Abstract
The relative efficacy and toxicity of the chemotherapeutic agents thioguanine (6TG) and etoposide (VP16) were assessed by a randomized comparison of the DAT (daunorubicin, cytarabine, thioguanine) versus ADE (daunorubicin, cytarabine, etoposide) regimens in the Medical Research Council's 10th acute myeloid leukaemia trial (MRC AML 10), which was open to patient entry from May 1988 to April 1995. In this, the largest reported trial of AML therapy to date, 1,857 eligible patients, mostly less than 56 years old, were randomized: 929 (including 143 children under 15 years old) were allocated to DAT and 928 (143 children) to ADE. The two groups were well matched for presentation features. The complete remission (CR) rate was 81% with DAT and 83% with ADE (P = .3). The percentages of remitters achieving remission after 1, 2, or more than 2 courses were 70%, 22%, and 8% for DAT and 74%, 21%, and 5% for ADE. The percentages failing to achieve a CR due to resistant disease were 11% with DAT versus 9% with ADE (P = .07). There was a slightly higher death rate in CR during consolidation chemotherapy with ADE (9%) than with DAT (6%) (P = .06). Patients receiving DAT took slightly but significantly longer to recover from neutropenia and thrombocytopenia but the median number of days in hospital were similar in each group. ADE patients experienced slightly more severe nonhematologic toxicity. There was also no significant difference between the groups in the longer-term measures of efficacy: disease-free survival at 6 years from CR was 42% (±4) for DAT and 43% (±4) for ADE (P = .8); relapse rate at 6 years was 50% (±4) for DAT and 49% (±5) for ADE (P = .6); survival at 6 years was 40% (±4) for both DAT and ADE (P = .9). Subgroup analysis failed to show any benefit for etoposide in patients with monocytic or myelomonocytic disease, or in any other diagnostic subgroup. In conclusion, DAT and ADE both achieve high remission rates and good long-term survival, and are equally effective chemotherapy regimens for the treatment of AML patients aged up to 55 years.
ALTHOUGH a substantial minority of patients with acute myeloid leukemia (AML) achieve complete remission (CR) and then appear to be cured, the majority are still not cured. Even in children or young adults about 10% to 20% have resistant disease and never achieve CR, another 5% to 10% die from infection or hemorrhage during aplasia, and even after remission has been achieved at least half (or more, at older ages) relapse and die. Therefore, trials are needed of treatments that might avoid primary resistance, limit early toxicity, and/or prevent relapse. In particular, toxicity has usually been assessed only by retrospective analysis of small groups of patients,1 but both toxicity and efficacy should be evaluated in large prospective trials.
It has been hypothesized2 that failure of therapy was often due to the selection and growth of drug resistant clones arising by spontaneous mutation. Hence, studies were undertaken to evaluate the delivery of multiple drugs simultaneously, the addition of high-dose cytarabine, and the introduction of newer drugs such as etoposide to induction regimens.3,4 Perhaps partly because of the small size of these studies, however, they produced no clear improvements in the rate of remission induction or survival. In many countries, therefore, the standard induction therapy remains daunorubicin and cytarabine. However, in the United Kingdom Medical Research Council (MRC) trials, the standard chemotherapy induction regimen has been DAT (daunorubicin, cytarabine, thioguanine).5 Thioguanine is a purine analog that leads to inhibition of DNA synthesis and accumulation of DNA. It also inhibits de novo purine biosynthesis by enzyme inhibition and may be associated with myelotoxicity and liver dysfunction.6 Etoposide acts in a different way. Its intracellular target is topoisomerase II and the drug stabilizes the clearable complex formed between this compound and DNA, leading to DNA strand breaks and, eventually, cell death.7,8 Etoposide has been claimed to be particularly effective in monocytic and myelomonocytic varieties of AML.9 Therefore, one aim of the MRC AML10 study was to compare the relative efficacy and toxicity of thioguanine with etoposide in the induction chemotherapy regimen in patients with AML aged less than 56 years old. The results are reported here.
MATERIALS AND METHODS
Patients.Between May 1988 and April 1995, a total of 1,966 patients was entered into the MRC AML 10 trial from 163 centers in the United Kingdom, Republic of Ireland, and New Zealand (Table 1), and 1,857 are evaluable. The trial was primarily designed for patients aged up to 55 years, though older patients could be entered if they were considered suitable for the intensive AML10 therapy. Based on UK incidence figures, recruitment to the trial represents about 40% of adults aged 15 to 55 and 75% of children (age 0 to 14) with AML in these countries.
Patient Population in MRC AML10
| No. entered | 1,966 |
| Not randomized DAT v ADE | 92 (18 adult, 74 children) |
| Randomized DAT v ADE | 1,874 |
| Ineligible | 17 (15 ALL, 1 lymphoma, |
| 1 malignant histocytosis) | |
| Evaluable | 1,857 (929 DAT, 928 ADE) |
| No. entered | 1,966 |
| Not randomized DAT v ADE | 92 (18 adult, 74 children) |
| Randomized DAT v ADE | 1,874 |
| Ineligible | 17 (15 ALL, 1 lymphoma, |
| 1 malignant histocytosis) | |
| Evaluable | 1,857 (929 DAT, 928 ADE) |
In addition to patients with AML, children with aggressive myelodysplasia (refractory anemia with excess blasts in transformation [RAEB-t]) were also eligible if AML type therapy was deemed appropriate. Secondary AML cases, either following prior cytotoxic chemotherapy or radiotherapy for other tumors or subsequent to a preceding hematologic disorder, were also eligible for entry.
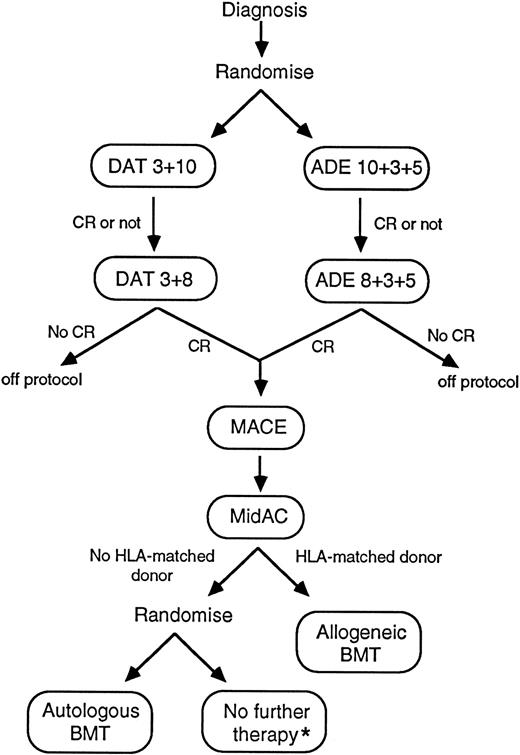
MRC-AML10: Protocol flow chart. *Autologous BMT reserved for second remission therapy if relapse occurred. DAT 3 + 10: Daunorubicin 50 mg/m2 slow intravenous (IV) push days 1, 3, 5; cytarabine 100 mg/m2 12-hourly IV push days 1 through 10; 6-thioguanine 100 mg/m2 12-hourly orally days 1 through 10. ADE 10 + 3 + 5: Daunorubicin 50 mg/m2 slow IV push days 1, 3, 5; cytarabine 100 mg/m2 12-hourly IV push days 1 through 10; etoposide (VP-16) 100 mg/m2 IV (1-hour infusion) days 1 through 5. DAT 3 + 8: As DAT 3 + 10 but cytarabine and 6-thioguanine days 1 through 8 only. ADE 8 + 3+ 5: As ADE 10 + 3 + 5 but cytarabine days 1 through 8 only. MACE: Amsacrine (m-amsa) 100 mg/m2 IV (1-hour infusion) days 1 through 5; cytarabine 200 mg/m2 IV (continuous infusion) days 1 through 5; etoposide 100 mg/m2 IV (1-hour infusion) days 1 through 5. MidAC: Mitozantrone 10 mg/m2 IV (short infusion) days 1 through 5; cytarabine 1.0 g/m2 IV (short infusion) days 1 through 5. NB: All doses were reduced by 25% for children less than 1 year old.
MRC-AML10: Protocol flow chart. *Autologous BMT reserved for second remission therapy if relapse occurred. DAT 3 + 10: Daunorubicin 50 mg/m2 slow intravenous (IV) push days 1, 3, 5; cytarabine 100 mg/m2 12-hourly IV push days 1 through 10; 6-thioguanine 100 mg/m2 12-hourly orally days 1 through 10. ADE 10 + 3 + 5: Daunorubicin 50 mg/m2 slow IV push days 1, 3, 5; cytarabine 100 mg/m2 12-hourly IV push days 1 through 10; etoposide (VP-16) 100 mg/m2 IV (1-hour infusion) days 1 through 5. DAT 3 + 8: As DAT 3 + 10 but cytarabine and 6-thioguanine days 1 through 8 only. ADE 8 + 3+ 5: As ADE 10 + 3 + 5 but cytarabine days 1 through 8 only. MACE: Amsacrine (m-amsa) 100 mg/m2 IV (1-hour infusion) days 1 through 5; cytarabine 200 mg/m2 IV (continuous infusion) days 1 through 5; etoposide 100 mg/m2 IV (1-hour infusion) days 1 through 5. MidAC: Mitozantrone 10 mg/m2 IV (short infusion) days 1 through 5; cytarabine 1.0 g/m2 IV (short infusion) days 1 through 5. NB: All doses were reduced by 25% for children less than 1 year old.
Presentation Features of Patients in MRC AML10
| Parameter . | Value . | No. of Patients . | Percent of Patients* . | |
|---|---|---|---|---|
| . | . | DAT . | ADE . | . |
| Age: | 0-1 | 27 | 25 | 3 |
| 2-14 | 116 | 118 | 13 | |
| 15-24 | 131 | 128 | 14 | |
| 25-34 | 147 | 149 | 16 | |
| 35-44 | 224 | 226 | 24 | |
| 45-55 | 280 | 278 | 30 | |
| 56+ | 4 | 4 | <1 | |
| Sex: | Male | 475 | 471 | 51 |
| Female | 454 | 457 | 49 | |
| Type of AML: | De novo | 863 | 859 | 93 |
| Secondary | 66 | 69 | 7 | |
| White blood cell count: (×109/L) | 0-9 | 417 | 412 | 45 |
| 10-99 | 366 | 385 | 40 | |
| 100-199 | 84 | 72 | 8 | |
| 200+ | 31 | 38 | 4 | |
| Unknown | 31 | 21 | 3 | |
| FAB type: | M0 | 16 | 12 | 2 |
| M1 | 136 | 172 | 17 | |
| M2 | 254 | 253 | 27 | |
| M3 | 143 | 130 | 15 | |
| M4 | 184 | 184 | 20 | |
| M5 | 79 | 81 | 9 | |
| M6 | 28 | 24 | 3 | |
| M7 | 24 | 18 | 2 | |
| RAEB-t | 13 | 17 | 2 | |
| Bilineage | 2 | 0 | <1 | |
| ALL | 2 | 6 | <1 | |
| Unknown | 48 | 31 | 4 | |
| Performance status: | Asymptomatic | 250 | 245 | 27 |
| Minimal symptoms | 485 | 489 | 52 | |
| Ill | 172 | 173 | 19 | |
| Very ill | 22 | 21 | 2 | |
| Cytogenetic group: | Favorable | 156 | 165 | 17 |
| Intermediate | 466 | 488 | 51 | |
| Adverse | 53 | 56 | 6 | |
| Unknown | 232 | 241 | 25 | |
| Parameter . | Value . | No. of Patients . | Percent of Patients* . | |
|---|---|---|---|---|
| . | . | DAT . | ADE . | . |
| Age: | 0-1 | 27 | 25 | 3 |
| 2-14 | 116 | 118 | 13 | |
| 15-24 | 131 | 128 | 14 | |
| 25-34 | 147 | 149 | 16 | |
| 35-44 | 224 | 226 | 24 | |
| 45-55 | 280 | 278 | 30 | |
| 56+ | 4 | 4 | <1 | |
| Sex: | Male | 475 | 471 | 51 |
| Female | 454 | 457 | 49 | |
| Type of AML: | De novo | 863 | 859 | 93 |
| Secondary | 66 | 69 | 7 | |
| White blood cell count: (×109/L) | 0-9 | 417 | 412 | 45 |
| 10-99 | 366 | 385 | 40 | |
| 100-199 | 84 | 72 | 8 | |
| 200+ | 31 | 38 | 4 | |
| Unknown | 31 | 21 | 3 | |
| FAB type: | M0 | 16 | 12 | 2 |
| M1 | 136 | 172 | 17 | |
| M2 | 254 | 253 | 27 | |
| M3 | 143 | 130 | 15 | |
| M4 | 184 | 184 | 20 | |
| M5 | 79 | 81 | 9 | |
| M6 | 28 | 24 | 3 | |
| M7 | 24 | 18 | 2 | |
| RAEB-t | 13 | 17 | 2 | |
| Bilineage | 2 | 0 | <1 | |
| ALL | 2 | 6 | <1 | |
| Unknown | 48 | 31 | 4 | |
| Performance status: | Asymptomatic | 250 | 245 | 27 |
| Minimal symptoms | 485 | 489 | 52 | |
| Ill | 172 | 173 | 19 | |
| Very ill | 22 | 21 | 2 | |
| Cytogenetic group: | Favorable | 156 | 165 | 17 |
| Intermediate | 466 | 488 | 51 | |
| Adverse | 53 | 56 | 6 | |
| Unknown | 232 | 241 | 25 | |
Percentages may not add to 100 because of rounding.
The trial had two randomizations (Fig 1); the first was between daunorubicin, cytarabine, and thioguanine (DAT) and daunorubicin, cytarabine, and etoposide (ADE) as induction regimens and the second, after the completion of a total of four courses of chemotherapy (drug dosages shown in the figure legend), compared high-dose therapy with autologous bone marrow transplant rescue (A-BMT) versus no further therapy. Patients who had an HLA-matched sibling donor were scheduled for an allogeneic bone marrow transplant after they had completed four courses of chemotherapy, and were not randomized with respect to A-BMT versus stop. The results of the second randomization will be reported separately.
Seventeen patients have been excluded as ineligible because of misdiagnoses that were reported to the trial office before therapy had commenced (Table 1). Eight patients who were rediagnosed as having acute lymphoblastic leukemia (ALL) after therapy had been started are, however, included in the analysis. Thus, this report deals with the outcome of 1,857 eligible patients, 929 of whom were allocated to DAT and 928 who were allocated to ADE.
Treatment.The schema of the MRC AML 10 trial is shown in Fig 1. Patients were randomized to receive “double induction” with either two courses of DAT or two courses of ADE (two courses were given even if remission was achieved after one course). If remission was achieved, two further consolidation courses (one MACE and one MidAC) were scheduled. In addition, in children, triple intrathecal therapy with methotrexate, cytarabine, and hydrocortisone was given as part of each course to a total of five doses. If CR was not achieved after two courses of DAT or ADE, and a further attempt to obtain remission was considered appropriate, clinicians could either continue with courses 3 and 4 as per protocol or could give alternative therapy. After completing protocol chemotherapy, it was recommended that patients with an HLA-matched sibling donor should then proceed to allogeneic transplant (allo-BMT) while the remainder should be randomized between either A-BMT or stopping treatment (with A-BMT reserved for second remission should relapse occur and the patient be successfully re-induced).
Remission Outcome by DAT Versus ADE
| . | Percent of Patients3-150 . | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | CR . | Induction Death . | Resistant Disease . | . | . | . | . | . | . | ||||||
| . | DAT . | ADE . | Total . | DAT . | ADE . | Total . | DAT . | ADE . | Total . | . | . | . | . | . | . |
| All patients | 81 | 83 | 82 | 8 | 9 | 9 | 11 | 9 | 10 | ||||||
| Age: | |||||||||||||||
| 0-14 | 89 | 93 | 91 | 6 | 3 | 5 | 6 | 3 | 5 | ||||||
| 15-24 | 83 | 88 | 85 | 9 | 5 | 7 | 8 | 7 | 7 | ||||||
| 25-34 | 81 | 90 | 85 | 9 | 5 | 7 | 10 | 5 | 7 | ||||||
| 35-44 | 81 | 77 | 79 | 7 | 11 | 9 | 12 | 13 | 12 | ||||||
| 45+ | 75 | 76 | 76 | 11 | 13 | 12 | 14 | 11 | 13 | ||||||
| . | Percent of Patients3-150 . | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | CR . | Induction Death . | Resistant Disease . | . | . | . | . | . | . | ||||||
| . | DAT . | ADE . | Total . | DAT . | ADE . | Total . | DAT . | ADE . | Total . | . | . | . | . | . | . |
| All patients | 81 | 83 | 82 | 8 | 9 | 9 | 11 | 9 | 10 | ||||||
| Age: | |||||||||||||||
| 0-14 | 89 | 93 | 91 | 6 | 3 | 5 | 6 | 3 | 5 | ||||||
| 15-24 | 83 | 88 | 85 | 9 | 5 | 7 | 8 | 7 | 7 | ||||||
| 25-34 | 81 | 90 | 85 | 9 | 5 | 7 | 10 | 5 | 7 | ||||||
| 35-44 | 81 | 77 | 79 | 7 | 11 | 9 | 12 | 13 | 12 | ||||||
| 45+ | 75 | 76 | 76 | 11 | 13 | 12 | 14 | 11 | 13 | ||||||
Percentages may not add to 100 because of rounding.
Deaths in CR During Consolidation by DAT Versus ADE
| . | Allocated Treatment . | ||
|---|---|---|---|
| . | DAT . | ADE . | |
| No. of patients in CR | 752 | 770 | |
| Deaths (% dying) in CR | 46 (6%) | 67 (9%) | P = .06 |
| No. dying after course: | |||
| 1 | 2 | 4 | |
| 2 | 5 | 10 | |
| 3 | 19 | 29 | |
| 4 | 17 | 21 | |
| Off therapy | 3 | 3 | |
| Cause of death: | |||
| Infection | 31 | 44 | |
| Hemorrhage | 3 | 11 | |
| Cardiac failure | 8 | 4 | |
| Other | 2 | 7 | |
| Unknown | 2 | 1 | |
| . | Allocated Treatment . | ||
|---|---|---|---|
| . | DAT . | ADE . | |
| No. of patients in CR | 752 | 770 | |
| Deaths (% dying) in CR | 46 (6%) | 67 (9%) | P = .06 |
| No. dying after course: | |||
| 1 | 2 | 4 | |
| 2 | 5 | 10 | |
| 3 | 19 | 29 | |
| 4 | 17 | 21 | |
| Off therapy | 3 | 3 | |
| Cause of death: | |||
| Infection | 31 | 44 | |
| Hemorrhage | 3 | 11 | |
| Cardiac failure | 8 | 4 | |
| Other | 2 | 7 | |
| Unknown | 2 | 1 | |
Definitions of endpoints.A normocellular bone marrow aspirate containing less than 5% leukemia blast cells and showing evidence of normal maturation of other marrow elements was the criterion for the achievement of CR. The persistence of myelodysplastic features did not exclude the diagnosis of CR. Remission failures were classified by the referring clinician as due either to induction death, ie, related to treatment and/or hypoplasia, or as resistant disease, ie, related to the failure of therapy to eliminate the disease (including partial remissions with 5% to 15% blasts). Where the clinician's evaluation was not available, deaths within 30 days of entry were classified as induction deaths and deaths at more than 30 days as resistant disease.
The following definitions are also used: overall survival is the time from entry to death; for remitters, disease-free survival (DFS) is the time from CR to first event (either relapse or death in CR); and, for remitters, the relapse risk is the cumulative probability of relapse, ignoring (ie, censoring) at death in CR.
Statistical methods.Randomizations were balanced by minimization. Remission rates and reasons for failure to achieve CR were compared using standard chi-squared tests. Kaplan-Meier life-tables were constructed for survival data and were compared by means of the log-rank test, with surviving patients being censored at September 1, 1995, when follow-up was up-to-date for all but 25 patients (the small number of patients lost to follow-up are censored at the date they were last known to be alive). All P values are two-tailed. Figures in parentheses after point estimates are 95% confidence intervals.
RESULTS
Patient characteristics.The presenting features of the patient population are given in Table 2. There were no statistically significant differences in the distribution of patients by age, gender, secondary leukemia, white blood cell count at diagnosis, French-American-British (FAB) type, performance status, and cytogenetic group between the two treatment groups. Of the 135 cases of secondary leukemia, 21 involved prior chemotherapy and/or radiotherapy for solid tumors, 79 preceding myelodysplasia, 1 prior ALL, 8 prior Hodgkin's disease, 2 prior chronic myeloid leukemia, 2 prior lymphoma, and 20 prior other hematologic disorders. FAB type10 11 was reviewed centrally in 64% of patients, the referring center classification being used in the remaining patients. Two percent of patients (7% of children and 1% of adults) had evidence of central nervous system disease at presentation.
Compliance with treatment allocation.Details of the induction therapy that was actually given are available for all but 51 patients (97% of the total). Compliance with allocated therapy was excellent, with 98% of patients in both arms receiving DAT or ADE as allocated. Of the noncompliers, 15 patients (8 DAT, 7 ADE) received no therapy, 3 patients allocated to DAT received ADE, 5 patients allocated to ADE received DAT, and 13 patients (8 DAT, 5 ADE) received alternative therapy. Among the 1,619 patients known to have been given a second course of therapy, 96% received DAT or ADE as originally allocated. Among the noncompliers, 7 patients received ADE instead of DAT, 7 received DAT instead of ADE, and 43 (23 DAT, 20 ADE) received other therapy. The latter group consisted mainly of patients who showed no response to their first course and were taken off protocol. All analyses are, however, by allocated treatment (ie, are “intention-to-treat”), so lack of compliance cannot affect the treatment group that a patient is in.
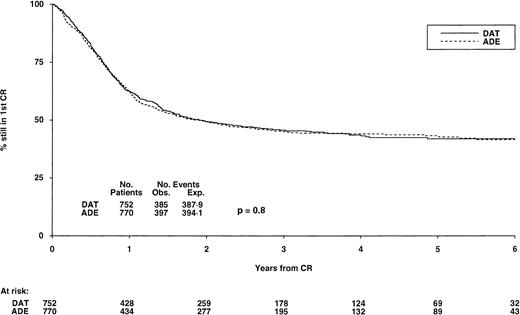
DFS from CR by induction treatment. At 6 years it is 42% (±4) for both DAT and ADE, P = .8. Under number of events, Obs. is the number observed in each arm, Exp. is the number expected (from log-rank analysis).
DFS from CR by induction treatment. At 6 years it is 42% (±4) for both DAT and ADE, P = .8. Under number of events, Obs. is the number observed in each arm, Exp. is the number expected (from log-rank analysis).
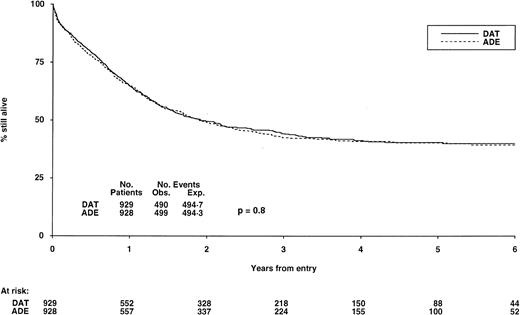
Survival from randomization by indution treatment. At 6 years it is 40% (±4) for DAT and 39% (±4) for ADE, P = .8. See legend to Fig 2 for explanation of Obs. and Exp. events.
Survival from randomization by indution treatment. At 6 years it is 40% (±4) for DAT and 39% (±4) for ADE, P = .8. See legend to Fig 2 for explanation of Obs. and Exp. events.
Remission rate.The overall CR rate was 82%, and it was 91% for children, 85% for adults aged 15 to 34, and 77% for adults aged 35+ (Table 3). The induction death rate was 9% overall, increasing from 5% in children to 12% in the over 45-year age group. Overall, 10% of patients had resistant disease and this also increased with age from 7% in children to 15% at age 45+. There was no significant difference in CR rate between those allocated DAT (81%) and those allocated ADE (83%, P = .3), nor was there any difference in the number of courses required to achieve CR. Seventy percent of those remitting with DAT achieved CR after one course versus 74% with ADE, a further 22% remitted on DAT after course two versus 21% with ADE and a third (or, in a few cases, fourth) course achieved a CR in a further 8% with DAT and 5% with ADE. There was slightly more resistant disease with DAT (11%) than with ADE (9%, P = .07), while 8% of DAT patients and 9% of ADE patients suffered induction death (P = .9).
Deaths in CR.There was a slightly higher death rate in CR during consolidation therapy following ADE (9%) compared with DAT (6%) (P = .06) (Table 4). This excess was not related to any particular course of therapy. Infection and hemorrhage were the main causes of death in CR, but both neutrophil and platelet recovery were both slightly quicker in the ADE arm (see below). Nor were there any differences between the two arms in nonhematologic toxicity after courses 3 and 4.
There were 231 allogeneic and 212 autologous BMTs performed in first CR. Procedural mortality did not differ following DAT or ADE induction: after allo-BMT it was 22% (26 of 117) with DAT and 22% (25 of 114) with ADE; after A-BMT, it was 14% (15 of 106) with DAT and 11% (12 of 106) with ADE.
DFS, relapses, and survival.Among those who achieved CR, DFS at 6 years was 42% (±4) for patients allocated DAT and 43% (±4) for those allocated ADE (P = .8) (Fig 2). If deaths in first CR are ignored, the relapse risk in the two groups was also very similar at 50% (±5) for DAT and 49% (±4) for ADE at 6 years (P = .6). Finally, overall survival from entry for all patients in the two groups was identical at 40% (±4) at 6 years for both DAT and ADE (P = .9) (Fig 3).
Relative efficacy of induction schedules in different patient subsets. Analysis of survival by age group (Table 5) or by FAB subtype (Table 6) showed no differences between thioguanine and etoposide in any subset (none of the 27 comparisons in Table 6 is significant). Mortality in the two treatment groups was also analyzed with regard to four other features at presentation and, again, no significant influence of gender, performance status, cytogenetic group, or white blood cell count was found. Patients with secondary AML fared worse than those with de novo disease (CR rates: 67% v 83%, P < .0001; DFS at 5 years: 31% v 44%, P = .001; overall survival at 5 years: 25% v 43%, P < .0001). There was no difference in CR rates between DAT (68%) and ADE (67%) in patients with secondary AML, but DFS at 5 years was somewhat better with ADE (41% v 25%, P = .04), as was overall survival (36% v 17%, P = .08), but these subgroup analyses should be interpreted with great caution (see Discussion).
Comparison of Survival in Different Age Groups by DAT Versus ADE
| Age (yr) . | Survival at 5 yr (%) . | |
|---|---|---|
| . | DAT . | ADE . |
| 0-14 | 60 | 53 |
| 15-24 | 47 | 46 |
| 25-34 | 52 | 46 |
| 34-44 | 33 | 33 |
| 45+ | 26 | 34 |
| All ages | 41 | 41 |
| Age (yr) . | Survival at 5 yr (%) . | |
|---|---|---|
| . | DAT . | ADE . |
| 0-14 | 60 | 53 |
| 15-24 | 47 | 46 |
| 25-34 | 52 | 46 |
| 34-44 | 33 | 33 |
| 45+ | 26 | 34 |
| All ages | 41 | 41 |
Test for heterogeneity (5 groups): χ24 = 3.4; P = .5. Test for trend: χ21 = 1.1; P = .3.
Relative Efficacy of Thioguanine and Etoposide in Different Morphologic Subclasses of AML
| Endpoint . | Allocated Treatment . | FAB Type . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | M0 . | M1 . | M2 . | M3 . | M4 . | M5 . | M6 . | M7 . | Other/Unknown . |
| CR rate (%) | TG | 63 | 76 | 85 | 85 | 79 | 84 | 89 | 70 | 69 |
| VP | 50 | 80 | 89 | 80 | 84 | 88 | 84 | 67 | 75 | |
| 5-yr DFS | TG | 27 | 38 | 40 | 52 | 47 | 40 | 34 | 16 | 27 |
| (from CR) | VP | 53 | 31 | 45 | 63 | 40 | 46 | 31 | 52 | 35 |
| 5-yr survival | TG | 16 | 34 | 41 | 59 | 39 | 33 | 44 | 23 | 27 |
| (all patients) | VP | 24 | 33 | 44 | 58 | 37 | 43 | 23 | 32 | 28 |
| Endpoint . | Allocated Treatment . | FAB Type . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | M0 . | M1 . | M2 . | M3 . | M4 . | M5 . | M6 . | M7 . | Other/Unknown . |
| CR rate (%) | TG | 63 | 76 | 85 | 85 | 79 | 84 | 89 | 70 | 69 |
| VP | 50 | 80 | 89 | 80 | 84 | 88 | 84 | 67 | 75 | |
| 5-yr DFS | TG | 27 | 38 | 40 | 52 | 47 | 40 | 34 | 16 | 27 |
| (from CR) | VP | 53 | 31 | 45 | 63 | 40 | 46 | 31 | 52 | 35 |
| 5-yr survival | TG | 16 | 34 | 41 | 59 | 39 | 33 | 44 | 23 | 27 |
| (all patients) | VP | 24 | 33 | 44 | 58 | 37 | 43 | 23 | 32 | 28 |
Tests for heterogeneity: CR rate: χ28 = 4.8, P = .8; DFS: χ28 = 7.5, P = .5; Survival: χ28 = 3.9, P = .9. Tests for interactions M4/M5 v M0/M1/M2/M3/M6/M7: CR rate: χ21 = 0.7, P = .4; DFS: χ21 = 0.3, P = .6; Survival: χ21 = 0.1, P = .7.
Nonfatal toxicity.Hematologic toxicity monitoring (Table 7) showed a slight but significant delay, of 1 or 2 days, in recovery of neutrophils and platelets after DAT compared with ADE, and this difference was also carried over to course 3. Conversely, ADE was associated with more, but not excessive, nonhematologic toxicity after course 1, eg, nausea (P = .01), alopecia (P < .0001), mucositis (P = .002), and diarrhea (P = .008), though not after course 2 where the only significant difference was for alopecia (P = .0001). Median hospital inpatient stay was the same for DAT and ADE at 27, 22, 23, and 23 days after courses 1 to 4, respectively.
Neutrophil and Platelet Recovery by Allocated Treatment
| After Course . | No. of Patients . | Median Days* to Neutrophils >1.0 × 109/L . | Median days* to Platelets >100 × 109/L . | ||||
|---|---|---|---|---|---|---|---|
| . | . | DAT . | ADE . | 2P Value7-151 . | DAT . | ADE . | 2P Value7-151 . |
| 1 | 1,746 | 19 | 18 | .002 | 17 | 17 | .005 |
| 2 | 1,492 | 19 | 17 | .001 | 18 | 17 | .007 |
| 3 | 1,287 | 23 | 21 | <.0001 | 29 | 26 | .002 |
| 4 | 990 | 29 | 28 | .1 | 37 | 36 | .5 |
| After Course . | No. of Patients . | Median Days* to Neutrophils >1.0 × 109/L . | Median days* to Platelets >100 × 109/L . | ||||
|---|---|---|---|---|---|---|---|
| . | . | DAT . | ADE . | 2P Value7-151 . | DAT . | ADE . | 2P Value7-151 . |
| 1 | 1,746 | 19 | 18 | .002 | 17 | 17 | .005 |
| 2 | 1,492 | 19 | 17 | .001 | 18 | 17 | .007 |
| 3 | 1,287 | 23 | 21 | <.0001 | 29 | 26 | .002 |
| 4 | 990 | 29 | 28 | .1 | 37 | 36 | .5 |
Measured from the end of the course.
Log-rank test.
DISCUSSION
The MRC AML10 trial is the largest trial of therapy for AML reported to date. Its overall results are better than in any previously reported large series of younger (age <56 years) patients with AML, with 42% of all patients and over half of all children surviving more than 6 years from diagnosis (Fig 3; Table 5). However, outcome was not materially influenced by the induction regimen given, and the remission rate, speed of remission attainment, DFS, relapse rates, and overall survival were not significantly different between DAT and ADE (Tables 3 and 4; Fig 2 and 3). Subgroup analyses likewise found no convincing significant differences (Tables 5 and 6) — undue weight should not be attached to the apparent benefit of ADE in patients with secondary AML because with multiple subgroup comparisons, P values of borderline significance could very easily arise by chance. The only other apparent difference was a trend toward a slightly higher rate of resistant disease on DAT (11%) compared with ADE (9%), but even this failed to reach conventional statistical significance (P = .07).
Analysis of toxicity showed a “swings and roundabouts” effect, with patients receiving DAT taking slightly longer to recover from pancytopenia (Table 7) (although the median number of days in hospital was not affected), whereas patients receiving ADE had slightly more nausea, alopecia, mucositis, and diarrhea. There was some suggestion of a slightly higher risk of death in CR during consolidation in the ADE group than in the DAT group (9% v 6%), mainly because of a small excess of deaths from infection and hemorrhage (Table 4). Because there was no greater toxicity (either hematologic or nonhematologic) after courses 3 and 4 with ADE, this may well be a chance finding because of small numbers.
The absence of any material differences in outcome between the DAT and ADE regimens raises the question as to whether thioguanine and etoposide are equally effective, or equally ineffective, when added to daunorubicin plus Ara-C. There have been two previous randomized trials that have investigated the effect of adding either thioguanine12 or etoposide13 to the induction regimen for AML. Preisler's series consisted of 668 patients who were randomly assigned to three initial therapy groups. They received either 7 days of cytarabine and 3 days of daunorubicin or the same regimen with thioguanine. The third group received 10 days of cytarabine and 3 days of daunorubicin. The remission rates were 53%, 57%, and 57% for the three groups (P = .6). There was no demonstrable difference in toxicity and no statistically significant difference in remission duration between patients treated on the three induction arms. Bishop's study included 264 adults who were randomized to receive either cytarabine by continuous infusion for 7 days and 3 days of daunorubicin or the same regimen with the addition of etoposide 75 mg/m2 per day for 7 days. The remission rate was 56% without etoposide and 59% with it. Survival was similar in the two arms but there was significantly improved remission duration with etoposide (mean 18 v 12 months, P = .01). These two trials were too small to provide conclusive evidence as to whether the addition of a third drug is beneficial, although in both studies the three-drug regimen did slightly better.
It has been claimed for a number of years that the epipodophyllotoxin group of drugs, and etoposide (VP16) in particular, had specific activity in the monocytic subtypes of acute monocytic leukemia.9,14 This finding was considered to be of particular importance for infants and young children who have a relatively high incidence of these AML subtypes and, in some series, a worse outcome.15 However, these claims were based on small numbers of patients, so they could easily be artifactual subgroup effects due to the play of chance. The large MRC AML10 trial has found no evidence that etoposide is more effective than thioguanine in any subset of patients including, in particular, those with monocytic disease.
Although there were no differences found between DAT and ADE, an important finding from MRC AML10 was the very good outcome in the trial as a whole. The CR rate was an exceptional 92% in children, and even in patients aged 45 or over it was 76%. Long-term outcome was similarly good, with 56% of children still alive 6 years after entry, with the corresponding figure for patients aged 45 or over being 35%. The DFS and overall survival curves (Figs 2 and 3) show clear plateaus beyond year 4, suggesting that over half of the children and about a third of the adults entered in AML10 may have been cured of their AML. Furthermore, these results have been obtained in a multicenter trial, in which patients were entered not only from large hospitals with much experience of treating AML, but also from many smaller centers that only see a few cases each year. It may be that the adoption of two courses of DAT or ADE (irrespective of whether CR was achieved after one course) and the use of two intensive consolidation courses were factors in the improved outcome in this study.
In conclusion, we have found no evidence that an etoposide-containing regimen (ADE) is any better than the previously standard MRC thioguanine regimen (DAT). In particular there was no additional benefit from the use of etoposide rather than thioguanine in patients with monocytic leukemias. The regimens are broadly equivalent with regard to toxicity, efficacy, and resource usage, and could thus be used interchangeably. The question as to whether or not the addition of either etoposide or thioguanine to a standard daunorubicin-cytarabine induction regimen is beneficial will have to await further randomized study.
ACKNOWLEDGMENT
We are grateful to the clinicians who entered their patients into MRC AML10 and for their wholehearted support, without which the trial would not have been such a success. The following institutions and clinicians participated (members of the MRC Adult and Childhood Leukaemia Working Parties are indicated by an asterisk):
Aberdeen Royal Infirmary: Dr N.B. Bennett, Dr A.A. Dawson, Dr D.J. King*; Addenbrooke's Hospital: Dr V.A. Broadbent*, Dr R. Marcus, Dr J.K.H. Rees*, Dr M.V. Williams*; Alder Hey Children's Hospital: Dr L. Ball, Dr J. Martin*, Dr H. McDowell; Alexandra Hospital: Dr D. Obeid; Auckland Hospital: Dr S. Palmer, Dr R. Varcoe*; Barnsley District General Hospital NHS Trust: Dr J.P. Ng; Beaumont Hospital: Dr J.R. O'Donnell; Belfast City Hospital: Dr Z.R. Desai; Birmingham Children's Hospital: Dr P.J. Darbyshire*, Dr F.G.H. Hill*, Dr J. Mann*; Birmingham General Hospital: Dr J.A. Holmes; Birmingham Heartlands Hospital: Dr M.J. Leyland, Dr D.W. Milligan*; Bradford Royal Infirmary: Dr L.A. Parapia, Dr A.T. Williams; Bristol Royal Infirmary: Dr G.L. Scott; Broadgreen Hospital: Dr P. Chu; Brook General Hospital: Dr R.M. Ireland; Central Middlesex Hospital: Dr S. Davies, Dr K. Ryan; Charing Cross Hospital: Dr M. Foadi, Dr D. McCarthy; Chelsea & Westminster Hospital: Dr C.E.M. Costello, Dr L. Sinclair; Cheltenham General Hospital: Dr R.G. Dalton; Christchurch Hospital: Dr D.N.J. Hart, Dr D. Heaton; Christie Hospital: Prof.O.B. Eden*; City Hospital NHS Trust: Dr D. Bareford; Conquest Hospital: Dr J. Beard; Crosshouse Hospital: Dr J.G. Erskine; Darlington Memorial Hospital: Dr P.J. Williamson; Department of Health: Dr M. Cuthbert*, Dr W. Maton-Howarth*, Dr A. Rejman*; Derby Childrens Hospital: Dr C.S. Nelson; Derbyshire Royal Infirmary: Dr D.C. Mitchell; Derriford Hospital: Dr A. Prentice*; Dewsbury and District Hospital: Dr M.R. Chapple; Dumfries & Galloway Royal Infirmary: Dr A. Stark; Dunedin Hospital: Dr C.H. Beresford, Dr C. Newhook; Eastbourne District General: Dr R.J. Grace; Edgware General Hospital: Dr D. Harvey; Falkirk & District Royal Infirmary: Dr A.D.J. Birch; Farnborough Hospital: Dr A.K. Lakhani, Dr I.R. Samaratunga; Frenchay Hospital: Dr P.J. Whitehead; Frimley Park Hospital: Dr J.A. Shirley, Dr J. VanDePette; George Eliot Hospital: Dr Narayanan; Glan Clwyd District General Hospital: Dr D.I. Gozzard; Glasgow Royal Infirmary: Dr I.M. Franklin*; Gloucester Royal Hospital: Dr J. Ropner; Good Hope District General Hospital: Dr M.S. Hamilton, Dr J. Tucker; Grantham & Kesteven General Hospital: Dr V.M. Tringham; Great Ormond Street Hospital: Prof J.M. Chessells*, Dr I.M. Hann*, Dr F. Vargha-Khadem*; Grimsby District General Hospital: Dr K.R. Speed; Guy's Hospital: Dr S.A. Schey*; Hammersmith Hospital: Dr J. Apperley, Prof D.A.G. Galton*, Prof.J.M. Goldman*, Dr E. Kanfer, Dr M. Laffan, Prof L. Luzatto, Dr D. Samson, Dr D. Swirsky*; Hartlepool General Hospital: Dr A. Youart; Hemel Hempstead General Hospital: Dr E.J. Gaminara, Dr J.F.M. Harrison; Hinchingbrooke Hospital: Dr C.E. Hoggarth; Horton General Hospital NHS Trust: Dr I.J. Durrant*; Hospital for Sick Children, Bristol: Dr A. Foot, Dr N. Foreman, Prof M.G. Mott, Dr A. Oakhill*; Hospital for Sick Children, Edinburgh: Dr A. Thomas*, Dr H. Wallace*; Hospital for Sick Children, Glasgow: Dr B. Gibson*; Huddersfield Royal Infirmary: Dr C. Carter; Ipswich Hospital: Dr N.J. Dodd*, Dr C.N. Simpson; Isle of Thanet District Hospital: Dr M. Leahy; King's College Hospital: Dr D.M. Layton, Dr G. Mufti*, Dr A. Pagliuca; King's Mill Hospital: Dr M. Auger; Law Hospital: Dr T.L. Allan, Dr J.D. Browning; Leeds General Infirmary: Dr J.A. Child*, Dr D.R. Norfolk, Dr G.M. Smith; Leicester Royal Infirmary: Dr C.S. Chapman, Dr C. Haworth*, Dr R.M. Hutchinson*, Dr V.E. Mitchell, Dr R.S. Shannon*, Dr J.K. Wood; Lincoln County Hospital: Dr M.A. Adelman, Dr D.R. Prangnell; Lister Hospital: Dr C. Tew, Dr S.M. Watkins; Llandough Hospital NHS Trust: Dr D. Webb*; London Hospital: Prof A.C. Newland*; Manchester Royal Infirmary: Dr J.A. Yin*; Mercy Hospital: Dr M. Madden*; Manor Hospital: Dr G.P. Galvin; Middlesbrough General Hospital: Dr J.E. Chandler, Dr G.P. Summerfield*; Middlesex Hospital: Prof D.C. Linch*; Milton Keynes General Hospital: Dr E.J. Miller, Dr D.J. Moir; Monklands District General: Dr E.J. Fitzsimons; National Childrens Hospital: Prof I.J. Temperley; Neath General Hospital: Dr A.C. Beddall; New Cross Hospital: Dr A. MacWhannell; Ninewells Hospital & Medical School: Prof M.J. Pippard; Ninewells Hospital: Dr P. Cachia, Dr A. Heppleston; Norfolk & Norwich Hospital: Dr A.J. Black, Dr J. Leslie; North Hampshire Hospital: Dr D.L. Aston, Dr A.E. Milne, North Staffs Hospital Centre: Dr P.M. Chipping, Dr R.M. Ibbotson; North Tees General Hospital: Dr R. Finney; Northampton General Hospital: Dr M.E. Haines, Dr J.R.Y. Ross, Dr S.S. Swart; Northern General Hospital: Dr J.T. Reilly; Northwick Park Hospital: Dr C.D.L. Reid, Dr P. Skacel; Nottingham City Hospital: Dr P.A.E. Jones, Dr N.H. Russell; Nottingham University Hospital: Dr J.M. Davies, Dr G. Dolan, Dr M. Hewitt*, Dr D. Walker*; Our Lady's Hospital: Dr F. Breatnach; Oxford Radcliffe Hospital: Dr P. Emerson*, Dr T.J. Littlewood*, Dr C.D. Mitchell*, Dr M.W. Moncrieff, Dr J.S. Wainscoat; Palmerston North Hospital: Dr B. Baker; Pembury Hospital: Dr D.S. Gillett; Pembury Hospital: Dr C. Taylor; Peterborough District Hospital: Dr S.A. Fairham, Dr J.Z. Wimperis; Pilgrim Hospital: Dr S. Sobolewski, Dr V.M. Tringham; Pinderfields General Hospital: Dr M.C. Galvin; Prince Philip Hospital: Dr R.V. Majer; Queen Alexandra Hospital: Dr M. Ganczakowski; Queen Elizabeth Hospital, Birmingham: Dr B.J. Boughton, Dr J.A. Holmes; Queen Elizabeth Hospital, Norfolk: Dr P. Coates,Dr J. Keidan; Queen Elizabeth Military Hospital: Dr J.M. Foxley; Queen Margaret Hospital: Dr A. Evan-Wong; Queen Mary's Hospital: Dr S. Bowcock; Dr E.L. Offerman; Rotherham District General: Dr P.C. Taylor; Royal Belfast Hospital: Dr S.I. Dempsey*; Royal Berkshire Hospital: Dr C. Burton*; Royal Bournemouth Hospital: Prof T.J. Hamblin*, Dr D.G. Oscier; Royal Chesterfield Hospital: Dr D.J. Clark, Dr R. Collin; Royal Cornwall Hospital (Treliske): Dr H.M. Daly, Dr A.R. Kruger; Royal Devon & Exeter Hospital: Dr M.V. Joyner, Dr R. Lee; Royal Free Hospital: Prof A.V. Hoffbrand*, Prof H.G. Prentice*; Royal Hallamshire Hospital: Dr D.A. Winfield; Royal Hampshire County Hospital: Dr W.O. Mavor; Royal Infirmary of Edinburgh: Dr C.A. Ludlam, Dr A.C. Parker*; Royal Liverpool University Hospital: Prof J.C. Cawley, Dr P. Chu, Dr R.E. Clark*, Dr J.M. Davies, Dr C.R.M. Hay; Royal Manchester Childrens Hospital: Dr M.E.M. Jenney, Dr R.F. Stevens*; Royal Manchester Childrens Hospital: Dr P. Morris-Jones*, Dr A. Will*; Royal Marsden Hospital: Prof D. Catovsky, Prof T.J. McElwain*, Dr S.T. Meller*, Prof J. Peto, Prof C.R. Pinkerton, Dr R.L. Powles*; Royal Shrewsbury Hospital: Dr N. O'Connor, Dr M.J. O'Shea; Royal Surrey County Hospital: Dr G. Robbins; Royal Sussex County Hospital: Dr J. Duncan, Dr M.W. Kenny; Royal United Hospital NHS Trust: Dr C.R.J. Singer, Dr J.G. Smith; Royal Victoria Hospital, Belfast: Dr F.G.C. Jones*, Dr E.E. Mayne; Royal Victoria Infirmary: Dr P. Hamilton, Dr J. Kernahan, Prof S.J. Proctor, Dr M. Reid*; Royal West Sussex Hospital: Dr P.C. Bevan, Dr P. Stross; Salisbury General Hospital: Dr H.F. Parry; Sandwell General Hospital: Dr S.I. Handa, Dr P.J. Stableforth; Scottish Home & Health Department: Dr Keele*; Scunthorpe General Hospital: Dr S. Jalihal, Dr R. Stewart; Seacroft Hospital: Dr S.M. Rajah; Selly Oak Hospital: Dr J.A. Murray, Dr W.N. Patten; Sheffield Children's Hospital: Prof.J.S. Lilleyman*; South Tyneside Hospital: Dr A.M. Hendrick; South Warwickshire Hospital: Dr P.E. Rose; Southampton University Hospital Trust: Dr A. Duncombe, Dr J. Kohler*, Dr A. Provan, Dr M. Radford*, Dr A.G. Smith*; Southmead Hospital: Dr J. Hows, Dr R.R. Slade; St. Albans City Hospital: Dr E.J. Gaminara; St. Bartholomew's Hospital: Dr J.E. Kingston; St. George's Hospital: Dr S. Ball, Dr D.H. Bevan; St. Helier Hospital: Dr J. Behrens, Dr M. Clarke; St. James Hospital: Prof S.R. McCann*, Prof I.J. Temperley; St. James's University Hospital: Dr C.C. Bailey*, Dr D.L. Barnard, Dr S.E. Kinsey*, Dr I.J. Lewis*, Dr B.A. McVerry; St. Mary's Hospital, London: Dr S.H. Abdalla, Dr B.J. Bain; St. Mary's Hospital, Portsmouth: Dr P.J. Green; St. Thomas' Hospital: Dr R. Carr, Prof.T.C. Pearson, Dr N.P. Slater; St. Vincent's Hospital: Prof J.J. Fennelly; Stobhill General Hospital: Dr R.L.C. Cumming, Dr R.B. Hogg; Stoke Mandeville Hospital: Dr A.M. O'Hea, Dr S.M. Sheerin; Sunderland Royal Infirmary: Dr P.J. Carey*, Dr D.K. Goff; Taunton & Somerset Hospital: Dr M.J. Phillips; Torbay Hospital: Dr B. Murphy*; University College Hospital, Galway: Prof E.L. Egan, Dr M. Murray; University College Hospital, London: Dr S. Devereux, Dr A.H. Goldstone*, Dr K.G. Patterson; University Hospital of Wales: Prof A.K. Burnett*, Dr C. Poynton, Dr E. Thompson*, Dr J.A. Whittaker*; University of Birmingham: Prof I.C. MacLennan*; Victoria Hospital, Kirkcaldy: Dr C.J. McCallum, Dr S.Y. Rogers; Victoria Hospital, Worksop: Dr B. Paul; Victoria Infirmary: Dr R.A. Sharp, Dr P.J. Tansey*; Waikato Hospital: Dr G. Corbett, Dr S. May; Walsgrave Hospital: Dr R. Harris, Dr M.J. Strevens; Walton Hospital: Dr P.A. Stevenson; Wellington Hospital: Dr J.C. Carter, Dr G. Green; West Hill Hospital: Dr V.E. Andrews; West Middlesex Hospital: Dr R.G. Hughes; West Suffolk Hospital: Dr P. Harper; Western General Hospital: Dr N.C. Allan*, Dr P. Ganly, Dr M.J. Mackie; Western Infirmary: Dr A. Barrett*, Dr I.L. Evans, Dr N.P. Lucie; Wexham Park Hospital: Dr C. Hatton; Whipps Cross Hospital: Dr C.C. Anderson, Dr C. DeSilva; Whiston Hospital: Dr G. Satchi, Dr J. Tappin; Whittington Hospital: Dr N.E. Parker; William Harvey Hospital: Dr D.G. Wells; Worcester Royal Infirmary: Dr A.H. Sawers, Dr R. Stockley; Wycombe General Hospital: Dr S. Kelly, Dr G.C. Rastogi; York District Hospital: Dr L.R. Bond; Ysbyty Gwynedd: Dr H.E.T. Korn, Dr D.H. Parry.
We also thank Rachel Clack, Sarah Griffin, Sue Knight, and Angela Radley for data management, and Libby Tute and Cathy Harwood for preparing the manuscript.
Address reprint requests to Ian M. Hann, MD, Department of Haematology & Oncology, Great Ormond Street Hospital for Children, London WC1N 3JH, UK.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal