Abstract
The bacterial superantigen staphylococcal enterotoxin A (SEA) is an efficient activator of cytotoxic T cells when presented on major histocompatibility complex (MHC) class II molecules of target cells. Our previous studies showed that such SEA-directed T cells efficiently lysed chronic B-lymphocytic leukemia (B-CLL) cells. Next, we made a mutated SEA–protein A (SEAm-PA) fusion protein with more than 1,000-fold reduced binding affinity for MHC class II compared with native SEA. The fusion protein was successfully used to direct T cells to B-CLL cells coated with different B lineage–directed monoclonal antibodies (MoAbs). In this communication, we constructed a recombinant anti-CD19-Fab-SEAm fusion protein. The MHC class II binding capacity of the SEA part was drastically reduced by a D227A point mutation, whereas the T-cell activation properties were retained. The Fab part of the fusion protein displayed a binding affinity for CD19+ cells in the nanomolar range. The anti-CD19-Fab-SEAm molecule mediated effective, specific, rapid, and perforin-like T-cell lysis of B-CLL cells at low effector to target cell ratios. Normal CD19+ B cells were sensitive to lysis, whereas CD34+ progenitor cells and monocytes/macrophages were resistant. A panel of CD19+ B-cell lines representing different B-cell developmental stages were efficiently lysed, and the sensitivity correlated with surface ICAM-1 expression. The anti-CD19-Fab-SEAm fusion protein mediated highly effective killing of tumor biopsy cells representing several types of B-cell non-Hodgkin's lymphoma (B-NHL). Humanized severe combined immune deficiency (SCID) mice carrying Daudi lymphoma cells were used as an in vivo therapy model for evaluation of the anti-CD19-Fab-SEAm fusion protein. Greater than 90% reduction in tumor weight was recorded in anti-CD19-Fab-SEAm–treated animals compared with control animals receiving an irrelevant Fab-SEAm fusion protein. The present results indicate that MoAb-targeted superantigens (SAgs) may represent a promising approach for T-cell–based therapy of CD19+ B-cell malignancies.
NON-HODGKIN'S LYMPHOMAS of the B-cell type (B-NHLs) represent a large and growing proportion of malignant neoplasms exhibiting great heterogeneity with respect to histology, immunophenotype, and clinical behavior. At present, about one third of the patients with high-grade disease are cured by high-dose chemoradiotherapy followed or not by stem cell rescue, whereas few, if any, cases with low-grade malignancy are permanently cured.1 Immunotherapy using naked monoclonal antibodies (MoAbs) has largely been unsuccessful,2 whereas toxin- and isotope-conjugated pan-B-cell MoAbs followed by stem cell rescue may produce encouraging results.3-8 Tumor vaccines based on patient-specific Ig idiotypes are presently being attempted and have so far provided proof of the principle.9-11
Staphylococcal enterotoxins A (SEA), B, C, D, E, and H are termed superantigens (SAgs) because of their capacity to stimulate a large proportion of T cells expressing particular T-cell receptor Vβ sequences.12 The SAg molecule binds outside the peptide binding cleft of the major histocompatibility complex (MHC) class II molecule with high affinity, and is presented to T cells as an unprocessed protein.13,14 Both CD4+ and CD8+ T cells respond to staphylococcal enterotoxins by proliferation, production of cytokines such as interleukin-2, interferon gamma, and tumor necrosis factor α (TNFα), and generation of strong T-cell cytotoxic capacity.15-17 SAgs have the ability to direct T-cell cytotoxicity against HLA-DR–positive (HLA-DR+) cells such as B cells, dendritic cells, and monocytes.18
SAg-directed T cells can lyse a variety of HLA-DR+ tumor target cells, whereas targets lacking the SAg receptor HLA-DR are resistant. We recently demonstrated that SEA-directed T cells kill primary chronic B-lymphocytic leukemia (B-CLL) cells in vitro, and sensitivity to lysis was dependent on HLA-DR, ICAM-1 (CD54), CD18, and CD72 surface molecules.19 We next demonstrated that the introduction of a point mutation in the SEA molecule reduced its HLA class II binding capacity more than 1,000-fold. Mutated SEA (SEAm) was then fused to protein A and used to screen for MoAbs capable of directing SAg-reactive T cells to leukemic cells.20 This fusion protein was 100-fold more potent in lysing B-CLL target cells coated with certain B-cell–specific/associated mAbs in comparison to uncoated HLA class II–positive (HLA class II+) B-CLL cells. The most promising antibody specificity turned out to be CD19.
In the present communication, we constructed a recombinant fusion protein between the Fab fragment of an anti-CD19 mAb and a SEA D227A mutant for preclinical in vitro studies of cytotoxic T-cell therapy for human B-cell malignancies. We also tested the potential in vivo antitumor effects of this fusion protein in severe combined immune deficiency (SCID) mice carrying Daudi lymphoma cells and human peripheral blood mononuclear cells as effectors.
MATERIALS AND METHODS
Construction of SEAm.The SEA gene was cloned by polymerase chain reaction (PCR) from the Staphylococcus aureus strain ATCC 8095 (obtained from American Type Culture Collection, Rockville, MD). The nucleotide sequence was found to be identical to the published sequence.21 Alanine substitution mutagenesis combined with the SEA crystal structure identified D227 as an essential amino acid in the C-terminal major HLA class II binding site.22 In SEAm, the mutation D227A was introduced by PCR by changing the Asp codon GAT into GCT (Ala). A diagnostic SpeI site was introduced at codons 232 to 233 by changing codon 232 from ACA to ACT. Binding studies with HLA class II+ cells demonstrated a kd of 10−8 mol/L for recombinant native SEA, whereas the kd for SEAm was estimated to be more than 10−5 mol/L.22
Cloning of the Fv part of anti-CD19 (CLB-B4/1) and expression in Escherichia coli. The Fv-encoding portions of the mAb CLB-B4/1 (anti-CD19 IgG/k) were cloned from the CLB hybridoma (kindly provided by Drs C. Melief, R.F. Tiebout, and A.M. Kruisbeek, Leiden, The Netherlands) using previously described methodology.23 Briefly, cDNA was made from the mRNA and coding regions of the entire variable domains, and parts of the signal sequences and the constant domains of the heavy and light chains were amplified by PCR. The oligonucleotides 5′-CAATTTTCTTGTCCACCTTGGTGC-3′ and 5′-ACTAGTCGACATGGIATGGAGCIGGATCTTTmTCTT-3′ were used for the heavy chain, resulting in a 563-bp fragment, and the oligonucleotides 5′-ACT AGT CGA CAT GGA TTT ICA G G T G C A G A T T w T C A G C TTC-3′ and 5′-GCGCCGTCTAGAATTAACACTCATTCCTGTTGAA-3′ were used for the light chain, yielding a 717-bp fragment. For each chain, three separate clones were sequenced and found to be identical. DNA fragments suitable for insertion into the expression vector were obtained in a second PCR step. To assemble a Fab-expression plasmid, the variable regions of CLB-B4/1 mAb were fused to sequences coding for the constant regions of the murine IgG1/k antibody C242.24 A region coding for the SEA D227A gene was fused after the heavy chain.
The expression plasmid used contained the kanamycin resistance gene and a Lac promoter inducible with isopropyl-βD-thiogalactopyranoside (IPTG). In the fermenter, anti-CD19-Fab-SEA D227A was produced in E coli at protein levels exceeding 100 mg/L, which was comparable to similar Fab-SAg constructs previously expressed in our laboratory.23 The anti-CD19-Fab-SEAm protein was purified with a protein G–based affinity chromatography and ion-exchange chromatography as described. The recombinant product demonstrated a molecular weight of about 82 kD on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE).
Iodination of anti-CD19-Fab-SEAm and cell binding assay.The anti-CD19-Fab-SEAm protein was 125I-labeled and used in a cell-binding assay with Daudi B-lymphoma cells.23 24 The dissociation constant (Kd) and the number of binding sites were determined by Scatchard analyses.
Other reagents.Recombinant SEA was produced as previously described.22 Anti-CD19 (CLB-anti-CD19) IgG mAb was a gift from Dr C. Melief (Leiden, The Netherlands). A control fusion protein (C215-Fab-SEAm) was constructed and purified to homogeneity as described previously.23 mAbs against the Apo-1/Fas antigen (CD95) were purchased from Immunotech (Marseille, France) and were as follows: ZB4 (blocking of apoptosis), CH-11 (induction of apoptosis), and UB2 (FITC-conjugated F(ab)2 fragment for staining procedures). FITC-conjugated MoAbs against ICAM-1 (CD54) and LFA-1 (CD11a) were obtained from Immunotech. Anti-CD22 (RFB4) was a gift from Professor G. Janossy (London, UK), and anti-CD24 (SWA11) was kindly provided by Dr U. Zwangermeister Witke (Zürich, Switzerland). Anti-PBC MoAb (reactive with a mitochondrial respiratory enzyme, PDH-E2) used as an irrelevant control was kindly provided by Dr A. Björkland (Department of Clinical Immunology, University of Uppsala). FITC- or PE-conjugated MoAbs used in two-color FACS analysis were as follows: anti-HLA-DR-FITC, anti-CD20-FITC, anti-CD3-FITC, anti-CD34-PE, and anti-CD16-PE from Becton Dickinson (Mountain View, CA) and anti-CD19-FITC, anti-CD19-PE, anti-CD4-PE, anti-CD8-PE, and anti-CD5-PE MoAbs from Ortho Diagnostic Systems (Raritan, NJ). Rabbit F(ab)2 antimouse Ig-FITC (RAM-FITC) and rabbit antimouse Ig (RAM) were obtained from Dakopatts (Glostrup, Denmark). Recombinant interleukin-2 and 51Cr were from Amersham (Buckingham, UK). 12-O-tetradecanoyl phorbol 13-acetate (TPA), EGTA, propidium iodide (PI), and 2-aminoethylisothiouronium bromide hydrobromide (AET) were purchased from Sigma (St Louis, MO). RPMI 1640 (Flow Laboratories, Glasgow, Scotland) supplemented with 10% fetal calf serum, 1 mmol/L nonessential amino acids, 0.1 mmol/L sodium pyruvate (both from GIBCO, Middlesex, UK), 100 U/mL penicillin, 100 μg/mL streptomycin, 10 mmol/L HEPES, 2 mmol/L L-glutamine, and 5 × 10−5 mol/L β-mercaptoethanol (Sigma) was used as complete medium.
Cell lines.Human peripheral blood mononuclear cells were isolated from a normal healthy subject by routine density centrifugation. A SEA-reactive T-cell line, SEA-T, was established by stimulation of these cells with SEA (1 ng/mL). The cell line was kept for weeks by repeated restimulation with SEA-coated, irradiated (137Cs, 4,000 rad) BSM B-lymphoblastoid cells and recombinant interleukin-2 (20 U/mL) in complete medium and then freeze-stored. These cells were thawed 1 to 2 weeks before use as effector T cells in the in vitro assays described later. The T-cell line was more than 98% CD3+, 80% to 90% CD8+, and 10% to 20% CD4+. Other cell lines were grown in log phase in complete medium at 37°C and 5% CO2 and were as follows: BSM (Epstein-Barr virus [EBV]-transformed lymphoblast); Raji, a HLA-DR–negative Raji mutant cell line RJ225, and Daudi (all Burkitt's lymphomas); NALM-1, NALM-16, NALL-1, and KM3 (acute lymphoblastic leukemias of pre-B type); U698 and MN60 (lymphoblastic lymphomas of B type); U715A and PL (follicle center cell lymphomas); and LP1 (myeloma).
Leukemia/lymphoma patient cells.Blood samples from six patients with untreated classic B-CLL (95% to 99% monoclonal CD19+ CD5+ cells) were Ficoll-separated (Pharmacia, Uppsala, Sweden) and freeze-stored in liquid nitrogen. Cells from three other B-CLL patients were used fresh in the FACS cytotoxicity assay. Cells from B-NHL patients were prepared from diagnostic lymph nodes using scissors plus steel mesh and kept in liquid nitrogen. The diagnoses (according to the Kiel classification25 ) were as follows: lymphoblastic lymphoma of B type (LB; one patient), centroblastic lymphoma (CB; three patients), centroblastic-centrocytic lymphoma (CB-CC; two patients), hairy cell leukemia (HCL; three patients), and immunocytic lymphoma (IC; three patients).
Highly purified CD34+ progenitor cells.CD34+ enriched progenitor cells were obtained from five patients with multiple myeloma. Progenitor cells were mobilized with the combination of cyclophosphamide (4 g/m2) plus rh-GSF (lenograstim, 5 μg/kg) and subsequently harvested by leukapheresis.26 CD34+ progenitor cells were isolated from the leukapheresis product with Ceprate SC technology (CellPro, Bothell, WA). The purity of the enriched fraction was 92% to 95%. Cells were kept in liquid nitrogen if not used fresh in the assays. All patients were from a multicentric phase III trial studying the efficacy of tumor depletion from the peripheral stem cell harvest by CD34+ enrichment. The study was approved by the local ethics committee.
Nonmalignant B cells.To obtain normal B cells (95% CD19+), tonsils were minced, T-cell–depleted by E-rosetting, and stored in liquid nitrogen until use.
Monocytes/macrophages.Normal donor blood was purified in four steps to generate a pure monocyte/macrophage (CD14+) cell population: (1) blood was routinely Ficoll-separated, (2) mononuclear cells were E-rosetted to eliminate T cells and natural killer cells, (3) cells were Percoll-separated (Pharmacia), and (4) magnetic beads were used together with MoAbs against CD19 and CD3 to reduce contaminating B and/or T cells. Greater than 95% of the remaining cells were CD14+.
Short-term cell cultures.Freeze-stored B-CLL cells were thawed and cultured for 3 days with or without TPA (1.6 × 10−7 mol/L) in complete medium and used as targets in the assays. B-CLL cells separated from fresh blood were cultured overnight and used as targets in the FACS-cytotoxicity assay.
B-NHL cells, CD34+ progenitor cells, and CD19+ tonsil cells were thawed and incubated overnight in complete medium before use. Purified monocytes/macrophages were similarly kept in medium overnight before use.
51Cr-release assay.Cytotoxicity was measured in a standard 4-hour 51Cr-release assay and expressed as follows: % specific lysis = 100 × (experimental − background cpm/maximal background cpm). Target cells were labeled for 2 hours with 51Cr (250 μCi/1 × 106 cells). The cells were then washed twice and seeded in triplicate in v-bottomed microtiter plates at a concentration of 2.5 × 103 cells per well. SEA, anti-CD19-Fab-SEAm, or control fusion protein was added directly to the assay. Indicated numbers of effector cells were added in 0.2 mL complete medium. The plates were incubated at 37°C and 5% CO2, supernatants were collected, and the released 51Cr was measured in a gamma counter (LKB-Wallac 1282; Stockholm, Sweden). Spontaneous release was estimated by incubation of target cells in medium alone, and maximum release by resuspending the wells with 0.1% Tween 20. Spontaneous release was typically less than 30% of maximum release.
FACS-cytotoxicity assay.Target and effector cells were seeded in flat-bottomed microtiter plates (50,000 to 100,000 target cells per well depending on B-cell content adjusted to an E:T cell ratio of 20:1) and incubated at 37°C and 5% CO2 with anti-CD19-Fab-SEAm or control fusion protein for 4 hours. Cells were then stained with an antibody cocktail consisting of anti-CD19,20,22,24, washed, and stained with the secondary antibody RAM-FITC. After a second wash, cells were stained with propidium iodide (PI) immediately before analysis in a fluorescence microscope or a FACScan flow cytometer (Becton Dickinson). FACS data from cells stained green (B cells) were collected and analyzed with the Lysis II software (Becton Dickinson). The number of B cells was correlated with a fixed total number of cells counted (500,000 cells). Double-stained cells were considered dead. B-cell viability remained high (75% to 90%) if cells were kept for 4 hours in medium alone or medium plus fusion protein without effector T cells.
Flow cytometric analysis of cell phenotype.Cell phenotype was determined by FACS analysis as described elsewhere.19 Appropriate isotypic control MoAbs were used to estimate the level of nonspecific surface binding.
SCID mice.Two- to 3-month-old female SCID mice (C.B-17) were obtained from Bommice (Ry, Denmark) and kept under pathogen-free conditions. The animals were injected intraperitoneally (IP) with 3 × 105 Daudi lymphoma cells and 5 days later with Ficoll-separated human peripheral blood mononuclear cells.24 Treatment with anti-CD19-Fab-SEAm or C215-Fab-SEAm control fusion protein was initiated at day 5 and given as four daily intravenous injections (100 μg per injection). Animals were killed after 40 days, and the macroscopically observed tumors in the peritoneal cavity were counted and total tumor mass in each animal was determined as described previously.27 Immunohistochemistry was used to confirm the presence of tumor cells. Tumors with a weight less than 5 mg were estimated as 2 mg, tumors with a weight greater than 5 mg and less than 10 mg as 7 mg, and tumors larger than 10 mg as the actual weight. All tumors larger than 1 mg were counted. Each treatment cohort contained five to seven mice to permit comparison to other treatment cohorts treated simultaneously with the same batch of effector cells. Statistical significance was determined by the Mann-Whitney U test.
RESULTS
Prokaryotic expression of anti-CD19-Fab-SEAm fusion protein.The variable (V) region of the H chain of the CLB-CD19 antibody cDNA was ligated to the first constant (C) region of a H chain gene fragment from a consensus murine IgG1. This Fd gene fragment was then fused to the SEAm gene and expressed as a bicistronic transcription unit with the gene encoding the V region of the CLB-CD19 κ light chain ligated to the first C region of a consensus κ chain. The pKP865 vector was used for expression of the Fab-SEAm fusion protein in E coli. The fusion protein was purified to more than 95% homogeneity as determined by SDS-PAGE and reverse-phase high-performance liquid chromatography (data not shown). Under nonreducing conditions, the protein migrated as an 82-kD band that dissociated into the light chain (26 kD) and Fd-SEAm fusion protein under reducing conditions.
Binding affinity of the fusion protein to the CD19 antigen and MHC class II molecules.The binding affinity of anti-CD19-Fab-SEAm to CD19 and HLA class II molecules was investigated using CD19+HLA-DR+ Daudi cells. Scatchard analysis showed an apparent Kd of 10−9 mol/L and demonstrated approximately 1.7 × 105 antigen sites per cell (Fig 1). Binding of anti-CD19-Fab-SEAm to HLA-DR+CD19− target cells failed to show a specific binding, and the Kd was estimated to be more than 10−5 mol/L (data not shown).
Binding of 125I-labeled anti-CD19-Fab-SEAm fusion protein to Daudi lymphoma cells. Daudi cells (3 × 105/mL) were incubated (1 hour at 22°C) with serially diluted 125I-anti-CD19-Fab-SEAm (0.7 to 300 nmol/L). Cell-bound radioactivity is shown as a Scatchard plot and as a saturation curve (insert) after correction for background binding (B/Fnonspecific = 0.02) in each point. Scatchard analysis showed an apparent Kd of 9.0 nmol/L and approximately 1.7 × 105 sites per cell (R = −.97). Each value is the mean of triplicate samples.
Binding of 125I-labeled anti-CD19-Fab-SEAm fusion protein to Daudi lymphoma cells. Daudi cells (3 × 105/mL) were incubated (1 hour at 22°C) with serially diluted 125I-anti-CD19-Fab-SEAm (0.7 to 300 nmol/L). Cell-bound radioactivity is shown as a Scatchard plot and as a saturation curve (insert) after correction for background binding (B/Fnonspecific = 0.02) in each point. Scatchard analysis showed an apparent Kd of 9.0 nmol/L and approximately 1.7 × 105 sites per cell (R = −.97). Each value is the mean of triplicate samples.
T-cell targeting to MHC class II+ and class II− Burkitt cell lines.To investigate residual HLA class II affinity of the anti-CD19-Fab-SEAm fusion protein, we performed cytotoxicity experiments with the SEA-T effector cell line against RJ225 (HLA-DRlowCD19+) and native Raji (DR++CD19+) target cells (Fig 2). A control fusion protein (C215-Fab-SEAm, directed against human colon cancer cells) or native SEA were used as controls. As expected, Raji but not RJ225 cells were efficiently killed by T cells plus native SEA. The irrelevant control fusion protein containing a D227A mutation in the SEA part mediated minimal background lysis of either target. In contrast, the anti-CD19-Fab-SEAm fusion protein was highly efficient in directing T-cell lysis of both RJ225 and Raji cells at very low concentrations of the protein. The lysis was clearly dose-dependent, and up to 75% cytotoxicity was recorded in the presence of 0.5 to 5.0 ng/mL of the fusion protein. Thus, residual MHC class II–dependent lysis with the anti-CD19-Fab-SEAm fusion protein was low.
Effects of anti-CD19-Fab-SEAm against 2 CD19+ lymphoma cell line targets. T-cell targeting to (A) Raji cells (CD19+HLA-DR++) and (B) RJ225 cells (CD19+HLA-DRlow) mediated by anti-CD19-Fab-SEAm fusion protein, native SEA, or C215-Fab-SEAm control fusion protein. Each value is the mean of triplicate samples. The E:T ratio was 40:1, and specific cytotoxicity was measured in a 51Cr-release assay.
Effects of anti-CD19-Fab-SEAm against 2 CD19+ lymphoma cell line targets. T-cell targeting to (A) Raji cells (CD19+HLA-DR++) and (B) RJ225 cells (CD19+HLA-DRlow) mediated by anti-CD19-Fab-SEAm fusion protein, native SEA, or C215-Fab-SEAm control fusion protein. Each value is the mean of triplicate samples. The E:T ratio was 40:1, and specific cytotoxicity was measured in a 51Cr-release assay.
Sensitivity of normal B cells, CD34+ cells, and monocytes/macrophages in T-cell/anti-CD19-Fab-SEAm fusion protein killing.To examine whether normal CD19+ B cells were sensitive to anti-CD19-Fab-SEAm fusion protein–mediated killing, we used B cells purified from tonsil tissue (95% CD19+ cells). Significant lysis (60%) of these B cells was seen when using anti-CD19-Fab-SEAm–targeted T cells compared with the control fusion protein (6%) (not shown).
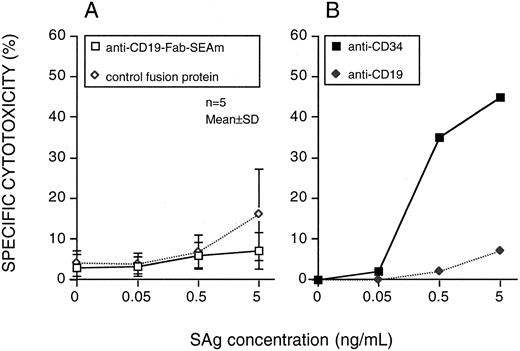
The sensitivity of CD34+CD19− progenitor cells (92% to 98% purity) from five myeloma patients was also tested. None of these cell preparations were sensitive to lysis by anti-CD19-Fab-SEAm plus T cells. Two CD34+ targets were also used in an indirect cytotoxicity system using mAbs against CD34 or CD19 together with PA-SEAm.20 The CD34 MoAb mediated efficient lysis, whereas the CD19 MoAb did not (Fig 3).
Progenitor cell sensitivity to SAg-directed T-cell killing. Highly purified CD34+ cells were used as targets. (A) Anti-CD19-Fab-SEAm fusion protein or C215-Fab-SEAm control fusion protein. Results are the mean ± SD % cytotoxicity. (B) An indirect system including MoAbs against CD34 or CD19 followed by RAM and PA-SEAm. Each value is the mean of triplicate samples. The E:T ratio was 40:1, and specific cytotoxicity was measured in a 51Cr-release assay.
Progenitor cell sensitivity to SAg-directed T-cell killing. Highly purified CD34+ cells were used as targets. (A) Anti-CD19-Fab-SEAm fusion protein or C215-Fab-SEAm control fusion protein. Results are the mean ± SD % cytotoxicity. (B) An indirect system including MoAbs against CD34 or CD19 followed by RAM and PA-SEAm. Each value is the mean of triplicate samples. The E:T ratio was 40:1, and specific cytotoxicity was measured in a 51Cr-release assay.
Monocytes/macrophages (>95% CD14+ cells) representing HLA class II+ CD19− normal target cells were used in anti-CD19-Fab-SEAm–directed T-cell lysis. Neither the anti-CD19-Fab-SEAm nor the control fusion protein mediated any cytotoxicity. In contrast, native SEA mediated a strong dose-dependent T-cell lysis of monocytes (results not shown).
SAg-mediated lysis of resting and activated B-CLL cells.We reported previously that T-cell lysis of B-CLL targets coated with native SEA or, alternatively, PA-SEAm (SEAm fused with protein A) combined with B-cell–reactive MoAb, was augmented if the target cells were preactivated with TPA.19 20 Therefore, B-CLL cells from six donors were cultured in the presence or absence of TPA for 3 days and used as targets in the assay. Anti-CD19-Fab-SEAm–targeted T cells lysed resting B-CLL cells as effectively as TPA-activated cells (Fig 4). In comparison, native SEA mediated slightly enhanced killing of TPA-activated B-CLL cells compared with nonactivated B-CLL cells (not shown). The irrelevant fusion protein was inactive against both target cells.
SAg-mediated T-cell killing of B-CLL target cells. Cells from 6 patients with B-CLL were cultured for 3 days with TPA (▪, ♦) or in medium alone (□, ⋄) and used as targets. Cytotoxic T cells were directed by addition of anti-CD19-Fab-SEAm fusion protein or C215-Fab-SEAm control fusion protein. Results are the mean ± SD. Each value is the mean of triplicate samples. The E:T ratio was 40:1, and specific cytotoxicity was measured in a 51Cr-release assay.
SAg-mediated T-cell killing of B-CLL target cells. Cells from 6 patients with B-CLL were cultured for 3 days with TPA (▪, ♦) or in medium alone (□, ⋄) and used as targets. Cytotoxic T cells were directed by addition of anti-CD19-Fab-SEAm fusion protein or C215-Fab-SEAm control fusion protein. Results are the mean ± SD. Each value is the mean of triplicate samples. The E:T ratio was 40:1, and specific cytotoxicity was measured in a 51Cr-release assay.
A dose-dependent response to anti-CD19-Fab-SEAm was obvious, with significant lysis already at 0.5 ng/mL of the anti-CD19 fusion protein against nonactivated and TPA-activated cells.
Impact of effector to target cell ratio in anti-CD19-Fab-SEAm–mediated killing of B-CLL cells.The impact of the E:T ratio was studied in cytotoxicity assays with four different B-CLL targets. The anti-CD19-Fab-SEAm fusion protein mediated significant lysis already at a low E:T ratio of 3.75:1, and maximal lysis was seen at an E:T ratio of 15:1. The irrelevant fusion protein mediated low levels of killing at E:T ratios varying from 3.75:1 to 60:1 (not shown).
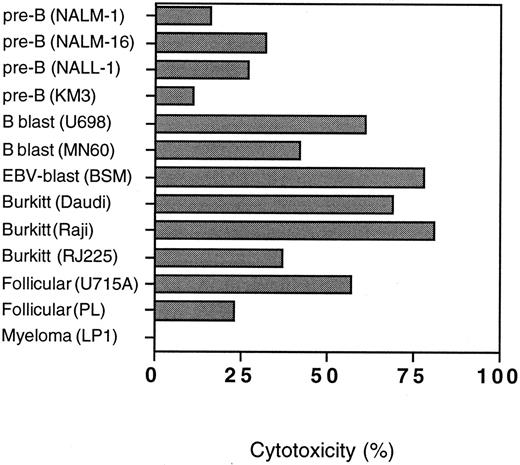
Killing of CD19+ B-cell lines.Thirteen B-cell lines representing different B-cell maturation stages were used as targets in anti-CD19-Fab-SEAm–mediated T-cell killing to evaluate whether lytic sensitivity was restricted to a particular differentiation step. The cell lines were categorized into six different groups: lymphoblasts of pre-B type (NALL-1, NALM-1, NALM-16, and KM3), lymphoblasts of B type (U698 and MN60), EBV-transformed lymphoblasts (BSM), Burkitt's lymphomas (Daudi, Raji, and RJ225), follicle center cell lymphomas (U715A, and PL), and myeloma (LP1). All cell lines were sensitive to anti-CD19-Fab-SEAm–directed T-cell killing, except for the CD19− myeloma cell line LP1. There was an anti-CD19-Fab-SEAm dose-dependent response against all sensitive targets (not shown). The control fusion protein mediated negligible levels of cytotoxicity at 0.5 ng/mL, but at high concentrations (5 ng/mL) some background was seen for cell lines expressing high amounts of HLA-DR and ICAM-1 (not shown). The specific cytotoxicity at an anti-CD19-Fab-SEAm protein concentration of 0.5 ng/mL is shown in Fig 5.
Anti-CD19-Fab-SEAm–directed lysis of 13 different B-cell lines. SAg concentration was 0.5 ng/mL and E:T ratio 40:1. Results are shown as specific cytotoxicity with the anti-CD19-Fab-SEAm fusion protein (minus cytotoxicity with C215-Fab-SEAm control fusion protein) as measured in a 51Cr-release assay. Each value is the mean of triplicate samples.
Anti-CD19-Fab-SEAm–directed lysis of 13 different B-cell lines. SAg concentration was 0.5 ng/mL and E:T ratio 40:1. Results are shown as specific cytotoxicity with the anti-CD19-Fab-SEAm fusion protein (minus cytotoxicity with C215-Fab-SEAm control fusion protein) as measured in a 51Cr-release assay. Each value is the mean of triplicate samples.
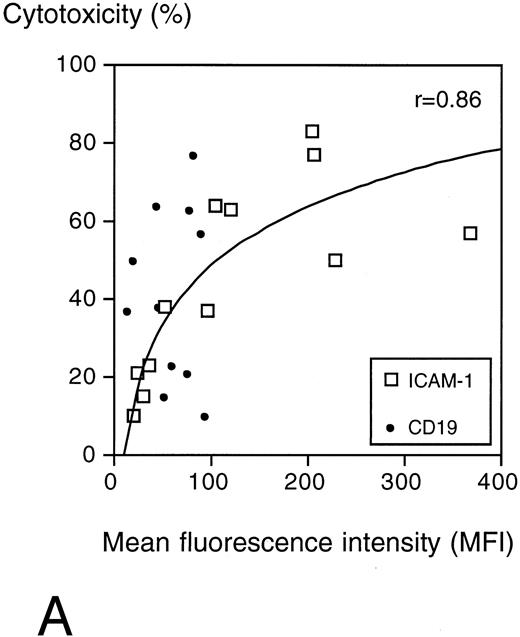
The specific cytotoxicity correlated with the surface expression of ICAM-1 (r = .86) but not with CD19 expression, which was low on all target cells (Fig 6A).
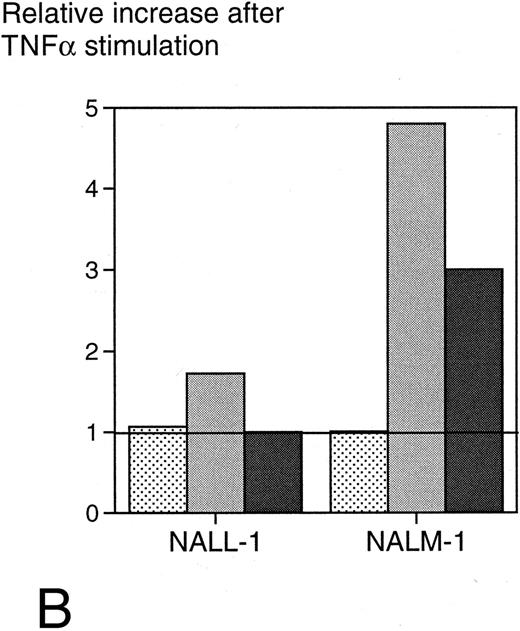
(A) Surface ICAM-1 and CD19 expression of 12 different CD19+ B-cell lines (expressed as MFI) correlated with their sensitivity to anti-CD19-Fab-SEAm plus T cells. A logarithmic curve fit is shown for the ICAM-1/cytotoxicity correlation. (B) Effect of TNFα treatment of 2 pre-B-cell lines. Cell surface CD19 and ICAM-1 expression and sensitivity to anti-CD19-Fab-SEAm–mediated T-cell lysis (fusion protein concentration 0.5 ng/mL) is shown. The pre-B-cell lines (NALL-1 and NALM-1) were stimulated with TNFα (1,000 U/mL for 40 hours). Surface antigen expression and sensitivity of nonstimulated cells is presented as 1.0 (horizontal bar) to allow comparison to stimulated cells. Each value is the mean of triplicate samples. ▧, CD19 expression; , ICAM-1 expression; ▪, sensitivity to lysis.
(A) Surface ICAM-1 and CD19 expression of 12 different CD19+ B-cell lines (expressed as MFI) correlated with their sensitivity to anti-CD19-Fab-SEAm plus T cells. A logarithmic curve fit is shown for the ICAM-1/cytotoxicity correlation. (B) Effect of TNFα treatment of 2 pre-B-cell lines. Cell surface CD19 and ICAM-1 expression and sensitivity to anti-CD19-Fab-SEAm–mediated T-cell lysis (fusion protein concentration 0.5 ng/mL) is shown. The pre-B-cell lines (NALL-1 and NALM-1) were stimulated with TNFα (1,000 U/mL for 40 hours). Surface antigen expression and sensitivity of nonstimulated cells is presented as 1.0 (horizontal bar) to allow comparison to stimulated cells. Each value is the mean of triplicate samples. ▧, CD19 expression; , ICAM-1 expression; ▪, sensitivity to lysis.
Since surface expression of ICAM-1 seemed to be of major importance for target cell sensitivity to SAg-mediated T-cell cytotoxicity, we wanted to evaluate whether upregulation of ICAM-1 expression could confer increased sensitivity to lysis. For this purpose, we treated target cell lines with TNFα, which has been reported to upregulate ICAM-1 on several cell types. The pre-B-cell lines NALL-1 and NALM-1, which exhibit low ICAM-1 expression (mean fluorescence intensity [MFI], 97 and 25, respectively) and moderate to low sensitivity (30% and 16% specific cytotoxicity) to anti-CD19-Fab-SEAm and T cells, were stimulated with TNFα28 (1,000 U/mL) for 40 hours and then analyzed for surface ICAM-1 expression. Stimulation resulted in a 1.7- and 4.8-fold increase, respectively (MFI, 167 and 120), in surface ICAM-1 expression, but did not alter CD19 expression. Target cell sensitivity increased moderately for NALL-1 (from 30% to 36%) but drastically for NALM-1 (from 16% to 49%) (Fig 6B). This was not due to an increased fragility of TNF-exposed targets, since the control fusion protein mediated minimal lysis of these cells (0% to 14%, not shown).
Anti-CD19-Fab-SEAm–directed T-cell lysis of B-NHL biopsy cells.Freeze-thawed cells originally prepared from diagnostic lymph nodes obtained from 12 patients with various B-cell lymphomas showed poor uptake of 51Cr in a pilot study. Further, these lymph node preparations consisted of a mixture of clonal B cells and normal T cells. These facts excluded the use of the 51Cr-release assay, and instead we developed a method based on fluorescence-labeled B cells. This flow cytometric method estimated living versus dead target B cells after a 4-hour incubation with effector cells and fusion proteins, and enabled us to investigate the sensitivity of malignant lymph node B cells to anti-CD19-Fab-SEAm–directed cytotoxicity.
All B-NHL targets were sensitive to T cells and anti-CD19-Fab-SEAm compared with the control fusion protein. Twenty-five percent to 80% specific cytotoxicity against malignant B cells was seen (Fig 7) after 4 hours of incubation with effector cells plus anti-CD19-Fab-SEAm fusion protein (0.5 ng/mL).
T-cell plus SAg killing of malignant B cells from 12 patients with B-NHL. Target cell viability was determined by FACS analysis after a 4-hour incubation with anti-CD19-Fab-SEAm plus T cells. SAg concentration was 0.5 ng/mL, and results are shown as % dead cells after incubation with anti-CD19-Fab-SEAm minus % dead cells after incubation with the C215-Fab-SEAm control fusion protein.
T-cell plus SAg killing of malignant B cells from 12 patients with B-NHL. Target cell viability was determined by FACS analysis after a 4-hour incubation with anti-CD19-Fab-SEAm plus T cells. SAg concentration was 0.5 ng/mL, and results are shown as % dead cells after incubation with anti-CD19-Fab-SEAm minus % dead cells after incubation with the C215-Fab-SEAm control fusion protein.
Freshly separated cells from three patients with B-CLL were incubated in medium overnight and used as targets in the FACS-based assay. These fresh leukemic target cells were extremely sensitive to anti-CD19-Fab-SEAm–mediated cytotoxicity, and more than 97% cytotoxicity was seen in each case (data not shown).
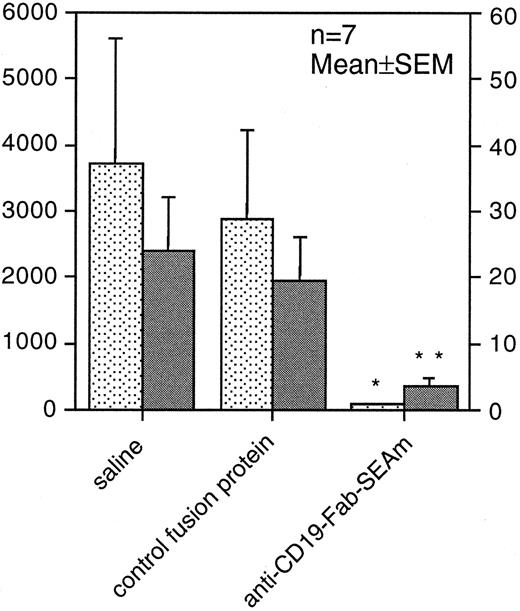
Anti-CD19-Fab-SEAm–based tumor therapy in SCID mice.To evaluate the therapeutic efficacy of the anti-CD19-Fab-SEAm fusion protein in vivo against human B-lymphoma cells, we used a humanized SCID model. SCID mice were injected IP with 3 × 105 Daudi lymphoma cells and were injected 5 days later IP with 3 × 105 human blood mononuclear cells. Anti-CD19-Fab-SEAm therapy was given as four daily intravenous treatments (100 μg per injection) from days 5 to 8. Control animals received PBS or an irrelevant control fusion protein. At day 40, the animals were killed and the number of tumors and the total tumor weight IP estimated. A significant antitumor effect was seen in the anti-CD19-Fab-SEAm–treated animals compared with control animals receiving effector cells only or effector cells combined with the irrelevant Fab-SEAm fusion protein. Anti-CD19-Fab-SEAm treatment resulted in greater than 90% reduction of the total tumor weight (P < .05) and a drastic decrease in the number of macroscopically detectable tumors (P < .01) (Fig 8).
Fab-SAg treatment of humanized SCID mice carrying B-lymphoma cells reduces tumor growth. SCID mice (7 animals per group) were injected IP with 3 × 105 Daudi B-lymphoma cells. Five days later, mice were injected IP with 3 × 105 normal human peripheral blood mononuclear cells. On days 5 to 8, mice were treated with daily intravenous injections (100 μg per injection) of anti-CD19-Fab-SEAm. Control animals received saline (PBS) or C215-Fab-SEAm control fusion protein, which does not bind to B-lymphoma cells. At day 40, the animals were killed and the (▪) number of tumors and (▧) total tumor weight (mg) were determined IP. Statistical evaluation was performed using the Mann-Whitney U test, where fusion protein–treated animals were compared with PBS-treated animals (**P < .01, *P < .05). One of 2 experiments producing similar results is shown as the mean ± SEM.
Fab-SAg treatment of humanized SCID mice carrying B-lymphoma cells reduces tumor growth. SCID mice (7 animals per group) were injected IP with 3 × 105 Daudi B-lymphoma cells. Five days later, mice were injected IP with 3 × 105 normal human peripheral blood mononuclear cells. On days 5 to 8, mice were treated with daily intravenous injections (100 μg per injection) of anti-CD19-Fab-SEAm. Control animals received saline (PBS) or C215-Fab-SEAm control fusion protein, which does not bind to B-lymphoma cells. At day 40, the animals were killed and the (▪) number of tumors and (▧) total tumor weight (mg) were determined IP. Statistical evaluation was performed using the Mann-Whitney U test, where fusion protein–treated animals were compared with PBS-treated animals (**P < .01, *P < .05). One of 2 experiments producing similar results is shown as the mean ± SEM.
DISCUSSION
In the present study, we extended our previous results with SAg-mediated T-cell lysis of B-CLL cells19,20 to construct a direct SAg-antibody fusion protein between an anti-CD19-Fab and a SEAm. The choice of a mAb directed against CD19 appeared justified, since CD19 is considered a strictly B-lineage–specific antigen that is expressed on the cell surface of the whole B-cell differentiation range starting with the IgH rearrangement up to plasmablasts. This would potentially enable T-cell therapy for a wide range of human B-cell malignancies. The anti-CD19-Fab-SEAm fusion protein showed high-affinity binding in the nanomolar range to the CD19 antigen. The mutated Fab-SEAm protein exhibited a 1,000-fold lower binding affinity for HLA class II compared with nonmutated SAg. This should favor CD19 antigen-specific targeting compared with interactions with HLA-DR+CD19− normal cells including monocytes, dendritic cells, and activated endothelial cells. It is anticipated that higher amounts of the mutated protein as compared with the wild-type protein could be administered in vivo before systemic immune activation and toxic side effects due to HLA-DR binding would occur.23
Our present in vitro studies with B-CLL cells as targets showed that such cells were efficiently lysed by low numbers of T cells and anti-CD19-Fab-SEAm. Lysis was rapid, specific, and mediated by surface CD19, but apparently not by MHC class II molecules. Importantly, with this fusion protein, no preactivation of the target cells was needed to increase their sensitivity, in contrast to our experience using other SAg constructs.19 20 Normal CD19+ B cells were also sensitive for SAg-directed T-cell killing, whereas CD34+ progenitor cells and CD14+ monocytes/macrophages were resistant. In vivo, an anti-CD19-Fab-SEAm protein would potentially eradicate all CD19+ B-lineage cells, including normal counterparts. However, current clinical transplant regimens including high-dose chemoradiotherapy followed by pan-B-cell–purged or, alternatively, CD34+ cell–enriched autografts effectively deplete the B-cell range with surprisingly little effect on posttransplant infection rates. Importantly, sparing of T-cell plus SAg-resistant CD34+ precursor cells should allow full regeneration of the B-cell compartment.
We extended the target cell range to include B-NHL biopsy cells representing several histologies, as well as a full range of established B-cell lines. All CD19+ cells and tumor biopsy cells were sensitive to T-cell/SAg–mediated lysis. The sensitivity of target cells was positively correlated with surface ICAM-1 expression but not with surface CD19 density. CD19 is required for anti-CD19-Fab-SEAm–mediated lysis, because only CD19+ target cells are lysed, but most likely very low levels of CD19 are sufficient for maximal cytotoxicity. This is consistent with the high potency of targeted SEA and recent views that only a few MHC/peptide complexes are required to activate T cells. The target cell surface expression of CD19 was found to be uniform on all cells examined. The expression was low (<100 MFI) compared with that of other B-cell surface markers like CD24 and CD40. CD19 expression did not change when cells were stimulated in vitro. Properties such as antigen shedding or internalization may play a role in antibody-directed killing, and these factors need to be investigated further. A role for ICAM-1 in SAg-induced cytotoxicity was supported by our previous studies using HLA-DR single and HLA-DR/ICAM-1 double transfectants.29 Stimulation of B-cell line target cells with TNFα led to a parallel increase in surface expression of ICAM-1 and target cell sensitivity to T-cell/anti-CD19-Fab-SEAm–mediated lysis. SEA is a potent inducer of TNFα in vitro16 and in vivo,17 suggesting that endogenously produced TNFα may enhance SAg-based lysis of neoplastic B cells in a therapeutic setting.
Freshly separated leukemia cells were extremely sensitive to anti-CD19-Fab-SEAm and T cells. The fact that many of the target cells in this study were obtained from patients displaying multiresistance to cytostatic drugs makes Fab-SEAm–mediated T-cell killing of B-cell tumor cells a potentially attractive adjuvant to conventional debulking therapy. The concept of Fab-SEA–based T-cell immunotherapy for lymphomas/leukemias is attractive also in other aspects: first, leukemic cells are easily accessible for mAb/T-cell targeting compared with solid tumors; second, B-lymphoma tissue frequently contains large numbers of T cells, which could mediate a local cytotoxic response without a requirement of effector cell homing into the tumor tissue; and third, the expression of surface ICAM-1 molecules by the proliferative tumor cell compartment in lymph nodes is higher than in blood,30 31 which might favor eradication of the clonogenic cells.
We have investigated the mechanisms of target cell lysis in T-cell/anti-CD19-Fab-SEAm fusion protein–mediated killing. Preliminary data indicate that lysis in the short-term 4-hour assay seems to be perforin-mediated, since the chelator EGTA, which binds Ca2+ needed for cytotoxic granulae release, totally abolished lysis. The addition of Fas-blocking antibodies did not alter SAg/T-cell lysis, and neither was there any correlation between target cell surface Fas expression and sensitivity for lysis (data not shown). SAg-triggered T cells release large amounts of TNFα, which is known to be toxic to several tumor types. However, we showed previously that high concentrations of TNFα had no adverse effect on B-CLL cells.19 SEA-induced cytotoxic T cells (CTL) appear rapidly in mice treated with a single injection of SEA17 or Fab-SEA.32 However, perforin knockout mice generate only marginal SEA-dependent CTL activity (J. Hansson, M. Dohlsten, unpublished observation, June 1996). Therefore, it remains likely that release of perforin/granzyme-containing granulae is important in SAg-dependent T-cell–mediated lysis of B-lineage tumor cells in vitro.
In vivo studies of the anti-CD19-Fab-SEAm fusion protein were performed in humanized SCID mice with IP growing Daudi cells. The growth of Daudi cells in SCID mice has previously been evaluated by Ghetie et al,33 who found disseminated IP growth, including engagement of the mesenteric lymph nodes. Tumor metastases in the liver and kidney were detected only at late stages. A similar disseminated growth pattern IP was seen after 3 to 4 weeks also in our study. At this time point, the tumor load in our model was mainly confined to the IP cavity. Because IP infusion of human peripheral blood mononuclear cells allows persistence of human lymphocytes in the peritoneal cavity for several weeks but only a marginal presence of these cells extraperitoneally (data not shown), the study setting was focused on evaluating antitumor effects against IP tumor growth. Treatment was initiated 5 days after tumor inoculation. Treated animals showed a dramatic reduction of tumor size and number compared with control animals. Further studies using syngeneic but “humanized” in vivo animal models are under development in our laboratory including hCD19cDNA-transfected murine B-cell leukemia cells and the use of hCD19 transgenic mice.
MoAb-targeted SAgs have been used to direct cytotoxic T cells to other types of human malignant cells in vitro. These studies include the use of MoAbs against CD7 and CD38 for lysis of acute T-lymphoblastic leukemia cell lines,34 anti–ganglioside GD2 human/mouse chimeric MoAb for lysis of human neuroectodermal tumor lines,35 and C242 MoAb against human colon carcinoma.24 Taken together, these results suggest that MoAb-targeted SAg may have a general applicability for a large variety of tumor types. Our present results with anti-CD19-Fab-SEAm argue for a further clinical development of targeted SAgs for T-cell immunotherapy of human B-lymphocyte–lineage malignancies.
ACKNOWLEDGMENT
We thank Professor C. Melief, Leiden, The Netherlands, for the kind gift of the anti-CD19 producer hybridoma. We also thank Dr L. Abrahmsén, Pharmacia Bioscience Centre, Stockholm, Sweden, for help with the construction of fusion proteins, and Dr M. Bengtsson, University Hospital, Uppsala, Sweden, for help with the progenitor cell purification.
Supported by grants from the Swedish Cancer Society, the Lion's Cancer Fund at the University Hospital, and the Leo Research Fund.
Address reprint requests to Thomas H. Tötterman, MD, PhD, Department of Clinical Immunology, University Hospital, S-751 85 Uppsala, Sweden.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal