Abstract
Sickle red blood cell (RBC) adhesion to the blood vessel wall is hypothesized to be the initiating event in the periodic vaso-occlusive episodes that characterize sickle cell disease (SCD). Thrombospondin-1 (TSP) and von Willebrand factor (vWF ) have each been implicated in the adhesion of sickle RBC to vascular endothelial cells (EC) and subendothelial matrices. To better understand the contributions of each of these adhesive glycoproteins, we examined the adhesion of sickle RBC to immobilized TSP and vWF using a parallel plate flow chamber. Under postcapillary venular shear stress (1 dyne/cm2), sickle RBC adhered preferentially to TSP. To explore potential interactive effects of vWF and TSP, we examined sickle RBC adhesion to mixtures of these proteins. Whether the proteins were first combined in solution or sequentially applied to the slide, the presence of vWF inhibited the binding of sickle RBC to TSP. The inhibition of adhesion by vWF was shown to be the result of specific and saturable binding of vWF to TSP. Furthermore, vWF in solution at normal plasma levels also inhibited RBC adhesion to immobilized TSP. These data indicate that sickle RBC adhesion in vivo may be significantly influenced by the relative concentrations of TSP and vWF in the vascular wall.
PERIODIC VASO-OCCLUSION is believed to be the principal pathophysiologic process responsible for the high level of morbidity in patients with sickle cell disease (SCD). Abnormal adhesive interactions between sickle erythrocytes and vascular endothelial cells (EC) and/or subendothelial matrix (SEM) are hypothesized to play the dominant role in the initiation of these events.1-4 It is likely that multiple mechanisms, including the activation of EC, platelets, leukocytes, and structural abnormalities intrinsic to sickle erythrocytes, are responsible for the enhanced adhesion.2,5 6
Thrombospondin-1 (TSP) and von Willebrand factor (vWF ) are among the proteins that have been implicated as mediators of the adhesive interactions between sickle erythrocytes and the blood vessel wall.7-12 TSP is a 420-kD trimeric multifunctional glycoprotein that interacts with structural elements of the SEM including collagen, fibronectin, and laminin and with a large number of cell surface molecules including proteoglycans, integrins, and nonintegrin receptors.13,14 It is constitutively synthesized and secreted by EC and certain other cell types in culture and is incorporated into the extracellular matrix of these cells.15-17 TSP is also present in the α-granules of platelets from which it is released during platelet activation.18
vWF is composed of disulfide-bonded subunits that form multimers ranging in size from 0.5 to 20 × 106 daltons.19 The two principal hemostatic functions of vWF are well characterized. First, it serves as a molecular bridge between the platelet glycoprotein Ib/IX receptor complex and subendothelial proteins under conditions of high shear stress. Second, it functions to promote the secretion and stability of factor VIII in the circulation.20 vWF is synthesized by vascular endothelium and megakaryocytes and is stored in EC Weibel-Palade bodies and in platelet α-granules.21,22 vWF is released from platelets, along with TSP, following platelet activation and from Weibel-Palade body stores in response to a number of physiologic agonists including thrombin,23 histamine,24 and the peptido-leukotrienes, LTC4 and LTD4 .25 The largest and most hemostatically potent forms of vWF are stored in these secretory granules.26 27
A role for vWF in the promotion of sickle erythrocyte adhesion to EC of various origins has been demonstrated using a variety of experimental techniques. Studies by Kaul et al12 and Fabry et al,28 using in vivo rat and ex vivo mesenteric vessel models, demonstrated that desmopressin infusion stimulated vWF secretion in the perfused vascular bed and increased the adhesion of sickle erythrocytes resulting in microvascular obstruction. The adhesion was abrogated by antibodies directed against vWF, but not thrombospondin or fibronectin. Wick et al10,11 demonstrated that the unusually large molecular weight forms of vWF that are released following stimulation of cultured EC increased adhesion of low-density sickle erythrocytes to human EC, primarily of large vessel (umbilical vein) origin. In these studies, antibodies specific for either platelet glycoprotein Ib or platelet glycoprotein IIb/IIIa blocked the adhesion or red blood cell (RBC) to EC.11
TSP has also been shown to promote sickle reticulocyte adhesion to human umbilical vein EC in a static adhesion assay7 and to microvascular EC under venular flow conditions.9 TSP immobilized on glass also supports sickle RBC adhesion in static7 and flow assays.29,30 There is experimental evidence that TSP-mediated SS RBC adhesion to vascular endothelium and sickle RBC binding to immobilized TSP involved different molecular determinants. CD36 (glycoprotein IV) is a nonintegrin TSP receptor located on the surface of reticulocytes and microvascular EC. Under venular flow conditions, anti-CD36 antibodies were found to block TSP-dependent sickle RBC adhesion to cultured microvascular EC,9 but failed to abrogate sickle RBC adhesion to immobilized TSP, which was instead blocked by anionic polysaccharides including dextran sulfate and chondroitin sulfate.29,30 In static assays, however, sickle RBC adhesion to EC or to immobilized TSP could be blocked by either anti-CD36 antibody or the TSP-derived peptide CSVTCG (derived from the CD36 binding site in the type 1 repeat domain).7
Reports of damaged endothelium31 32 and increased circulating EC33 in patients with SCD suggest that the SEM may become exposed during vaso-occlusive events. TSP and vWF are present together in the SEM and additional quantities of both proteins are likely to be deposited into the SEM by activated platelets at vascular injury. In the present study, we have undertaken an examination of sickle erythrocyte adhesion under dynamic flow conditions to immobilized vWF and TSP, individually and in combination, to better understand the possible contribution of these adhesive proteins to sickle vaso-occlusive processes.
MATERIALS AND METHODS
Materials.TSP was either purchased from GIBCO (Grand Island, NY), or purified as previously described.34 Polyclonal antisera against full-length TSP (R1)34 and a recombinant TSP type III domain fusion protein (R5)35 were raised in rabbits as previously described. Purified plasma-derived vWF was purchased from Haematologic Technologies, Inc (Essex Jct., VT).
vWF expression and purification.The establishment of the cell lines expressing recombinant vWF have been previously described.36,37 Recombinant vWF was purified from cell culture conditioned media as described by Cruz et al.38 The purified protein was assessed by silver-stained electrophoresis gels, protein assay (BCA; Pierce Chemical, Rockford, IL), and vWF enzyme-linked immunosorbent assay (ELISA). Our recombinant vWF was found to be equivalent to purified plasma vWF with respect to multimeric composition and functionality as determined by gel electrophoresis and ristocetin-induced platelet aggregation.37
Immobilization of purified proteins.Fifty microliters of a concentrated solution of purified protein(s) was applied to the center of a 25 × 75-mm glass slide. Following incubation at 37°C for 1 hour in 95% humidity, the entire slide was blocked with 1% bovine serum albumin (BSA) in Hanks' buffered saline solution (HBSS) for 1 hour under the same conditions. To prepare slides partially coated with both TSP and vWF, the first protein was applied to the center of the slide as described above, after which the slide was placed on top of a sheet of Parafilm containing 200 μL of the second protein in solution. The slide was then incubated at 37°C and 95% humidity for 1 hour, and blocked with BSA as described above.
Quantitation of immobilized protein.A dot-blot ELISA was developed to quantitate immobilized vWF and TSP. Glass slides were subjected to four successive rounds of scrape harvesting into a 250-μL solution containing 10% sodium dodecyl sulfate (SDS), 50 mmol/L Tris-HCl, 150 mmol/L NaCl, pH 7.6 (Tris buffered saline, [TBS]). Samples, along with standard controls, were then applied to nitrocellulose paper using a dot-blot manifold (Schleicher and Schuell, Keene, NH). After application to the manifold, the paper was blocked with 5% dried milk in TBS then incubated with either a monoclonal antibody to TSP [MA-II17] and a horseradish peroxidase (HRP)-conjugated antimouse IgG or a polyclonal HRP-conjugated anti-vWF (Dako Corp, Glostrup, Denmark). The ELISA signal was developed by chemiluminescence (Dupont NEN, Boston, MA) and exposed to autoradiography film.
Preparation of RBC.After obtaining informed consent, blood was drawn into heparinized tubes from patients with homozygous (Hb SS) SCD who were not in crisis and from normal volunteers without hemoglobinopathies. After centrifugation at 1,000g for 10 minutes, the plasma and buffy coat were removed, the RBC pellet was washed three times with HBSS, and then resuspended to 1% hematocrit in the same medium.
Erythrocyte adherence assay.A parallel plate flow chamber with a protein-coated slide as its base was used to assess the adhesion of RBCs under defined fluid dynamic conditions. The flow chamber and the glass slide were held together by a vacuum maintained at the periphery of the slide forming a channel of parallel plate geometry. The height of the flow channel, as determined by a silastic gasket, was 100 μm. After assembling the protein-coated slide with the flow chamber, RBC suspensions were drawn into the chamber from a reservoir maintained at 37°C in a water bath, by a syringe pump (Model 956; Harvard Apparatus, South Natick, MA) at a controlled flow rate to give a venular wall shear stress of 1 dyne/cm2. The flow chamber was mounted on an inverted phase contrast microscope (DIAPHOT-TMD; Nikon, Garden City, NY) equipped with a CCD video camera (Model 72; Dage-MTI). The microscope stage was maintained at 37°C by a thermostatic air bath (Model ASI-400; Nicholson Precision Instruments, Gaithersburg, MD). All of the experiments were recorded in real-time on a 0.5 in video cassette recorder (Model BV-1000, Mitsubishi, Cypress, CA) and displayed on a high resolution monitor (Model PM-127; Ikegami, Maywood, NJ). A PC-based image processing system (Optimas; Bioscan, Edmunds, WA) was used for digitization of video recordings and for further image processing and analysis. For each flow assay, the protein-coated slide was rinsed for 2 minutes with HBSS, followed by a 10-minute perfusion of the RBC suspension. Nonadherent RBC were removed by a 10-minute rinse with HBSS. The number of adherent erythrocytes remaining after the rinse period were counted in a minimum of 24 fields and reported as the number of adherent cells per mm2.
Binding assays.TSP in TBS containing 10 mmol/L CaCl2 , pH 8.0 (TBS-Ca) was coated onto plastic microtiter wells (Immulon-4, Dynatech, Chantilly, VA) at a concentration of 10 μg/mL. Wells were washed with TBS-Ca containing 0.05% Tween-20 and blocked with 1% BSA in TBST-Ca. Wells were washed and then incubated with vWF in TBST-Ca/BSA for 1 hour at 37°C. After washing, the wells were incubated with the HRP-conjugated anti-vWF described above. Color was developed with an o-phenylenediamine substrate and detected at 490 nm using an ELISA plate reader (Bio-Tek Instruments, Winooski, VT). Nonspecific binding was determined in duplicate wells not coated with TSP.
RESULTS
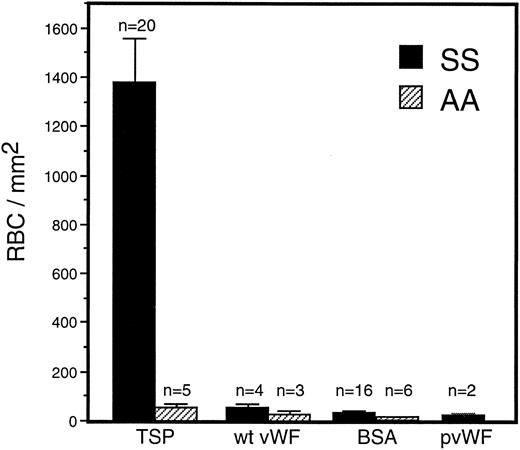
Adhesion of sickle RBC to vWF and TSP.Immobilized TSP and vWF were first compared for their ability to support sickle and normal erythrocyte adhesion under dynamic flow conditions (1 dyne/cm2 ) approximating the shear stress found in postcapillary venules, the predominant site of RBC adhesion in vivo. There was markedly greater adherence of sickle erythrocytes to TSP than to vWF or to BSA (Fig 1). In contrast, normal erythrocytes were only modestly adhesive to TSP, and still less so to vWF or BSA. In comparison to normal erythrocytes, sickle erythrocytes demonstrated a significantly increased adherence to TSP, while exhibiting only slight adherence to recombinant or plasma-derived vWF, which was not statistically different from BSA. Increasing the coating concentration of vWF from 20 μg/mL to 100 μg/mL did not result in increased adhesion of RBC.
Sickle and normal RBC adherence to immobilized TSP and vWF. Glass microscope slides, which form the base of the parallel plate flow chamber, were coated with either TSP (50 μg/mL) or vWF (20 or 100 μg/mL) and then blocked with 1% BSA for 60 minutes. A washed suspension of RBC (1% Hct in serum free medium) was perfused over the immobilized proteins for 10 minutes, followed by a 10-minute rinse with cell free medium at a shear stress of 1 dyne/cm2. Results demonstrate that sickle RBC adhered preferentially to immobilized TSP in comparison to vWF and BSA (nonspecific control). Similar levels of sickle RBC adhesion were observed for recombinant vWF (wt vWF ) and plasma-derived vWF (pvWF ) at coating concentrations of 20 μg/mL and 100 μg/mL. Data are shown for wt vWF at 20 μg/mL and for pvWF at 100 μg/mL. Error bars are standard error of mean (SEM).
Sickle and normal RBC adherence to immobilized TSP and vWF. Glass microscope slides, which form the base of the parallel plate flow chamber, were coated with either TSP (50 μg/mL) or vWF (20 or 100 μg/mL) and then blocked with 1% BSA for 60 minutes. A washed suspension of RBC (1% Hct in serum free medium) was perfused over the immobilized proteins for 10 minutes, followed by a 10-minute rinse with cell free medium at a shear stress of 1 dyne/cm2. Results demonstrate that sickle RBC adhered preferentially to immobilized TSP in comparison to vWF and BSA (nonspecific control). Similar levels of sickle RBC adhesion were observed for recombinant vWF (wt vWF ) and plasma-derived vWF (pvWF ) at coating concentrations of 20 μg/mL and 100 μg/mL. Data are shown for wt vWF at 20 μg/mL and for pvWF at 100 μg/mL. Error bars are standard error of mean (SEM).
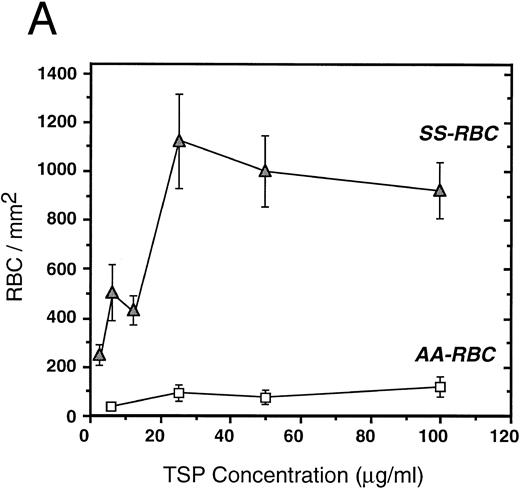
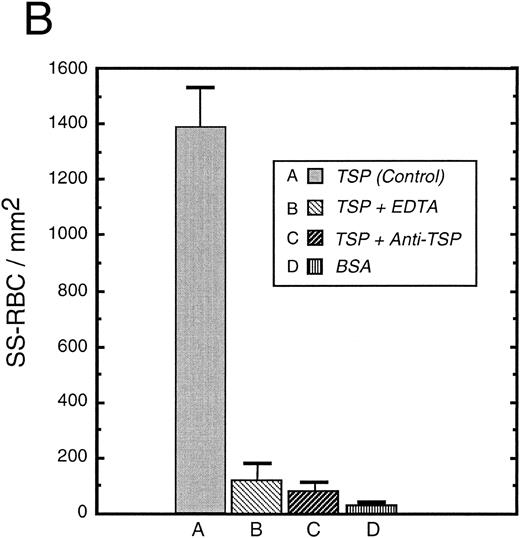
To further elucidate the adherence of sickle erythrocytes to TSP, we examined the effect of protein-coating concentration as shown in Fig 2A. Sickle erythrocytes adhered to TSP in a concentration-dependent and saturable manner, while normal erythrocyte adherence remained at a low level irrespective of coating concentration. The specificity of adherence was demonstrated by inhibition with an anti-TSP antiserum (Fig 2B). The specificity of RBC binding to TSP was further supported by the observation that the presence of a second antibody directed against the type 3 repeat of TSP35 resulted in approximately 70% inhibition of adhesion [288 ± 56 (with R5) v 982 ± 332 (control)]. In the presence of EDTA, at a coating concentration of 50 μg/mL, sickle erythrocyte adhesion to TSP was abolished (Fig 2B), consistent with the requirement for calcium in the interaction of TSP with other proteins.17 39
Sickle and normal RBC adherence to immobilized TSP. (A) Glass slides were coated with varying concentrations of TSP (2.5 to 100 μg/mL) and adhesion of normal and sickle RBC assessed under flow conditions as described in Fig 1. Error bars are standard deviation (SD) of 10 randomly chosen microscopic fields from one representative experiment. (B) Slides were coated with TSP (50 μg/mL) or BSA as described in Materials and Methods. In some experiments, the slides were additionally treated with EDTA (5 mmol/L) or a 1:2 dilution of a rabbit polyclonal anti-TSP antiserum (R1)34 before RBC perfusion. Error bars are SEM.
Sickle and normal RBC adherence to immobilized TSP. (A) Glass slides were coated with varying concentrations of TSP (2.5 to 100 μg/mL) and adhesion of normal and sickle RBC assessed under flow conditions as described in Fig 1. Error bars are standard deviation (SD) of 10 randomly chosen microscopic fields from one representative experiment. (B) Slides were coated with TSP (50 μg/mL) or BSA as described in Materials and Methods. In some experiments, the slides were additionally treated with EDTA (5 mmol/L) or a 1:2 dilution of a rabbit polyclonal anti-TSP antiserum (R1)34 before RBC perfusion. Error bars are SEM.
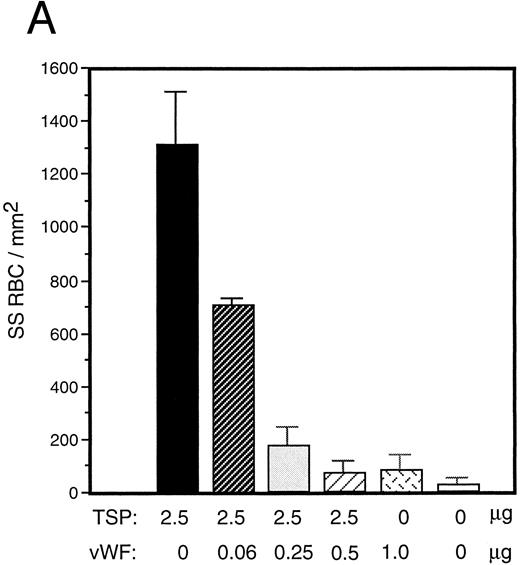
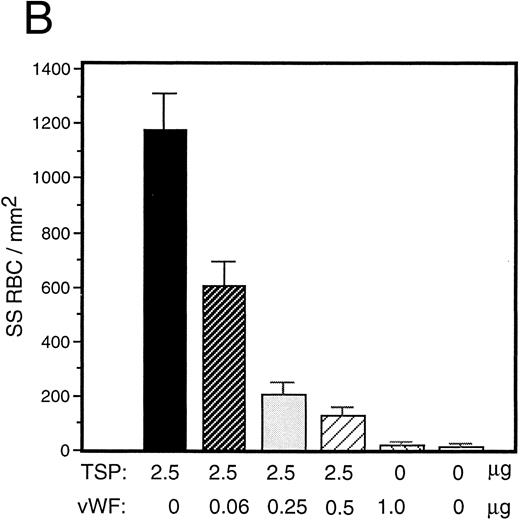
vWF inhibits sickle RBC binding to TSP.Because TSP and vWF are both components of the subendothelial matrix, it was of interest to explore the potential for their mutual interaction with respect to the promotion of sickle erythrocyte adherence. We initiated a series of experiments involving mixtures of these proteins expecting their effects to be additive. TSP and vWF were combined in solution and then coimmobilized on glass. Surprisingly, we found that rather than promoting adhesion, the presence of vWF in combination with TSP served to inhibit RBC binding to TSP. Representative experiments for recombinant and plasma-derived forms of vWF are shown in Fig 3A and B, respectively. vWF inhibition of RBC adhesion was concentration-dependent and was observed at ratios of vWF to TSP as low as 1:40 (wt:wt).
Sickle RBC adhesion to mixtures of TSP and vWF. TSP and vWF were combined in solution at various ratios and coated onto glass microscope slides. Sickle RBC adhesion was assessed as in Fig 1. In combination with a fixed concentration of TSP (50 μg/mL), vWF exerted a concentration-dependent inhibitory effect. The effects of both recombinant and plasma vWF are shown in (A) and (B), respectively. Indicated weights represent total amounts of protein present in the mixture. Error bars are SEM.
Sickle RBC adhesion to mixtures of TSP and vWF. TSP and vWF were combined in solution at various ratios and coated onto glass microscope slides. Sickle RBC adhesion was assessed as in Fig 1. In combination with a fixed concentration of TSP (50 μg/mL), vWF exerted a concentration-dependent inhibitory effect. The effects of both recombinant and plasma vWF are shown in (A) and (B), respectively. Indicated weights represent total amounts of protein present in the mixture. Error bars are SEM.
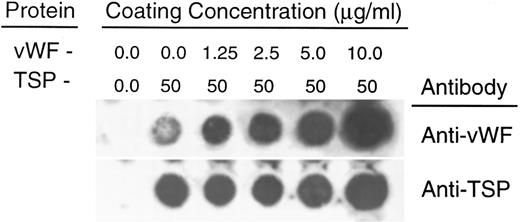
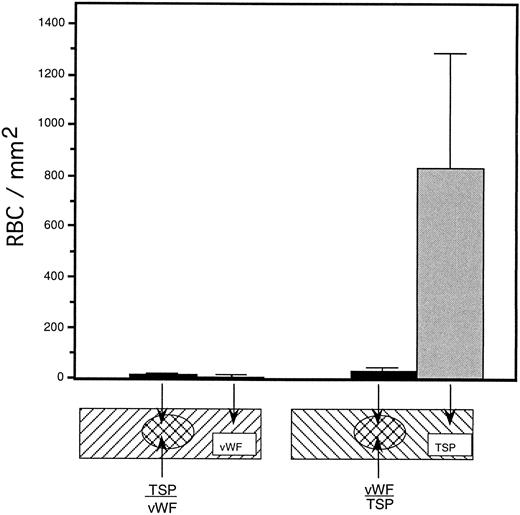
To rule out the possibility that the observed inhibition was simply due to the preferential binding of vWF to glass, thereby displacing binding of TSP, we performed a dot-blot analysis to approximate the amount of TSP bound to the slide in the presence of increasing concentrations of vWF (Fig 4). The results indicated that the presence of vWF did not inhibit TSP binding, the amount of TSP bound to the slide remained relatively constant at all ratios of vWF:TSP examined. Our initial observation that vWF inhibited the adhesion of sickle RBC to TSP was obtained by mixing solutions of the two proteins before their application to the slide. To determine whether vWF would inhibit erythrocyte binding to TSP, which had been previously immobilized on glass, we devised a reciprocal coating procedure. In this two-step process, the first protein was applied in the center of the slide and the entire slide was then coated with the second protein. Thus, the center area of the slide contained an overlay of the two proteins, while the coating on the outer edges consisted of only the protein that was applied last. Sickle RBC binding was then assessed under flow conditions, the results of which are shown in Fig 5. Irrespective of the order of protein addition, erythrocyte binding to TSP was dramatically decreased by the presence of vWF. Moreover, erythrocyte adhesion to the outer portions of the reciprocally-coated slides confirm the previous finding that TSP is significantly more adhesive than vWF to sickle RBC. Similarly, we observed that the addition of vWF (5 to 10 μg/mL) to RBC suspensions resulted in an 86.5% ± 10.8% (n = 4) reduction in adhesion to TSP immobilized on glass (data not shown). The inhibitory action of vWF with respect to TSP thus appeared to be equivalent irrespective of whether the proteins were first combined in solution and immobilized together or applied to the slide sequentially.
Quantitation of protein deposition by dot-blot ELISA. Glass slides were prepared as described in Fig 3. Immobilized protein was eluted by repeated scrape harvesting into a Tris-HCl buffer containing 10% SDS and applied to nitrocellulose membranes as described in Materials and Methods. Duplicate strips were allowed to react with antibodies against either vWF (upper row) or TSP (lower row). The weak signal generated by the anti-vWF antibody against TSP alone was less than 5% of the signal generated by reaction against the lowest concentration of vWF (1.25 μg/mL) (determined by densitometry). In separate experiments, neither the anti-vWF nor the anti-TSP antibody demonstrated detectable cross-reactivity against either TSP or vWF, respectively.
Quantitation of protein deposition by dot-blot ELISA. Glass slides were prepared as described in Fig 3. Immobilized protein was eluted by repeated scrape harvesting into a Tris-HCl buffer containing 10% SDS and applied to nitrocellulose membranes as described in Materials and Methods. Duplicate strips were allowed to react with antibodies against either vWF (upper row) or TSP (lower row). The weak signal generated by the anti-vWF antibody against TSP alone was less than 5% of the signal generated by reaction against the lowest concentration of vWF (1.25 μg/mL) (determined by densitometry). In separate experiments, neither the anti-vWF nor the anti-TSP antibody demonstrated detectable cross-reactivity against either TSP or vWF, respectively.
Sickle RBC adhesion to slides sequentially coated with TSP and vWF. TSP and vWF were sequentially coated onto glass microscope slides (as depicted). A washed suspension of RBC (1% hematocrit in serum-free medium) was perfused into the flow chamber for 10 minutes, followed by a 10-minute rinse with cell-free medium at a shear stress of 1 dyne/cm2. The number of adherent RBC remaining at the end of the rinse period was determined using video microscopy. vWF-induced inhibition of sickle RBC binding to TSP was observed irrespective of the order of protein application to the slide.
Sickle RBC adhesion to slides sequentially coated with TSP and vWF. TSP and vWF were sequentially coated onto glass microscope slides (as depicted). A washed suspension of RBC (1% hematocrit in serum-free medium) was perfused into the flow chamber for 10 minutes, followed by a 10-minute rinse with cell-free medium at a shear stress of 1 dyne/cm2. The number of adherent RBC remaining at the end of the rinse period was determined using video microscopy. vWF-induced inhibition of sickle RBC binding to TSP was observed irrespective of the order of protein application to the slide.
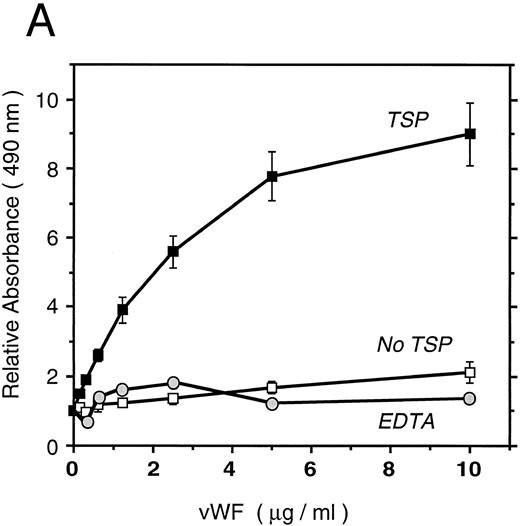
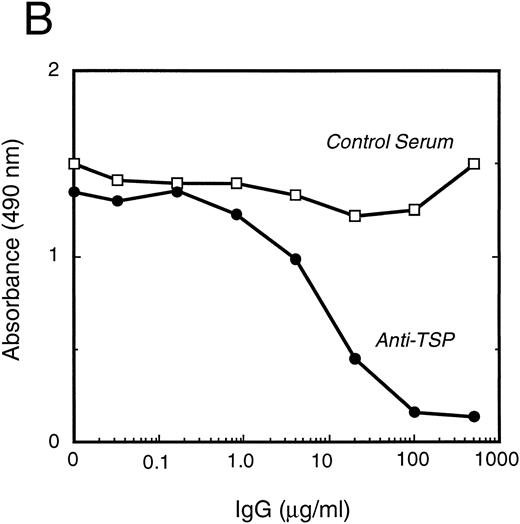
vWF binds specifically to TSP.To further elucidate the mechanism by which vWF inhibits TSP-mediated RBC binding, we used an ELISA-based microtiter binding assay. Our results demonstrate saturable binding of vWF to immobilized TSP with apparent half-maximal binding occurring at 2 μg/mL of vWF (Fig 6A). It was further demonstrated that this interaction was specifically inhibited by anti-TSP antiserum (Fig 6B).
vWF binding to immobilized TSP. (A) Results of a microtiter dish binding assay. vWF bound to immobilized TSP in a concentration-dependent manner (TSP, ▪). Nonspecific binding to BSA-coated wells (no TSP,□) and lack of binding in the presence of EDTA (circles) are also shown. (B) Inhibition of binding by anti-TSP antiserum. vWF (2.5 μg/mL) binding to immobilized TSP in the presence of increasing amounts of anti-TSP antiserum (circles) or control serum (squares) is indicated.
vWF binding to immobilized TSP. (A) Results of a microtiter dish binding assay. vWF bound to immobilized TSP in a concentration-dependent manner (TSP, ▪). Nonspecific binding to BSA-coated wells (no TSP,□) and lack of binding in the presence of EDTA (circles) are also shown. (B) Inhibition of binding by anti-TSP antiserum. vWF (2.5 μg/mL) binding to immobilized TSP in the presence of increasing amounts of anti-TSP antiserum (circles) or control serum (squares) is indicated.
DISCUSSION
Periodic painful crises and end organ damage in SCD are thought to arise principally from the occlusive events in the microvascular circulation. In the currently favored model, abnormal adherence of sickle reticulocytes and deformable discocytes to the postcapillary vessel wall is proposed to impede the flow of dense and irreversibly sickled RBC, which are less intrinsically adherent.9,12,28 The resultant increase in the capillary transit time is predicted to be sufficient to induce SS hemoglobin polymerization, morphologic sickling of the denser RBC, and persistent microvascular blockade in a relatively hypoxic environment. Activated EC may provide a critical stimuli to initiate this process through the surface expression of receptors including vascular cell adhesion molecule-1 (VCAM-1) and E-selectin,40-42 as well as the rapid elaboration of adhesive molecules, such as unusually large forms of vWF. Reversible or irreversible formation of intercellular gaps exposing subendothelial matrix components is also likely to be an important determinant of vaso-occlusive events in SCD.
The cytoadhesive proteins, vWF and TSP, have each been implicated in the adhesion of sickle and normal (Hb AA) erythrocytes to components of the vascular wall. We found that TSP and vWF, whose secretion is differentially regulated by vascular EC,16,27 also differ in their ability to support erythrocyte adhesion. Sickle erythrocytes are considerably more adherent to immobilized TSP than to vWF and the level of sickle erythrocyte adhesion to TSP is significantly greater than that observed for normal erythrocytes. To explore potential interactive effects of vWF and TSP, we examined sickle RBC adherence to mixtures of these proteins. Contrary to expectation, the binding of sickle RBC to immobilized TSP was found to be inhibited by vWF. Our data are most consistent with a model in which vWF binds to and blocks one or more RBC binding sites on TSP. This model is supported by the fact that an anti-TSP antiserum, which is directed against the type 3 repeats of TSP,17 inhibits both vWF and RBC interactions with TSP.
In SCD patients, endothelial matrix proteins may become exposed as a result of damage to the EC monolayer induced by sickle RBC42,43 or in response to locally elevated concentrations of agonists that induce EC gap formation, such as thrombin and histamine.44 The relative abundance of vWF and TSP may play an important role in regulating sickle erythrocyte-vessel wall interactions. The normal plasma concentration of vWF is 10 μg/mL, while that of TSP is approximately 500 ng/mL. In sickle cell patients presenting with painful crises, plasma TSP levels are twofold to fivefold higher45 and concentrations of TSP as high as 50 μg/mL have been measured in serum, presumably as a result of its release from activated platelets.46 Even at these elevated concentrations of TSP, our studies indicate that physiologic levels of vWF would be predicted to inhibit RBC adhesion. On the other hand, levels of vWF and TSP found in the extracellular matrix can be influenced by local factors, such as the induction of platelet18,21,22 and EC secretion.15,16 Vaso-occlusive crises are frequently encountered in the setting of inflammation and infection, at which times elevated levels of inflammatory cytokines such as interleukin-1 (IL-1), tumor necrosis factor-α (TNF-α), and interferon-γ may also influence EC secretion of vWF and TSP and the deposition of vWF in the matrix.16 47-50 Our in vitro study of RBC adhesion to immobilized vWF/TSP mixtures was intended to model the interaction of RBCs with the subendothelium. Based on our findings, we speculate that the presence of vWF may significantly reduce sickle RBC-subendothelial matrix interactions. However, the influence of vWF and TSP-mediated interactions of RBC with intact EC monolayers was not examined in this study and may be different from those that we observed with purified, immobilized proteins.
Wick et al10,11 demonstrated that high molecular weight multimers of vWF derived from human umbilical vein EC promoted the adhesion of sickle erythrocytes to EC monolayers. Similarly, Kaul et al12 showed that high molecular weight multimers of vWF released in response to desmopressin stimulation of ex vivo mesocecal vasculature markedly enhanced the adhesion of sickle RBC.12 The low level of sickle erythrocyte adhesion to immobilized recombinant vWF (and plasma-derived vWF ) demonstrated in the present study is consistent with the observation that the lower molecular weight vWF, derived from plasma, appears to be a poor promoter of RBC adhesion to cultured EC.10,11 A more recent study employing a dynamic flow assay similar to ours, also demonstrated that sickle RBC adhesion is not supported by immobilized, plasma-derived vWF.29 Plasma-derived and recombinant vWF display similar multimeric composition and are each fully able to support platelet adhesion under conditions of high shear stress.51 It remains possible that the unusually large multimers of vWF that are acutely released from EC in response to a variety of agonists may interact with TSP or sickle RBC in a qualitatively different manner.
TSP is a “matricellular” protein,14 capable of functional interactions with a variety of matrix proteins and cell types.52-54 Among these matrix proteins are included vWF,55 fibronectin,56 fibrinogen,56-58 laminin,55 and type V, but not types I, III, or IV collagen.55 Our results confirm the earlier observation that TSP binds to vWF and demonstrate that vWF binding to TSP is saturable and specific. The binding of vWF to TSP requires that one of the two proteins first be immobilized. This finding is consistent with previous observations that vWF and TSP undergo important alterations in functional capacity upon immobilization.59 60 Most important to the aim of the present study was the finding that the TSP/vWF complexes that are formed fail to support TSP-mediated sickle RBC adhesion under dynamic flow conditions.
The interaction that we observed between vWF and TSP provides a novel mechanism by which sickle RBC adhesive events may be regulated by products of the vascular endothelium. TSP is thought to play a dominant role in mediating sickle erythrocyte adhesion to EC.7,9 The present study demonstrates that the ability of TSP to promote RBC adhesion is inhibited by vWF at normal plasma concentrations. Constitutive levels of plasma vWF increase in response to a range of viral, bacterial, and parasitic infections,61 and locally high concentrations of vWF may be generated through the stimulation of EC by inflammatory mediators and coagulation proteins.62 Overall, the present data suggest that variations in the levels of TSP and vWF and possibly the molecular nature of vWF may exert a significant modulatory effect on TSP-mediated RBC adhesion and thus contribute to the pathophysiology of sickle vaso-occlusion.
Supported by National Institutes of Health Grants No. HL15157 (G.A.B., K.A.B., R.J.W., and B.M.E.), HL28749 (J.L.), HL30160 (R.P.H.), HL45629 (B.M.E.) and National Science Foundation Grant No. BCS-9110461 (G.A.B.).
Address reprint requests to Gilda A. Barabino, PhD, Department of Chemical Engineering, Northeastern University, 342 Snell Engineering Center, Boston, MA 02115.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal