and no circulating PBMC were detected 3 months after transplantation of unirradiated SCID mice with HFBM or HFL cells (data not shown). Third, significant BM engraftment with human cells and significant numbers of human PBMC were not observed in irradiated SCID mice that were IV injected with human fetal spleen cells (data not shown). Finally, mature human lymphocytes injected by the IV route into irradiated SCID mice are rapidly cleared from the circulation. Significant numbers of human cells were not detected in the PB or the BM of irradiated SCID mice 48 hours after they were injected IV with an equivalent number (4 × 107) of human PBMCs (data not shown). Similar results were reported by Lapidot et al,3 and the rapid clearance of IV injected human PBMC are probably the result of their entrapment in the SCID mouse lung.28 Therefore, taken together these data indicate that peripheral reconstitution of the SCID-hu mouse with human cells was due to the successful engraftment of the BM with human precursor cells and the source of the circulating human PBMC was the ongoing human hematopoietic activity occurring in the SCID mouse BM.
Although mature B cells expressing sIgM and sIgD were detected in the PB of the BM-SCID-hu and Liv-SCID-hu mice, only minimal levels of human IgM or IgG were detected in the mouse serum (data not shown). Isotype switching by human B cells requires two separate signals, the first mediated by a cognate interaction between CD40-ligand, a membrane protein expressed by T cells and CD40, a protein on the surface of B cells,29 and the second provided by T-cell secreted cytokines such as IL-4 and IL-10.30,31 Despite the presence of circulating human T cells in the PB of BM-SCID-hu mice implanted with human fetal thymus, only low levels of human Ig were detected. Human fetal T cells may have a limited capacity to stimulate B-cell Ig production,32 which may be due to impairment of function of human fetal spleen CD45RO+ memory T cells, the predominant provider of B-cell help.33 Similarly, human neonatal B cells also appear to exhibit defective IgG production,32 most likely due to the inability of neonatal T cells to provide adequate T-cell help.34 This is further indicated by the observation that while minimal serum levels of human IgM and IgG were detected in SCID mice injected intraperitonealy with human cord blood mononuclear cells, significant serum levels of human IgM and IgG were detected in SCID mice injected with a mixture of human cord blood B cells and adult PB T cells.35 The defective capacity of neonatal T cells to induce neonatal B-cell IgG production may be a result of a compromised capacity to provide the CD40 signal to B cells due to their markedly diminished expression of CD40-ligand.36 Because the maturational state and functional activity of the circulating T cells present in the BM-thy-SCID-hu mice are probably equivalent to that of human fetal and neonatal T cells, it is likely that they are also incapable of providing adequate T-cell help. Another explanation for the minimal levels of human Ig in the serum of the BM-SCID-hu and Liv-SCID-hu mice may involve an intrinsic B-cell maturational block in human fetal B cells. For example, CD5+ B cells that comprise 40% to 60% of human fetal splenic B cells display a defective capacity to produce IgG and human fetal and cord blood B cells display minimal isotype switching after stimulation with CD40 agonists.31
Circulating human T cells were present in SCID mice transplanted with HFBM cells and then implanted with human thymic tissue but not in SCID mice that were only implanted with human thymic tissue or SCID mice transplanted with HFBM cells alone. For murine thymopoiesis, precursor cells from the BM are required to populate implanted murine thymic tissue as shown in SCID mice where normal thymopoiesis and population of the PB with mouse T cells occurred only in SCID mice that were also transplanted with normal mouse BM.38 Similar results were reported for human thymopoiesis where depletion and involution of immature thymocytes in the implant occurred in greater than 90% of SCID mice implanted with human fetal thymus alone.20 The involution of human fetal thymic fragments implanted in SCID mice can be prevented if they are reconstituted by intraimplant microinjection with human CD34+ cells.39,40 We have previously shown that mouse T-cell precursors from the BM can home to human thymus implanted into athymic NIH-beige-nude-xid mice and then mature into normal mouse T cells capable of repopulating the peripheral lymphoid compartment.10 Therefore, we investigated whether transplantation with HFBM permitted continued thymopoiesis in human fetal thymic tissue implanted in SCID mice as described for SCID mice implanted with mouse thymus and transplanted with murine BM.38 When SCID mice were implanted with thymic tissue alone, very few peripheral human T cells were detected 3 months later. In contrast, when SCID mice implanted with human thymic tissue were also transplanted with HFBM, a significant population of human T cells as well as human B cells were present in the PB of BM-SCID-hu mice 3 months after implantation. It is likely that the presence of human T cells in the periphery of the HFBM-transplanted SCID mice was due to reconstitution of the human thymic implant with precursor cells that migrated from the mouse BM that was engrafted with human hematopoietic precursors as described for SCID mice implanted with mouse thymus and transplanted with mouse BM.38 This was confirmed by retroviral tagging of HFBM cells before transplantation and demonstration of integrated retroviral sequences in sorted immature CD4+CD8+ thymocytes and mature CD4+ thymocytes present in the thymic implant as well as in mature CD4+ lymphocytes present in the PB. Taken together these data showed that human precursors from the engrafted mouse BM homed to the human thymic implant, matured and populated the PB with mature human T cells.
We also showed that human precursor cells transduced with a retroviral vector could successfully engraft in the mouse BM for up to 7 months after transplantation. PBMC that contained integrated retroviral sequences were detected in some of the mice up to 7 months after transplantation. This indicated that retrovirally-transduced human precursor cells could engraft in the SCID mouse BM and populate the PB of the mouse with mature human cells containing the integrated retroviral vector. In addition, we showed that retrovirally transduced precursor cells can produce pre-T cells that could migrate to the implanted human thymus, differentiate into mature T cells and populate the PB with human T cells containing integrated retrovirus. This should provide us with a valuable model for examining the in vivo efficacy of genetic therapy strategies for “intracellular immunization” to protect T cells from human immunodeficiency virus (HIV) infection such as those using vectors expressing ribozymes directed to HIV sequences.17,41 42 A major advantage of the model that we have described is that following transduction, human hematopoiesis comparable to that which occurs in the human BM, occurs in the mouse BM without the requirement for the provision of exogenous human cytokines. These transduced human precursor cells mature and populate the periphery of the mice with human cells.
The model presented in the current report differs significantly from that previously reported using SCID mice that were implanted subcutaneously with human fetal bone fragments43 or with human fetal bone fragments and human fetal thymus and spleen.44 Although mice in these models exhibited long-term human hematopoiesis and thymopoieis, these activities were localized to the implanted fusion graft with no evidence presented of population of the PB with mature human cells. In contrast, in our model we showed active human hematopoiesis after engraftment of the mouse BM that was associated with population of the PB with mature human cells. In addition, thymopoiesis in our model was mediated by migration of human precursors from the engrafted mouse BM into the distally implanted human thymus. Therefore, our model better reflects the in vivo human hematopoietic program involving population of the periphery with mature human cells and migration of T-cell precursors to the thymus where they differentiate and populate the periphery with mature human T cells. Our SCID mouse model also differs from another model where CD34+ cells obtained from HFL were transduced with a retroviral vector and then directly injected into human thymus and liver tissue implanted in SCID mice.45 In that model, no evidence of ongoing hematopoiesis by retrovirally-transduced stem/precursor cells was shown and no demonstration of population of the PB with transduced human PBMC was presented. In contrast, in the current report we show significant engraftment of the mouse BM with transduced human hematopoietic precursors that undergo the normal hematopoietic differentiation program and populate the mouse PB with human T cells containing integrated retroviral sequences.
Active human hematopoiesis associated with the maturation of human B cells and monocytes in the BM of the BM-SCID-hu and Liv-SCID-hu mice described in this report occurred without treatment with exogenous human cytokines and was associated with reconstitution of the peripheral lymphoid compartment of these mice with human B cells and monocytes. Provision of the BM-SCID-hu mice with a human thymic environment resulted in the population of the PB of the mice with human T cells as well. Although long-term engraftment of beige/nude/xid mice was observed after cotransplantation with retrovirally transduced human CD34+ cells and human IL-3 producing stromal cells, the mice were not implanted with human thymus and therefore no human T cells were observed in the periphery.46 Even though mature human T cells were detected in the PB of SCID mice exhibiting BM engraftment after transplantation with human cord blood, the source of the mature human T cells was attributed to engraftment with mature human T cells in the original donor cord blood.47 To the best of our knowledge this is the first report of active human hematopoiesis and thymopoiesis resulting in the population of the PB with human B cells and T cells occurring in a xenogeneic background without treatment with exogenous growth factors. In addition to providing a model for studying hematopoiesis, these mice may also provide a valuable model for assessing the effectiveness of in vivo gene therapy using human stem and precursor cells. Furthermore, these mice should prove useful for examining the in vivo pathophysiology of the effects of HIV-1 infection on human hematopoiesis and the in vivo effectiveness of gene therapy for protecting human cells from HIV infection.
ACKNOWLEDGMENT
We appreciate the kind gifts of SCF, IL-6, and PIXY321 from Immunex Corp, rh-Epo from Amgen Inc, rhGM-CSF from Genetics Institute. We thank D. Gebhardt for assistance in the flow cytometry, A. Watford for assistance in animal care, and T.C. Chang for performing the statistical analysis. Flow cytometry was performed in the Flow Cytometry Core Facility and oligonucleotides were synthesized in the Oligonucleotide Synthesis Core Facility supported by the Cancer Center Grant (5P30CA13330).
Supported by the National Institutes of Health (National Institute of Allergy and Infectious Diseases Centers for AIDS Research Grants No. AI-27741, AI-36664, and HE-53754) and SPIRAT award to F.W.-S.
Address reprint requests to Harris Goldstein, MD, Albert Einstein College of Medicine, Chanin Bldg, Room 601, 1300 Morris Park Ave, Bronx, NY 10461.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal