Abstract
Despite multiple disparate prognostic risk analysis systems for evaluating clinical outcome for patients with myelodysplastic syndrome (MDS), imprecision persists with such analyses. To attempt to improve on these systems, an International MDS Risk Analysis Workshop combined cytogenetic, morphological, and clinical data from seven large previously reported risk-based studies that had generated prognostic systems. A global analysis was performed on these patients, and critical prognostic variables were re-evaluated to generate a consensus prognostic system, particularly using a more refined bone marrow (BM) cytogenetic classification. Univariate analysis indicated that the major variables having an impact on disease outcome for evolution to acute myeloid leukemia were cytogenetic abnormalities, percentage of BM myeloblasts, and number of cytopenias; for survival, in addition to the above, variables also included age and gender. Cytogenetic subgroups of outcome were as follows: “good” outcomes were normal, −Y alone, del(5q) alone, del(20q) alone; “poor” outcomes were complex (ie, ≥3 abnormalities) or chromosome 7 anomalies; and “intermediate” outcomes were other abnormalities. Multivariate analysis combined these cytogenetic subgroups with percentage of BM blasts and number of cytopenias to generate a prognostic model. Weighting these variables by their statistical power separated patients into distinctive subgroups of risk for 25% of patients to undergo evolution to acute myeloid leukemia, with: low (31% of patients), 9.4 years; intermediate-1 (INT-1; 39%), 3.3 years; INT-2 (22%), 1.1 years; and high (8%), 0.2 year. These features also separated patients into similar distinctive risk groups for median survival: low, 5.7 years; INT-1, 3.5 years; INT-2, 1.2 years; and high, 0.4 year. Stratification for age further improved analysis of survival. Compared with prior risk-based classifications, this International Prognostic Scoring System provides an improved method for evaluating prognosis in MDS. This classification system should prove useful for more precise design and analysis of therapeutic trials in this disease.
A MULTIPLICITY OF DISPARATE METHODS has been developed for evaluating the potential clinical outcomes for patients with myelodysplastic syndromes (MDSs). After the initial French-American-British (FAB) Morphology Group classification in 1982,1 at least six additional risk classification systems have been used regarding prognostic classification of MDSs and their potential for survival and evolution to acute myeloid leukemia (AML).2-7 These classification methods, in addition to the bone marrow (BM) morphological classification as used by the FAB group, have included clinical variables such as BM myeloblast percentage, BM biopsy features, specific cytopenias, age, lactate dehydrogenase level, and BM cytogenetic pattern.1-9 A number of other cytogenetic classification methods have also been used to categorize patients with MDS.8,10 11 However, imprecision associated with varying degrees of sensitivity and specificity persists with these classification systems regarding clinical outcomes.
To attempt to improve the clinical and prognostic use of these systems and to develop a consensus prognostic risk-based analysis system, an International MDS Risk Analysis Workshop was convened. In this workshop, cytogenetic, morphological and clinical data were combined and collated from patients with primary MDS from seven previously reported studies that used independent risk-based prognostic systems.2-7 13 The combined data obtained from these patients were analyzed centrally, and a global analysis was performed. Critical prognostic variables were then re-evaluated using this data set to generate a prognostic system, in particular, using a more refined BM cytogenetic classification combined with relevant clinical parameters.
The goals of this study were (1) to obtain a database from a large representative group of well-defined untreated primary MDS patients with a prolonged follow-up period, (2) to refine the BM cytogenetic subgroups evaluated, (3) to combine statistically defined major clinical and cytogenetic parameters for evaluating clinical outcomes, (4) to define and weight prognostic risk categories utilizing multivariate analyses, (5) to generate an International Prognostic Scoring System (IPSS) for MDS based on these findings, and (6) to compare this IPSS with prior classification methods.
MATERIALS AND METHODS
Patients.Table 1 provides a summary of the clinical data obtained from 816 primary MDS patients evaluated in this study who had also been evaluated in prior individual risk-based studies regarding their clinical outcomes.2-7,13 Of these patients, 759 individuals were also analyzed for AML evolution. Data are presented on demographic, clinical, and cytogenetic variables, including three of the most widely used MDS risk evaluation systems (ie, FAB, Spanish, and Lille).1,3 5 For each of these variables, the number and percentages of patients in each category, as well as the estimated median (time to 50%) survival and time to 25% of patients evolving to AML are given (Tables 1 and 2) using Kaplan-Meier analyses (see below, Statistics). These tables also include log-rank statistics with indications of statistical significance. Patients who had previously received intensive chemotherapy and those with secondary MDS were excluded from analysis. However, patients who had received prior short courses (ie, ≤3 months) of low-dose oral chemotherapy (43 patients) or hemopoietic growth factor exposure (20 patients) were included in the analysis. Investigators from the seven participating institutions completed a standard comprehensive registration form for each patient detailing the patients' clinical and cytogenetic features at presentation and clinical outcomes (ie, survival time from diagnosis and time until AML evolution). Cytopenias were defined as a hemoglobin level of less than 10 g/dL, an absolute neutrophil count of less than 1,500/μL, and a platelet count of less than 100,000/μL. These data were then submitted to the Workshop Central Statistical Analyst (C.C.) to provide a comprehensive database and to permit further analysis.
Clinical Variables of MDS Patients Related to Survival and AML Evolution
| Variable . | No. of Patients . | % of Patients . | Median Survival (yr) . | Log Rank . | No. of Patients . | % of Patients . | 25% AML (yr) . | Log Rank . |
|---|---|---|---|---|---|---|---|---|
| Cytogenetics* | 816 | 99† | 759 | 71† | ||||
| Good | 570 | 70 | 3.8 | 521 | 69 | 5.6 | ||
| Intermediate | 112 | 14 | 2.4 | 107 | 14 | 1.6 | ||
| Poor | 134 | 16 | 0.8 | 131 | 17 | 0.9 | ||
| Gender | ||||||||
| Female | 325 | 40 | 3.9 | 15† | 300 | 40 | 2.9 | 0.4‡ |
| Male | 491 | 60 | 2.5 | 459 | 60 | 3.1 | ||
| Age (yr) | ||||||||
| ≤60 | 205 | 25 | 4.6 | 29† | 187 | 25 | 2.6 | 0.3‡ |
| >60 | 611 | 75 | 2.5 | 572 | 75 | 3.2 | ||
| FAB | ||||||||
| RARS | 125 | 15 | 6.9 | 222† | 109 | 14 | 10.1 | 202† |
| RA | 294 | 36 | 4.2 | 272 | 36 | 4.7 | ||
| RAEB | 208 | 26 | 1.5 | 198 | 26 | 1.4 | ||
| RAEB-T | 61 | 8 | 0.6 | 60 | 8 | 0.2 | ||
| CMMLρ | 126 | 15 | 2.4 | 118 | 16 | 2.9 | ||
| Cytopenia | ||||||||
| 0 | 152 | 19 | 5.3 | 96† | 140 | 18 | 7.6 | 39† |
| 1 | 322 | 39 | 4.0 | 292 | 39 | 5.6 | ||
| 2 | 224 | 27 | 1.6 | 213 | 28 | 1.6 | ||
| 3 | 118 | 15 | 1.3 | 114 | 15 | 1.3 | ||
| Hb | ||||||||
| <10 g/dL | 442 | 54 | 1.9 | 62† | 409 | 54 | 2.1 | 17† |
| ≥10 g/dL | 373 | 46 | 4.5 | 349 | 46 | 4.4 | ||
| ANC | ||||||||
| <1.5 K/μL | 366 | 46 | 2.5 | 4‡ | 379 | 50 | 2.1 | 121-155 |
| ≥1.5 K/μL | 439 | 54 | 3.5 | 380 | 50 | 4.7 | ||
| Plts | ||||||||
| <100 K/μL | 303 | 37 | 1.6 | 57† | 289 | 38 | 1.6 | 14† |
| ≥100 K/μL | 512 | 63 | 3.9 | 469 | 62 | 3.8 | ||
| BM blasts | ||||||||
| <5% | 483 | 59 | 4.4 | 220† | 437 | 58 | 7.6 | 169† |
| 5%–10% | 183 | 22 | 2.1 | 178 | 23 | 2.1 | ||
| 11%–20% | 114 | 14 | 0.7 | 112 | 14 | 0.7 | ||
| 21%–30% | 36 | 5 | 0.6 | 35 | 5 | 0.2 | ||
| Lille | ||||||||
| Low | 382 | 47 | 4.8 | 272† | 565 | 75 | 5.5 | 172† |
| Intermediate | 329 | 41 | 2.4 | 153 | 20 | 2.9 | ||
| High | 100 | 12 | 0.5 | 39 | 5 | 0.2 | ||
| Total | 811 | 757 | ||||||
| Spanish | ||||||||
| Low | 391 | 48 | 4.9 | 226† | 477 | 63 | 5.5 | 90† |
| Intermediate | 311 | 38 | 2.4 | 161 | 21 | 1.6 | ||
| High | 111 | 14 | 0.6 | 118 | 16 | 0.7 | ||
| Total | 813 | 756 |
| Variable . | No. of Patients . | % of Patients . | Median Survival (yr) . | Log Rank . | No. of Patients . | % of Patients . | 25% AML (yr) . | Log Rank . |
|---|---|---|---|---|---|---|---|---|
| Cytogenetics* | 816 | 99† | 759 | 71† | ||||
| Good | 570 | 70 | 3.8 | 521 | 69 | 5.6 | ||
| Intermediate | 112 | 14 | 2.4 | 107 | 14 | 1.6 | ||
| Poor | 134 | 16 | 0.8 | 131 | 17 | 0.9 | ||
| Gender | ||||||||
| Female | 325 | 40 | 3.9 | 15† | 300 | 40 | 2.9 | 0.4‡ |
| Male | 491 | 60 | 2.5 | 459 | 60 | 3.1 | ||
| Age (yr) | ||||||||
| ≤60 | 205 | 25 | 4.6 | 29† | 187 | 25 | 2.6 | 0.3‡ |
| >60 | 611 | 75 | 2.5 | 572 | 75 | 3.2 | ||
| FAB | ||||||||
| RARS | 125 | 15 | 6.9 | 222† | 109 | 14 | 10.1 | 202† |
| RA | 294 | 36 | 4.2 | 272 | 36 | 4.7 | ||
| RAEB | 208 | 26 | 1.5 | 198 | 26 | 1.4 | ||
| RAEB-T | 61 | 8 | 0.6 | 60 | 8 | 0.2 | ||
| CMMLρ | 126 | 15 | 2.4 | 118 | 16 | 2.9 | ||
| Cytopenia | ||||||||
| 0 | 152 | 19 | 5.3 | 96† | 140 | 18 | 7.6 | 39† |
| 1 | 322 | 39 | 4.0 | 292 | 39 | 5.6 | ||
| 2 | 224 | 27 | 1.6 | 213 | 28 | 1.6 | ||
| 3 | 118 | 15 | 1.3 | 114 | 15 | 1.3 | ||
| Hb | ||||||||
| <10 g/dL | 442 | 54 | 1.9 | 62† | 409 | 54 | 2.1 | 17† |
| ≥10 g/dL | 373 | 46 | 4.5 | 349 | 46 | 4.4 | ||
| ANC | ||||||||
| <1.5 K/μL | 366 | 46 | 2.5 | 4‡ | 379 | 50 | 2.1 | 121-155 |
| ≥1.5 K/μL | 439 | 54 | 3.5 | 380 | 50 | 4.7 | ||
| Plts | ||||||||
| <100 K/μL | 303 | 37 | 1.6 | 57† | 289 | 38 | 1.6 | 14† |
| ≥100 K/μL | 512 | 63 | 3.9 | 469 | 62 | 3.8 | ||
| BM blasts | ||||||||
| <5% | 483 | 59 | 4.4 | 220† | 437 | 58 | 7.6 | 169† |
| 5%–10% | 183 | 22 | 2.1 | 178 | 23 | 2.1 | ||
| 11%–20% | 114 | 14 | 0.7 | 112 | 14 | 0.7 | ||
| 21%–30% | 36 | 5 | 0.6 | 35 | 5 | 0.2 | ||
| Lille | ||||||||
| Low | 382 | 47 | 4.8 | 272† | 565 | 75 | 5.5 | 172† |
| Intermediate | 329 | 41 | 2.4 | 153 | 20 | 2.9 | ||
| High | 100 | 12 | 0.5 | 39 | 5 | 0.2 | ||
| Total | 811 | 757 | ||||||
| Spanish | ||||||||
| Low | 391 | 48 | 4.9 | 226† | 477 | 63 | 5.5 | 90† |
| Intermediate | 311 | 38 | 2.4 | 161 | 21 | 1.6 | ||
| High | 111 | 14 | 0.6 | 118 | 16 | 0.7 | ||
| Total | 813 | 756 |
Degrees of freedom for the log-rank χ2 test are one less than the number of categories of the variable.
Abbreviations: Hb, hemoglobin level; ANC, absolute neutrophil count; Plts, platelet count; K, 1,000.
Cytogenetic subgroups: Good, normal, del(5q) only, del(20q) only, −Y only; Intermediate, +8, single miscellaneous, double abnormalities; Poor, complex (ie, ≥3 anomalies) or chromosome 7 abnormalities.
P < .0001.
P value not statistically significant.
ρ Excluding patients with WBC counts > 12,000/μL.
P < .0005.
Major Multivariate Variables Related to Clinical Outcome in MDS Patients Using Proportional Hazards Regression Analysis: International MDS Risk Analysis Workshop
| Variable . | No. of Categories . | χ2 Values* . | |
|---|---|---|---|
| . | . | Survival . | AML Evolution . |
| BM blasts | 4 | 97 | 102 |
| Cytogenetics | 3 | 63 | 70 |
| Cytopenias | 4 | 46 | 43 |
| Age | 2 | 38 | 31 |
| Gender | 2 | 24 | 19 |
| Variable . | No. of Categories . | χ2 Values* . | |
|---|---|---|---|
| . | . | Survival . | AML Evolution . |
| BM blasts | 4 | 97 | 102 |
| Cytogenetics | 3 | 63 | 70 |
| Cytopenias | 4 | 46 | 43 |
| Age | 2 | 38 | 31 |
| Gender | 2 | 24 | 19 |
Categories of variables are defined in Table 1 and text.
P < .0001.
BM morphology.BM morphology was evaluated by each institution using the FAB classification system.1 Patients had clinically stable MDS and cytopenias for ≥4 weeks for inclusion in the study. The FAB classification system defined MDS as showing BM dysplasia in at least two of the hemopoietic cell lines; patients with refractory anemia (RA) had less than 5% BM myeloblasts; those with RA with excess blasts (RAEB) had 5% to 20% blasts; and those with RAEB in transformation (RAEB-T) had 21% to 30% blasts. Patients with RA with ringed sideroblasts (RARS) had 15% of the erythroid cells with RS as well as less than 5% BM blasts. Patients were also subdivided into those with BM blasts less than 5%, 5% to 10%, 11% to 20%, and 21% to 30%. In our study, patients with chronic myelomonocytic leukemia (CMML), ie, with monocytosis of greater than 1,000/μL, were subdivided into “proliferative” and “nonproliferative” subtypes. Proliferative-type CMML (ie, patients with white blood cell [WBC] counts >12,000/μL) were excluded from this analysis, because these individuals were believed to predominantly represent myeloproliferative disorders (MPDs) rather than MDS.14 Nonproliferative CMML patients had WBC counts ≤12,000/μL, as well as other features of MDS, and were included in this analysis. A representative proportion of slides from each center (35 cases, randomly selected) was separately reviewed by three members of the Workshop Morphology Committee (J.B., Chair; T.H. and T.V.). Highly significant (P < .0004) concordance between reviewers was found regarding the percentage of BM blasts enumerated, with blinded independent evaluations, as determined by correlation analysis.
Cytogenetic pattern.Cytogenetic analysis of BM specimens was performed at the individual centers; the results were reviewed and collated centrally by the Workshop Cytogenetics Committee (M.M.L., Chair). Inclusion in the study required the analysis of ≥10 metaphase cells per patient. The criteria defined by the International System for Human Cytogenetic Nomenclature, 199515 were used for identification of abnormal clones. For cytogenetic categorization, patients were divided into those with normal karyotypes or those with single recurring, double recurring, or complex (ie, ≥3 anomalies) recurring, or miscellaneous (nonrecurring) abnormalities. The individual cytogenetic abnormalities were classified according to 1 of 12 different cytogenetic categories. Subsequently, based on the outcome analyses indicated in Table 1 and shown below, the patients were placed into good, intermediate, and poor risk subgroups.
Statistics.Each clinical and cytogenetic variable was analyzed by the Workshop Statistical Committee (C.C., Chair) using Kaplan-Meier curves and log-rank tests (for univariate analysis, see Tables 1 and 2). P values less than .05 were considered statistically significant. The committee then generated information regarding multivariate analysis of the parameters evaluated, using proportional hazards regression analysis. The final set of risk variables for the prognostic model was chosen on both statistical and clinical grounds. Weighting each of the prognostic variables (to express their relative importance) using regression coefficients from the proportional hazards regression analysis permitted generation of a categorical risk-scoring system. The numbers provided for the risk-scoring values were approximations to the nearest 0.5 unit. These scores were combined as explained below to create risk categories to develop the IPSS. The predictive power of this IPSS was also compared with those of the FAB, Spanish, and Lille classification systems for MDS.1,3 5 Correlation analysis was performed for evaluation of concordance between morphological reviewers.
RESULTS
Univariate analyses.The proportion of patients in this study with FAB classification subtypes was 15% RARS, 36% RA, 26% RAEB, 8% RAEB-T and 15% CMML. The median age of the patients was 69 years (range, 16 to 96 years). The male:female ratio was 1.5:1; 25% of the patients were ≤60 years of age, and 55% were ≤70 years of age (Table 1). The median follow-up time for these patients was 1.9 years (range, 0.1 to 17 years). Tables 1 and 2 indicate the factors determined by univariate analysis to be relevant for survival and AML evolution in the MDS patients studied. This analysis showed that the major variables predictive of outcome for survival were FAB classification, percentage of BM blasts, cytogenetic pattern, age, gender, and number of cytopenias. The major features predictive of AML evolution were FAB classification, percentage of BM blasts, cytogenetic pattern, and number of cytopenias.
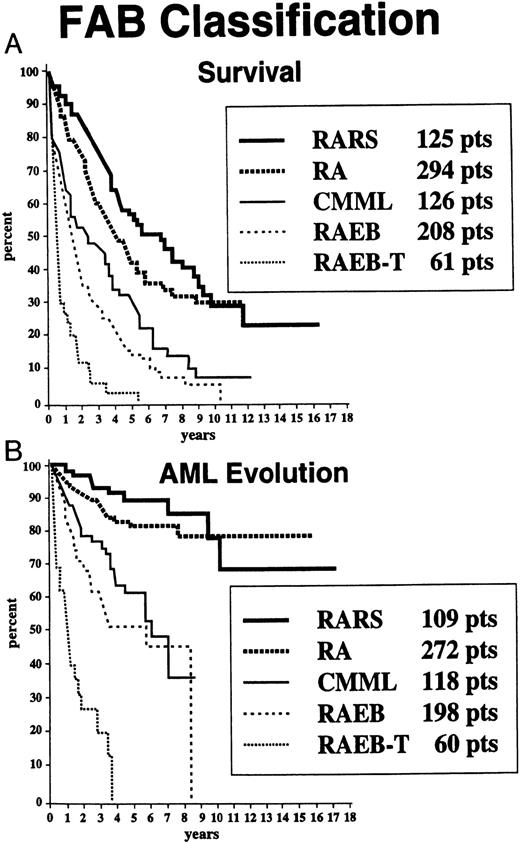
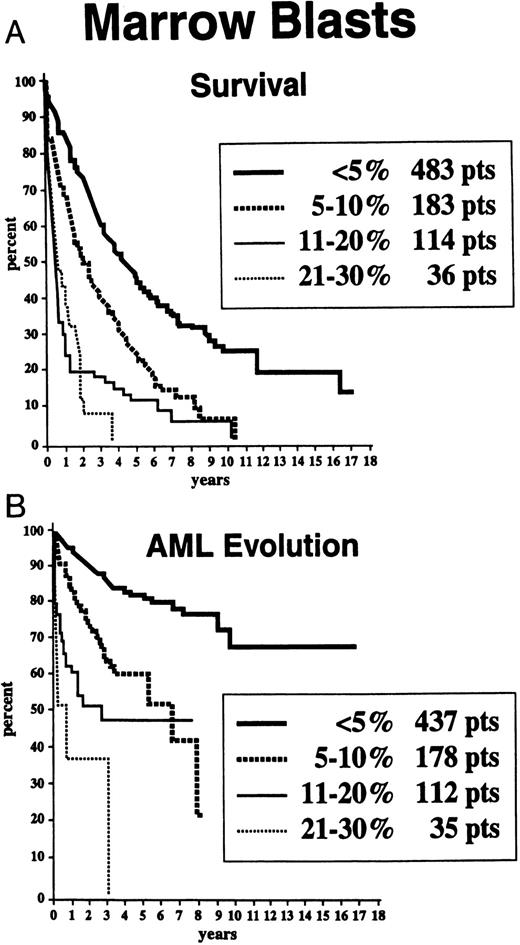
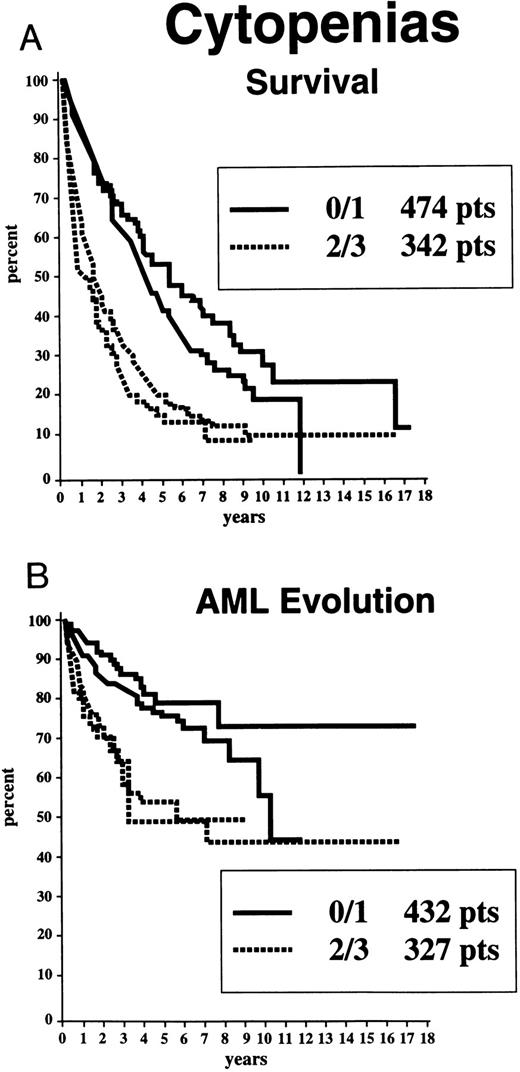
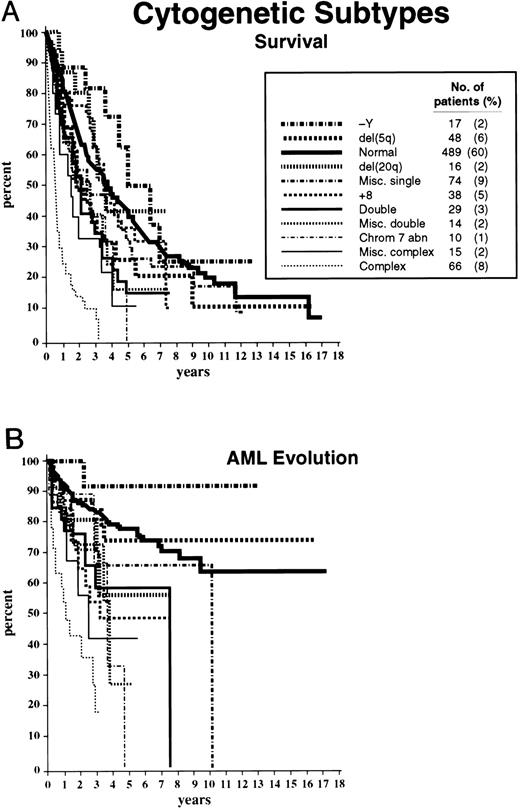
Survival and AML evolution times for patients are shown as they related to their initial FAB classification (Fig 1), BM blast percentages (Fig 2), and the number of cytopenias (Fig 3). These clinical outcomes as they related to individual cytogenetic subgroups are shown in Figs 4A and 4B. These data showed relatively poorer prognoses for patients with RAEB or RAEB-T, greater than 10% BM blasts, and 2 to 3 cytopenias and for those with complex cytogenetic abnormalities or chromosome 7 anomalies. Table 2 provides detailed information regarding survival and AML evolution as they related to BM cytogenetic subcategories and indicates the proportion of patients with various cytogenetic abnormalities. A normal karyotype was found in 60% of the patients. The most common single abnormalities were del(5q) and trisomy 8 (≈5% to 6% for each).
Survival (A) and freedom from AML evolution (B) of MDS patients related to their FAB classification subgroup (Kaplan-Meier curves).
Survival (A) and freedom from AML evolution (B) of MDS patients related to their FAB classification subgroup (Kaplan-Meier curves).
Survival (A) and freedom from AML evolution (B) of MDS patients related to the percentage of their marrow myeloblasts (Kaplan-Meier curves).
Survival (A) and freedom from AML evolution (B) of MDS patients related to the percentage of their marrow myeloblasts (Kaplan-Meier curves).
Survival (A) and freedom from AML evolution (B) of MDS patients related to the number of cytopenias initially present (Kaplan-Meier curves). The two solid lines in each graph indicate patients with 0 or 1 cytopenia; the two dashed lines indicate patients with 2 or 3 cytopenias, respectively.
Survival (A) and freedom from AML evolution (B) of MDS patients related to the number of cytopenias initially present (Kaplan-Meier curves). The two solid lines in each graph indicate patients with 0 or 1 cytopenia; the two dashed lines indicate patients with 2 or 3 cytopenias, respectively.
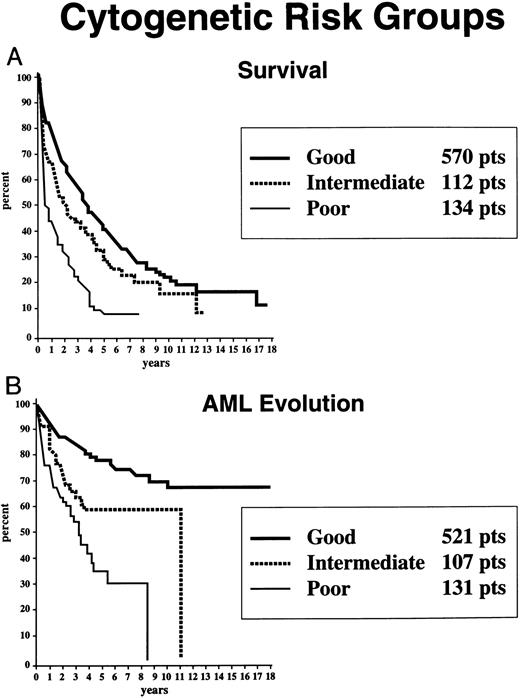
Survival (A) and freedom from AML evolution (B) of MDS patients related to their individual cytogenetic categories (Kaplan-Meier curves). The number of patients in each category and their proportional representation (in parentheses) are given for the 816 patients analyzed for survival (A). Virtually identical proportions of cytogenetic abnormalities were found for the 759 patients analyzed for AML evolution (B).
Survival (A) and freedom from AML evolution (B) of MDS patients related to their individual cytogenetic categories (Kaplan-Meier curves). The number of patients in each category and their proportional representation (in parentheses) are given for the 816 patients analyzed for survival (A). Virtually identical proportions of cytogenetic abnormalities were found for the 759 patients analyzed for AML evolution (B).
Based on the univariate analysis, the patients were separated for both survival and AML evolution into three prognostic subgroups related to their BM cytogenetic pattern: good, intermediate, or poor (Table 1). Patients with BM karyotypes that were normal, del(5q), del(20q) and −Y had relatively good prognoses (70%), whereas relatively poor prognoses were present in patients with complex abnormalities (ie, ≥ 3 anomalies) or chromosome 7 or chromosome anomalies (16%). The remaining patients were intermediate in outcome (14%). Of the 66 patients in the “complex” category, 63 patients had chromosome 5 and/or 7 abnormalities in addition to other anomalies (33, abnormalities of chromosome 5; 7, abnormalities of chromosome 7; 23, abnormalities of both chromosomes). The median survival times of patients within these three cytogenetic subgroups groups were 3.8, 2.4, and 0.8 years, respectively, and the times for 25% of the patients to undergo AML evolution were 5.6, 1.6, and 0.9 years (Table 1 and Fig 5).
Survival (A) and freedom from AML evolution (B) of MDS patients related to their risk-based categorical cytogenetic subgroups: Good, Intermediate, and Poor. Good, normal, del(5q) only, del(20q) only, −Y only; Poor, complex (ie, ≥3 anomalies) or chromosome 7 abnormalities; Intermediate, other abnormalities (Kaplan-Meier curves).
Survival (A) and freedom from AML evolution (B) of MDS patients related to their risk-based categorical cytogenetic subgroups: Good, Intermediate, and Poor. Good, normal, del(5q) only, del(20q) only, −Y only; Poor, complex (ie, ≥3 anomalies) or chromosome 7 abnormalities; Intermediate, other abnormalities (Kaplan-Meier curves).
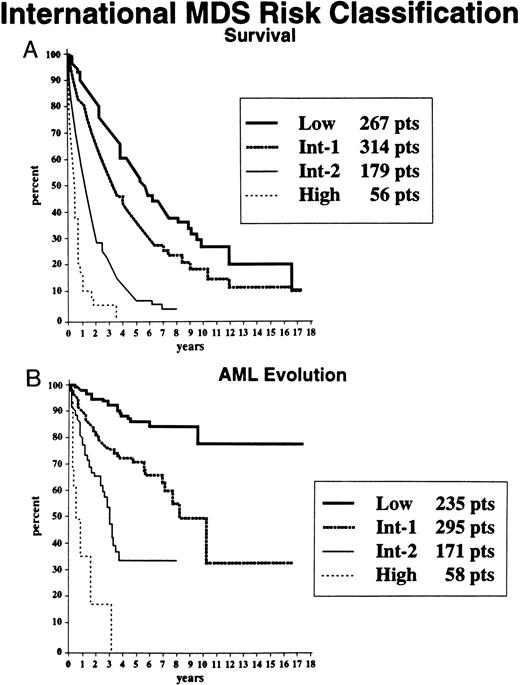
Multivariate analyses.Using proportional hazards regression multivariate analysis, the most significant independent variables for determining outcome were BM blast percentage, number of cytopenias, cytogenetic subgroup (ie, good, intermediate, or poor), age, and gender (Table 2). The final set of risk variables used for the prognostic model were BM blast percentage, number of cytopenias, and cytogenetic subgroup (chosen on both clinical and statistical grounds, these were significant variables for both survival and AML evolution). Risk scores for each significant variable were generated with weighting relative to the statistical power (ie, using coefficients from the proportional hazards regression analysis), and an IPSS for MDS was developed. The risk scores for BM blast percentage, cytogenetic subgroup, and number of cytopenias were evaluated, and the weighted scores are shown in Table 3 (categories are defined in the text and Table 1). By combining the risk scores for these three major variables, patients were stratified into four distinctive risk groups regarding both survival and AML evolution, with their risk scores being: low, 0; intermediate-1 (INT-1), 0.5 to 1.0; INT-2, 1.5 to 2.0; and high, ≥2.5. Table 4 indicates the proportions of patients in each risk group, their median survival times, and times to 25% AML evolution. Figure 6 shows the Kaplan-Meier curves depicting survival and freedom from AML times for patients in these prognostic subgroups. Much less precise discrimination between the four subgroups occurred when either cytopenias or cytogenetic subtypes were omitted from the classification (data not shown).
IPSS for MDS: Survival and AML Evolution
| Prognostic Variable . | Score Value . | ||||
|---|---|---|---|---|---|
| . | 0 . | 0.5 . | 1.0 . | 1.5 . | 2.0 . |
| BM blasts (%) | <5 | 5-10 | — | 11-20 | 21-30 |
| Karyotype3-150 | Good | Intermediate | Poor | ||
| Cytopenias | 0/1 | 2/3 | |||
| Prognostic Variable . | Score Value . | ||||
|---|---|---|---|---|---|
| . | 0 . | 0.5 . | 1.0 . | 1.5 . | 2.0 . |
| BM blasts (%) | <5 | 5-10 | — | 11-20 | 21-30 |
| Karyotype3-150 | Good | Intermediate | Poor | ||
| Cytopenias | 0/1 | 2/3 | |||
Scores for risk groups are as follows: Low, 0; INT-1, 0.5-1.0; INT-2, 1.5-2.0; and High, ≥2.5.
Good, normal, −Y, del(5q), del(20q); Poor, complex (≥3 abnormalities) or chromosome 7 anomalies; Intermediate, other abnormalities.
Age-Related Survival and AML Evolution of MDS Patients Within the IPSS Subgroups
| . | No. of Patients . | Low . | Int-1 . | Int-2 . | High . |
|---|---|---|---|---|---|
| A.Median Survival (yr) | |||||
| Total no. of patients (%) | 816 | 267 (33) | 314 (38) | 176 (22) | 59 (7) |
| Median (yr) | 5.7 | 3.5 | 1.2 | 0.4 | |
| Age (yr) | |||||
| ≤60 | 205 (25) | 11.8 | 5.2 | 1.8 | 0.3 |
| >60 | 611 | 4.8 | 2.7 | 1.1 | 0.5 |
| ≤70 | 445 (54) | 9.0 | 4.4 | 1.3 | 0.4 |
| >70 | 371 | 3.9 | 2.4 | 1.2 | 0.4 |
| B.25% AML Evolution (yr) | |||||
| Total no. of patients (%) | 759 | 235 (31) | 295 (39) | 171 (22) | 58 (8) |
| Median (yr) | 9.4 | 3.3 | 1.1 | 0.2 | |
| Age (yr) | |||||
| ≤60 | 187 (25) | >9.4 (NR) | 6.9 | 0.7 | 0.2 |
| >60 | 572 | 9.4 | 2.7 | 1.3 | 0.2 |
| ≤70 | 414 (55) | >9.4 (NR) | 5.5 | 1.0 | 0.2 |
| >70 | 345 | >5.8 (NR) | 2.2 | 1.4 | 0.4 |
| . | No. of Patients . | Low . | Int-1 . | Int-2 . | High . |
|---|---|---|---|---|---|
| A.Median Survival (yr) | |||||
| Total no. of patients (%) | 816 | 267 (33) | 314 (38) | 176 (22) | 59 (7) |
| Median (yr) | 5.7 | 3.5 | 1.2 | 0.4 | |
| Age (yr) | |||||
| ≤60 | 205 (25) | 11.8 | 5.2 | 1.8 | 0.3 |
| >60 | 611 | 4.8 | 2.7 | 1.1 | 0.5 |
| ≤70 | 445 (54) | 9.0 | 4.4 | 1.3 | 0.4 |
| >70 | 371 | 3.9 | 2.4 | 1.2 | 0.4 |
| B.25% AML Evolution (yr) | |||||
| Total no. of patients (%) | 759 | 235 (31) | 295 (39) | 171 (22) | 58 (8) |
| Median (yr) | 9.4 | 3.3 | 1.1 | 0.2 | |
| Age (yr) | |||||
| ≤60 | 187 (25) | >9.4 (NR) | 6.9 | 0.7 | 0.2 |
| >60 | 572 | 9.4 | 2.7 | 1.3 | 0.2 |
| ≤70 | 414 (55) | >9.4 (NR) | 5.5 | 1.0 | 0.2 |
| >70 | 345 | >5.8 (NR) | 2.2 | 1.4 | 0.4 |
Abbreviation: NR, not reached.
Survival (A) and freedom from AML evolution (B) of MDS patients related to their classification by the IPSS for MDS: Low, INT-1, INT-2, and High (Kaplan-Meier curves).
Survival (A) and freedom from AML evolution (B) of MDS patients related to their classification by the IPSS for MDS: Low, INT-1, INT-2, and High (Kaplan-Meier curves).
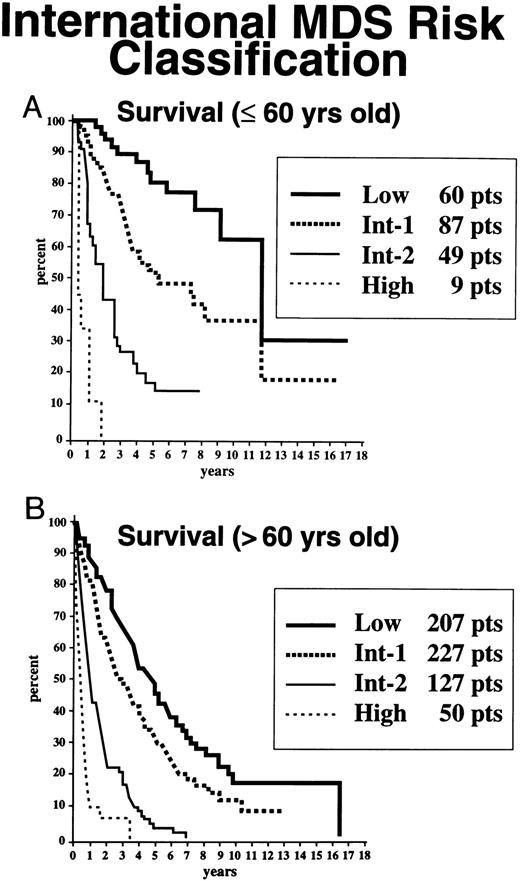
Age- and gender-related effects.Because age at diagnosis was shown to be an important variable for survival of these patients (but not for AML evolution in univariate analysis), the patients were stratified for this outcome for this parameter. Table 4 indicates the proportions of patients stratified by age in each risk group, their median survival times, and times to 25% AML evolution. As shown in Fig 7 and Table 4, the survival curves for patients aged 60 and under or greater than 60 years of age in the high-risk or INT-2–risk groups did not differ substantially whether patients were elderly or younger; however, in the low-risk and INT-1–risk groups, there were striking differences in survival times, with poorer survival times occurring in the relatively older subgroup. Similar findings for individuals in these risk groups were present in patients aged 70 and under or greater than 70 years of age (Table 4).
Survival, based on age ≤60 (A) v <60 years (B), of MDS patients related to their classification by the IPSS for MDS: Low, INT-1, INT-2, and High (Kaplan-Meier curves).
Survival, based on age ≤60 (A) v <60 years (B), of MDS patients related to their classification by the IPSS for MDS: Low, INT-1, INT-2, and High (Kaplan-Meier curves).
Moderate gender-related differences for survival were noted in the low-risk patients, with women having slightly more prolonged survival rates than men (6.3 v 5 years, respectively). These differences were less pronounced and less statistically significant than those that were age-related (Tables 1 and 2). Similar proportions of men versus women were represented within the four risk groups (low, 33% v 33%; INT-1, 37% v 40%; INT-2, 22% v 21%; and high, 8% v 6%). This similarity of representation within the risk groups was also present for patients aged 60 and under or greater than 60 years of age.
Leukemia-free survival (LFS).Patients were also analyzed for LFS to determine the impact of AML evolution on overall survival. Of the 759 patients analyzed for leukemic outcome, 492 patients (65%) died; of these individuals, 149 patients (30%) died with leukemia (20% overall; Table 5). As shown in Table 5, differences were noted in the proportion of these patients who died with leukemia in relation to their initial prognostic risk group. Of the patients who died, the proportions who died with leukemia in the low-risk, INT-1–risk, INT-2–risk, and high-risk groups were 19%, 30%, 33%, and 45%, respectively. The risk of death from AML in the low-risk group was less than that for the other combined groups (P = .0001). The median LFS time for the low-risk group was also longer than that for the remaining combined groups (5.7 v 1.9 years; P < .0001). The LFS times for these four patient risk groups were 5.7, 2.7, 0.95, and 0.3 years, respectively.
Survival of MDS Patients With or Without AML Evolution: Leukemia-Free Survival
| Subgroups . | No. of Patients . | No. of Patients Who . | ||
|---|---|---|---|---|
| . | . | Died (%) . | Died With Leukemia (%) . | Died Without Leukemia (%) . |
| Low | 235 | 113 (48) | 22 (19) | 91 (81) |
| Int-1 | 295 | 181 (61) | 55 (30) | 126 (70) |
| Int-2 | 171 | 147 (86) | 49 (33) | 98 (67) |
| High | 58 | 51 (88) | 23 (45) | 28 (55) |
| Total | 759 | 492 (65) | 149 (30) | 343 (70) |
| Subgroups . | No. of Patients . | No. of Patients Who . | ||
|---|---|---|---|---|
| . | . | Died (%) . | Died With Leukemia (%) . | Died Without Leukemia (%) . |
| Low | 235 | 113 (48) | 22 (19) | 91 (81) |
| Int-1 | 295 | 181 (61) | 55 (30) | 126 (70) |
| Int-2 | 171 | 147 (86) | 49 (33) | 98 (67) |
| High | 58 | 51 (88) | 23 (45) | 28 (55) |
| Total | 759 | 492 (65) | 149 (30) | 343 (70) |
Comparison of prognostic systems.Patients were analyzed for clinical outcomes based on their categorization using the FAB (based on BM blast percentage),1 Spanish (BM blasts, age, and platelet count),3 and Lille (platelet count, BM blasts, and karyotype)5 prognostic risk systems, in addition to the IPSS. The IPSS effectively discriminated between the defined subgroups of these other categorization systems. For patients classified by the FAB system, MDS patients with the RA subtype were separable into low-risk, INT-1–risk, and INT-2–risk subgroups, and RARS patients were separable into low-risk and INT-1–risk subgroups (proportions not shown). The RAEB patients were predominantly separated into INT-1– and INT-2–risk subgroups, with a low proportion in the high-risk group. Patients with RAEB-T were separated into INT-1–, INT-2–, and high-risk groups. CMML patients were present in all four risk groups. The Spanish and Lille systems had their high-risk patients distributed between the IPSS INT-1–, INT-2–, and high-risk groups. The intermediate groups in both of these systems were well-separated into the IPSS high-risk, INT-2–risk, INT-1–risk, and, to a lesser degree, low-risk patients; whereas the low-risk patients were separated into INT-1–, INT-2–, and low-risk groups. The IPSS had smaller P values after it and the three other prognostic systems were used to predict both survival (P < .0001 in all comparisons vP = .001 to < .0001) and AML evolution (P < .0001 in all comparisons vP = .043 to < .0001), indicating its greater discriminating power compared with that of the other systems.
DISCUSSION
We herein report the results of the IPSS for MDS regarding critical factors related to clinical outcome in a large group of well-defined untreated primary MDS patients with a prolonged follow-up period. Three refined risk-based cytogenetic subgroups (good, poor, and intermediate) were found to relate to clinical outcomes, both for survival and AML evolution. The categorical IPSS we used was based on statistical weighting of risk scores of the critical clinical and cytogenetic variables using proportional hazards regression analysis. We combined the statistically defined major clinical features such as BM blasts and cytopenias with these refined cytogenetic parameters and analyzed the patients for their clinical outcomes. Using these features, the International MDS Risk Analysis Workshop generated an IPSS with four subgroups of patients who showed significantly different clinical outcomes (low-risk, INT-1–risk, INT-2–risk, and high-risk groups; see Tables 1 and 4 and Fig 6). The use of these four risk categories was particularly helpful in separating the intermediate prognostic groups defined by these prior systems into two discernible subgroups. Table 3 shows this scoring system and may be readily used to evaluate clinical prognostic risk categories for MDS patients. We also found age-related categorization to be important for evaluation of survival but not for AML evolution (Tables 1 and 4 and Fig 7). In comparison with three other major prior classification systems (FAB, Spanish, and Lille),1,3 5 the IPSS was shown to have better discriminatory power regarding the patients' clinical outcomes.
Our study showed generally similar proportions of patients in the FAB classification subgroups in comparison with those of prior reports, although with slightly less advanced (ie, RAEB, RAEB-T) patients as compared with those previously reported,2,3,16-19 and as reviewed in a recent meta-analysis.20 In addition, the survival times and time to AML evolution for our patients according to their FAB subtype were similar to those previously reported. These findings suggest that, despite more rigorous entry criteria for our study (ie, exclusion of previously treated patients from our study), we evaluated the natural history of a representative group of MDS patients.
Regarding clinical outcomes, prior studies have suggested the importance of a variety of clinical features, including differing numbers or types of cytopenias (ie, less than versus more than 1 or 2 cytopenias, thrombocytopenia, anemia versus neutropenia),2-6 BM blast percentages,1-6,8,10, 21 and cytogenetic abnormalities.5,6,8 10-12 Our study confirmed the importance of these features as critical variables. We extended the use of these clinical and biological variables for prognostic evaluation by combining them statistically, using a more refined cytogenetic analysis.
By evaluating a relatively large group of patients, our study permitted risk-related analysis of a greater number of cytogenetic subgroups regarding survival and AML evolution, particularly for those chromosomal abnormalities that were relatively uncommon. Our study showed that patients with del(20q) only, del(5q) only, −Y, or normal karyotypes had improved outcomes. These findings regarding del(20q) as the sole abnormality are similar to those recently reported for a smaller group of MDS patients.22 Of note are the results of a recent report in which most MDS patients with a del(20q) had complex karyotypes, an advanced MDS stage or AML, and a poor prognosis.23 Together, these data suggest that the del(20q) may be associated with a favorable outcome when noted as a sole abnormality but with less favorable prognosis in the setting of a complex karyotype. As described below, this phenomenon is analogous to that observed with the del(5q).
Patients with del(5q) as the sole karyotypic abnormality have previously been well defined as having relatively good prognoses, whereas poor prognoses were found when it was combined with other anomalies.10,12,24,25 Loss of the Y chromosome in elderly men has been described in the BMs of patients with hematologic malignancies, but this abnormality has also been noted in BM samples from hematologically normal elderly men.26 Thus, this finding alone does not indicate the presence of a myeloid clonal hemopathy. However, once the diagnosis has been established by other means, our findings indicate that this feature confers an improved clinical outcome. Conversely, chromosome 7 anomalies and complex cytogenetic abnormalities (variously defined) have previously been associated with poor prognoses.10-12,27 In our series, the vast majority of patients in this group had abnormalities of chromosomes 5, 7, or both, together with other abnormalities. Multiple clones were common in this group, reflecting genetic instability. The presence of abnormalities of chromosomes 5 and 7 has been associated with poor outcomes in MDS and AML.5,6,10-12,27 Other anomalies were associated with intermediate risk. Our study showed karyotypic prognostic findings of these cytogenetic abnormalities in MDS patients similar to those reported in several smaller groups of MDS patients, many of whom were included in this analysis.5 6 Our data confirm these findings and extend their prognostic utility by using a more extensive karyotypic analysis and by combining such biological parameters with clinical features to provide a risk-scoring system. These cytogenetic correlative findings also suggest that genomic instability and biologically important genes may be present on these chromosomes that alter survival and the potential for leukemic evolution in MDS patients.
Because the vast majority of primary MDS patients are elderly, age-stratified morbidity and mortality figures are needed regarding their clinical outcomes. The median life expectancy of individuals 65 years old in the world's more developed countries is 13 to 16 years for men and 15 to 20 years for women.28 Each further decade of life is associated with decrements in expected survival. Our data regarding age-related outcomes of these individuals showed that marked differences were apparent in survival for patients in the low-risk and INT-1–risk subgroups (but not in the INT-2–risk or high-risk subgroups), with poorer prognosis in the relatively elderly cohorts of patients (ie, age, >60 v ≤60 years; age, >70 v ≤70 years). Coexisting diseases in the elderly patients contribute substantially to their poorer survival. Our data are similar to those recently reported, which further indicate the prognostic importance of age for survival in patients with MDS.29 Thus, to provide insights into disease-specific versus age-related impact on survival, age-stratified and normalized evaluations are needed to aid in decision-making regarding patient management. Gender also needs to be considered, although this is of lesser importance than age in this setting.
To determine the impact of AML evolution on survival, as it related to risk group, we evaluated LFS for these patients. Our data showed that the risk of death related to leukemic evolution was decreased in the low-risk group as compared with that for the more advanced groups (19% v 30% to 45%; Table 5). Thus, mortality from complications of BM failure (without leukemic evolution) and comorbid conditions plays a dominant role in the clinical outcome of the low-risk patients in contrast to the more prominent role of leukemic evolution in the higher risk patients.
The IPSS provides an improved method for predicting survival and AML evolution in MDS compared with those found with the FAB, Spanish, and Lille1,3,5 categorization methods. This improved method was the result of several features of the International Workshop model: the more refined cytogenetic categorizations, inclusion of cytopenias, improved subdivision of BM blast percentages, the four defined outcome subgroups, and the separate stratification for age. Regarding the difficulty of using the FAB classification for assessing prognosis in MDS, a wide variability of outcomes have been found in the RAEB and CMML subtypes defined by these criteria.16-19,21 Problems with the FAB classification's ability to provide more precise prognostic information (in addition to its relying on BM morphology alone for its classification) relate to the relatively wide proportion of BM blasts (ie, 5% to 20% and 1% to 20% blasts, respectively) present in these two clinical subgroups and in the nature of the proliferative form of CMML. The proliferative form of CMML (ie, with high WBC counts, hepatosplenomegaly, and constitutional symptoms) is more akin to an MPD than to MDS.14,30,31 This disorder differs in its major clinical features from the nonproliferative subtype of CMML (ie, in MDS patients) who have monocytosis but relatively low WBC counts and who previously were likely to be considered to have RAEB or RA with monocytosis. Clinical outcomes in CMML have been shown to be more closely related to their proportion of BM blasts than to their peripheral monocyte numbers.30 31 Further analyses will be needed to determine whether this or other classification systems for MDS or MPD will be useful for evaluating prognosis in this subset of CMML patients.
This IPSS defines critical prognostic features of MDS, shows the importance of age-related stratification, and provides improved prognostic evaluation compared with those of prior systems. This classification system should improve our ability to define clinical outcome in MDS and should provide a framework for future studies determining the possible role of molecular determinants (eg, oncogenes, tumor suppressor genes, and cytokine expression and responsiveness) for evaluating prognosis in this disorder. This system will likely prove useful for the design and analysis of therapeutic trials in MDS as well as aiding in the management of these patients.
ACKNOWLEDGMENT
With much appreciation, the authors wish to thank K. Bourgeois (Rochester, NY) for statistical assistance; Dr U. Germing (Dusseldorf, Germany) and R. Chapman (Bournemouth, UK) for data collation; and K. Heptinstall (Crosswicks, NJ) for administrative assistance.
Supported in part by the Myelodysplastic Syndrome Foundation, Crosswicks, NJ, by Amgen (Thousand Oaks, CA), and by the Fondo de Investigaciones Sanitarias de la Seguridad Social (FISS no. 93/0542), Ministerio de Sanidad y Consumo, Spain.
Address reprint requests to Peter Greenberg, MD, Hematology Division, Room S-161, Stanford University Medical Center, Stanford, CA 94305.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal