Abstract
We evaluated different culture conditions to obtain a lineage-selected proliferation of clonogenic megakaryocytic progenitors (MP). In low-density (LD) or CD34+ cell cultures, the best results were obtained in serum-free medium in the presence of megakaryocyte growth and development factor, stem cell factor, interleukin-3 (IL-3), IL-6, IL-11, FLT-ligand, and macrophage inflammatory protein-1α. In paired studies, expansion of LD cells was less effective than expansion of CD34+ cells, and pre-enrichment of CD34+ cells using negative depletion of lineage-positive cells produced significantly larger quantities of MP than pre-enrichment using positive selection. MP proliferation peaked on day 7 in culture, and an 8- ± 5-fold expansion of CD34+/CD61+ cells, a 17- ± 5-fold expansion of colony-forming units-megakaryocytes, and a 58- ± 14-fold expansion of the total number of CD61+ cells was obtained. In a feasibility clinical study, 10 cancer patients (8 with breast cancer and 2 with non-Hodgkin's lymphoma) undergoing autologous peripheral blood progenitor cell (PBPC) transplant received MP generated ex vivo (range, 1 to 21 × 105/kg CD61+ cells) together with unmanipulated PBPC. Eight patients received a single allogeneic platelet transfusion, whereas platelet transfusion support was not needed in 2 of the 4 patients receiving the highest doses of cultured MP. This result compares favorably with a retrospective control group of 14 patients, all requiring platelet transfusion support. Adverse reactions or bacterial contamination of cell cultures have not been observed. In conclusion, MP can be expanded ex vivo and safely administered to autologous transplant recipients. Further clinical trials will indicate the reinfusion schedule able to consistently abrogate the need for allogeneic platelet transfusion support in autologous transplantation.
THROMBOCYTOPENIA is a complication of chemotherapy-based cancer treatment and particularly of high-dose chemotherapy (HDCT) followed by autologous or allogeneic transplantation of hematopoietic stem/progenitor cells. Because the demand for platelet transfusion support has been almost tripled in the past 12 years1 and there is a lack of other treatment for severe thrombocytopenia induced by chemotherapy, investigators are evaluating the ability of cytokines such as interleukin-11 (IL-11) or megakaryocyte growth and development factor (MGDF ) to prevent or reduce the need for platelet transfusion support in patients undergoing chemotherapy.2-4 We report here a different (and possibly complementary) approach based on the ex vivo generation of clonogenic megakaryocytic progenitors (MP). First, culture conditions for the growth of human MP have been defined; second, in a clinical feasibility trial, 10 cancer patients undergoing autologous peripheral blood progenitor cell (PBPC) transplant received escalating doses of autologous MP generated ex vivo by liquid culture in the presence of cytokines.
MATERIALS AND METHODS
In vitro studies: cell collection and processing.Cancer patients undergoing PBPC autologous transplantation were treated with 7 g/m2 cyclophosphamide (CTX) and subsequent administration of 5 μg/kg/d granulocyte colony-stimulating factor (G-CSF ). PBPC collection by apheresis was started when the postnadir CD34+ cell count was greater than 50/μL. The approval for use of human subjects in in vitro studies was obtained by the Institutional Review Board, and cells were obtained after informed consent was received. An aliquot of less than 10% of the apheresis product was processed by Ficoll (1.077 g/dL; Cedar Lane, Hornby, Ontario, Canada) and the Ficoll fraction was processed for CD34+ cell enrichment using two different procedures. According to the manufacturer's instructions, Ceprate (CellPro, Bothell, WA) immunoaffinity columns were used for positive selection of CD34+ cells. At the end of the procedure, CD34+ cell recovery was 53% to 87% and purity was in the range of 48% to 97%. Following a different approach, CD34+ cell enrichment was obtained by negative depletion of lineage-positive cells. Briefly, cells were resuspended in phosphate-buffered saline (PBS) without Ca+Mg+ supplemented by 5% autologous serum and further incubated in ice with a mixture of anti-glycophorin A, anti-CD3, anti-CD2, anti-CD56, anti-CD24, anti-CD19, anti-CD14, anti-CD16, and anti-CD66b tetrameric antibody complexes (Stem Cell Technologies, Vancouver, British Columbia, Canada). These tetrameric antibody complexes are bispecific cross-linkers that bind the described antigen and dextran. After 30 minutes of incubation, 20 nm magnetic colloidal iron/dextran particles were added, and, after 30 more minutes of incubation, cells were processed through the StemSep device for depletion of targeted cells according to the manufacturer's instructions (Stem Cell Technologies). At the end of the negative depletion procedure, CD34+ cell recovery was 51% to 77% and purity was in the range of 37% to 94%.
MP cell culture.The Ficoll fraction (low-density [LD] cells) and CD34+ cells purified by positive selection or negative depletion of lineage-positive cells were cultured in 25-cm2 flasks in serum-free X-vivo 10 medium (BioWhittaker, Walkersville, MD) or in Iscove's modified Dulbecco's medium (IMDM) supplemented by 5% AB human serum rendered platelet-free by high-speed centrifugation and 0.2-μm filtration. Both media were supplemented by 40 μg/mL ciprofloxacin (Bayer, Leverkusen, Germany). LD cells were seeded at 0.1 to 1 × 106 cells/mL. Purified CD34+ cells were seeded at 50 × 103 cells/mL. In CD34+ cell cultures, the cell concentration was checked on days 5, 7, and 10 and readjusted by addition of fresh medium and splitting of the culture in two flasks (with no further cytokine feeding) when the cell concentration exceeded 150 × 103 cells/mL. In pilot studies, we determined the best cytokine concentration for MP proliferation, ie, 10 ng/mL for MGDF (a pegylated, truncated molecule related to thrombopoietin), stem cell factor (SCF ), IL-3, IL-6, and IL-11 and 20 ng/mL for FLT-ligand (FL) and macrophage inflammatory protein-1α (MIP-1α). Lower or higher cytokine concentrations were found to be less effective for MP expansion. In our hands, the presence in the cytokine combination of myeloid-specific growth factors such as G-CSF or granulocyte-macrophage colony-stimulating factor (GM-CSF ) or the delayed addition of MGDF was associated with a more profound switch of the cultured cells to the myeloid lineage, so myeloid-specific cytokines were not included in the cocktails. MGDF and SCF used in in vitro studies were a kind gift of Amgen (Thousand Oaks, CA). FL used in vitro was generously provided by Immunex (Seattle, WA). Other cytokines were purchased from Peprotech (London, UK) or R&D (Abingdon, UK).
Evaluation of the clonogenic potential.Because the various clonogenic assays for MP reported in the literature have major drawbacks, three different procedures were used in triplicate to evaluate the samples generated in this study. In a fibrin-clot assay, 1 to 3 × 105 nucleated cells or 5 to 20 × 103 purified CD34+ cells in IMDM were resuspended in a solution of 10% L-asparagine, 10% bovine serum albumin (BSA), 10% human AB serum, 10% bovine citrate plasma, 10% CaCl2 , and 10 ng/mL IL-3, IL-6, and MGDF. The solution was seeded in 24-well plates and incubated at 37°C in 5% CO2 . Moreover, two commercially available kits for burst-forming units-megakaryocyte (BFU-mega) and colony-forming units-megakaryocyte (CFU-mega) enumeration were used. In the first (Easymega; Hemeris, Sassenage, France), the medium contained 0.1% human collagen proteins, 1.5% BSA, 2 mmol/L L-glutamine, 100 μmol/L 2β mercaptoethanol, 0.1 mmol/L pyruvic acid, 250 μmol/L CaCl2 , 4% lipids, 10 μg/mL bovine pancreatic insulin, 300 μg/mL human transferrin, 2.5 ng/mL IL-3, 10 ng/mL IL-6, and 50 ng/mL MGDF. In the second, (Megacult HCC4701K; StemCell Technologies), 10 ng/mL IL-3, IL-6, and MGDF were added to the fetal bovine serum (FBS)-free base growth medium. Following instructions given by the manufacturers, 1 mL of commercially available media containing 1 to 3 × 105 nucleated cells or 5 to 20 × 103 purified CD34+ cells were seeded in triplicate in Petri dishes and incubated at 37°C in 5% CO2 . CFU-mega, ie, colonies of 5 to 50 CD41+ cells, were evaluated after 10 to 11 days of culture; BFU-mega, ie, colonies of 50 or more CD41+ cells, were evaluated after 18 days of culture. Briefly, wells were fixed in ice with methanol and acetone and subsequently incubated, first with anti-CD41 monoclonal antibody or an irrelevant control antibody (PharMingen, San Diego, CA) and then with a commercial alkaline-phosphatase anti-alkaline phosphatase (APAAP) complex (rabbit antimouse mononoclonal IgG antibodies and APAAP kit; Dako, Glostrup, Denmark). After staining, colonies were evaluated by microscopy as pure mega colonies, mixed mega colonies, or non-mega colonies, and only pure mega colonies were enumerated. The absolute number of colonies grown at each time point during the liquid culture was calculated by multiplying their incidence (per cell seeded in clonogenic assays) by the number of viable cells present in each liquid culture. Overall, BFU-mega and CFU-mega results of the two commercial kits were similar, whereas fibrin clots cultures tended to lyse after 12 to 14 days and the BFU-mega enumeration was sometimes not possible. Because the growth of the absolute number of BFU-mega paralleled the growth of the absolute number of CFU-mega (ratio of 1 BFU-mega to 7 to 14 CFU-mega), for the sake of brevity the results of clonogenic assays are reported throughout the study as the average of the three methods used and as the total number of CFU-mega, ie, the sum of BFU-mega and CFU-mega. BFU-erythrocyte (BFU-E), CFU–granulocyte-macrophage (CFU-GM), and CFU–granulocyte, erythrocyte, monocyte, megakaryocyte (CFU-GEMM) were also evaluated by means of a commercially available kit (Stem Cell Technologies HCC 4434) as previously described in detail.5
Flow cytometry.Phycoerythrin (PE)- or fluorescein isothiocyanate (FITC)-labeled anti-CD15 (Becton Dickinson, Mountain View, CA), anti-CD34 (Becton Dickinson), anti-CD41 (PharMingen), anti-CD61 (Becton Dickinson), anti-glycophorin A (Dako), and anti-cytokeratin (Dako) monoclonal antibodies were used to study MP proliferation in culture and to enumerate breast cancer cells possibly contaminating PBPC collections and MP cultures. A total of 100 to 500 × 103 cells were incubated at 22°C for 30 minutes in PBS-1% BSA with monoclonal antibodies. In samples for cytokeratin-positive cell detection, cells were previously permeablized by a detergent commercial solution (Micromet, Planegg, Germany). By means of flow cytometry (FACScan; Becton Dickinson), the percentage of stained cells was determined as compared with PE- or FITC-conjugated mouse isotypic control. A portion of each sample was incubated with the appropriate isotype control antibodies to establish the background level of nonspecific staining, and positivity was defined as being greater than the nonspecific background staining. Because the expression of the CD41+ phenotype paralleled the expression of the CD61+ phenotype, throughout the study only the number of CD61+ or CD34+/CD61+ cells is given. Cell viability was evaluated by staining cells with ethidium bromide and acridine orange. Dead cells were gated out based on orange fluorescence. Moreover, the percentage of cells undergoing apoptosis was evaluated by 7-amino actinomycin D (7AAD) staining following the method recently described by Philpott et al.6 The method of Tomer et al7 was slightly modified to evaluate ploidy. Briefly, cells were labeled with FITC-anti CD61 and then fixed in 0.5% paraformaldehyde. In the presence of Triton X-100, DNA was then stained with propidium iodide. RNA was digested, and samples were analyzed gating on peak versus integral red fluorescence to exclude doublets.
Electron microscopy.MP suspensions were fixed overnight with 2% glutaraldehyde in PBS at 4°C. A rinse in PBS of the specimen preceded the 1 hour of postfixation in 1% OsO4 in PBS at 4°C and wash in distilled water. On the same occasions, fixed MP were incubated with anti-CD61 antibodies (clone VPL2; kindly provided by W. Knapp, Wien, Austria) and with goat antimouse IgG conjugated with 15-nm colloidal gold particles (Jansen, Beerse, Belgium). Dehydration was performed using a graded ethanol series from 70% up to 100% and propylene oxide. After embedding in Araldite, ultrathin sections (silver or very pale gold) were obtained with a Reichert Jung ultramicrotome, counterstained with uranyl acetate and lead citrate, and observed with a CM10 transmission electron microscopy at 80 kV (Philips, Eindhoven, The Netherlands).
Immunohistochemistry.Cytospin preparations of MP culture cells and appropriate controls were fixed in acetone or buffered formol-acetone (BFA) for 5 minutes and incubated for 30 minutes with unconjugated monoclonal antibodies reacting with CD61 (Dako) or glycophorin A (Dako). Breast cancer cell contamination was evaluated by means of a commercially available APAAP-based kit for the detection of cytokeratin-positive cells (Micromet). By means of rabbit antimouse monoclonal antibodies (Dako) and APAAP complexes (Dako), immunoreactivity was shown with three rounds of APAAP. Cells were counterstained with hematoxylin.
Clinical study.A total of 10 patients (8 with breast cancer [Bc] and 2 with non-Hodgkin's lymphoma [NHL]) were enrolled after obtaining signed informed consent approved by the Institutional Review Board and by the Ethical Committee. The PBPC mobilization regimen included a single dose of CTX (7 g/m2) and 5 μg/kg G-CSF administered daily up to the last apheresis. The CD34+ cell count in peripheral blood was monitored, and PBPCs were collected by apheresis when CD34+ cells exceeded 50/μL. At least 5 × 106 CD34+ cells/kg of body weight (BW) were collected by means of 1 or 2 apheresis procedures. Table 1 summarizes the patients' prior therapy and the results of the mobilization treatment. An aliquot of PBPCs collected by apheresis, containing 1 to 10 × 109 nucleated cells, was processed by Ficoll and cryopreserved in vials in 10% dimethylsulfoxide (DMSO) after controlled rate freezing. Seven days before the reinfusion of autologous PBPC, the aliquot was thawed, CD34+ cells were enriched by CellPro columns (CD34+ cell recovery of 38% to 56% and purity of 80% to 98%), and cells were cultured in 25-cm2 flasks in serum-free medium (X-vivo 10; BioWhittaker) in the presence of 20 ng/mL MIP-1α and FL and 10 ng/mL each of IL-3, IL-6, IL-11, SCF, and thrombopoietin. Cytokines used in the clinical trial were purchased from Peprotech or R&D. Bacterial contamination was excluded by the Bactec method (Becton Dickinson), and expansion of cytokeratin-positive breast cancer cells was evaluated by flow cytometry and immunohistochemistry. HDCT included thotepa at 600 mg/m2, L-PAM at 160 mg/m2 in BC patients, and Mitox at 60 mg/m2 and L-PAM at 180 mg/m2 in NHL patients. Three hours after the reinfusion of the unmanipulated apheresis (containing a range of 5 to 16 × 106 autologous CD34+ cells/kg BW), patients received autologous MP generated ex vivo in 7 days of culture. Patients no. 1 through 6 received 1 to 1.5 × 105 CD61+ cells/kg, whereas patients no. 7 through 10 received escalating CD61+ cell doses (2.5 to 21 × 105/kg). Before reinfusion, cultured MP were washed and resuspended in Plasma-Lyte A (Baxter, Deefield, IL) supplemented by 1% human albumin (Baxter). Cell viability was evaluated by flow cytometry. Vital signs were registered, and toxicity observations included cardiopulmonary, neurologic and gastrointestinal abnormalities, anaphylaxis, and myalgias. Patients received 5 μg/kg/d G-CSF up to a neutrophil count greater than 1,000 μL. The platelet count was evaluated every 12 hours, and platelet transfusions were administered for prophylaxis when the platelet count was less than the traditional trigger value of 20,000/μL on two consecutive occasions.
Patient Characteristics and Mobilization
| Patient No. . | Diagnosis . | Age (yr) . | Prior Chemo . | Prior Radiotherapy . | Total Collected CD34+/kg (×106) . | No. of Apheresis . |
|---|---|---|---|---|---|---|
| Study group | ||||||
| 1 | NHL | 23 | Yes* | No | 8.5 | 2 |
| 2 | NHL | 42 | Yes* | No | 5.0 | 2 |
| 3 | BC | 49 | Yes† | No | 10.9 | 1 |
| 4 | BC | 48 | Yes† | No | 14.1 | 1 |
| 5 | BC | 31 | Yes† | No | 9.1 | 1 |
| 6 | BC | 48 | Yes† | No | 6.7 | 2 |
| 7 | BC | 47 | Yes† | No | 16.5 | 1 |
| 8 | BC | 57 | Yes† | No | 10.2 | 1 |
| 9 | BC | 46 | Yes† | Yes | 5.2 | 1 |
| 10 | BC | 53 | Yes† | No | 5.7 | 2 |
| Mean ± 1SD | 44 ± 10 | 9.1 ± 3.8 | ||||
| Control group | ||||||
| 1 | NHL | 39 | Yes* | No | 7.5 | 2 |
| 2 | NHL | 29 | Yes* | No | 12.1 | 2 |
| 3 | BC | 43 | Yes† | No | 9.3 | 3 |
| 4 | BC | 45 | Yes† | No | 7.8 | 1 |
| 5 | BC | 48 | Yes† | No | 9.8 | 2 |
| 6 | BC | 32 | Yes† | No | 10.4 | 1 |
| 7 | BC | 54 | Yes† | No | 5.1 | 2 |
| 8 | BC | 47 | Yes† | No | 5.6 | 3 |
| 9 | BC | 48 | Yes† | No | 10.0 | 2 |
| 10 | BC | 52 | Yes† | No | 7.2 | 3 |
| 11 | BC | 47 | Yes† | No | 6.0 | 2 |
| 12 | BC | 53 | Yes† | No | 8.6 | 2 |
| 13 | BC | 41 | Yes† | No | 11.0 | 2 |
| 14 | BC | 51 | Yes† | No | 8.4 | 2 |
| Mean ± 1SD | 41 ± 13 | 8.4 ± 2.1 |
| Patient No. . | Diagnosis . | Age (yr) . | Prior Chemo . | Prior Radiotherapy . | Total Collected CD34+/kg (×106) . | No. of Apheresis . |
|---|---|---|---|---|---|---|
| Study group | ||||||
| 1 | NHL | 23 | Yes* | No | 8.5 | 2 |
| 2 | NHL | 42 | Yes* | No | 5.0 | 2 |
| 3 | BC | 49 | Yes† | No | 10.9 | 1 |
| 4 | BC | 48 | Yes† | No | 14.1 | 1 |
| 5 | BC | 31 | Yes† | No | 9.1 | 1 |
| 6 | BC | 48 | Yes† | No | 6.7 | 2 |
| 7 | BC | 47 | Yes† | No | 16.5 | 1 |
| 8 | BC | 57 | Yes† | No | 10.2 | 1 |
| 9 | BC | 46 | Yes† | Yes | 5.2 | 1 |
| 10 | BC | 53 | Yes† | No | 5.7 | 2 |
| Mean ± 1SD | 44 ± 10 | 9.1 ± 3.8 | ||||
| Control group | ||||||
| 1 | NHL | 39 | Yes* | No | 7.5 | 2 |
| 2 | NHL | 29 | Yes* | No | 12.1 | 2 |
| 3 | BC | 43 | Yes† | No | 9.3 | 3 |
| 4 | BC | 45 | Yes† | No | 7.8 | 1 |
| 5 | BC | 48 | Yes† | No | 9.8 | 2 |
| 6 | BC | 32 | Yes† | No | 10.4 | 1 |
| 7 | BC | 54 | Yes† | No | 5.1 | 2 |
| 8 | BC | 47 | Yes† | No | 5.6 | 3 |
| 9 | BC | 48 | Yes† | No | 10.0 | 2 |
| 10 | BC | 52 | Yes† | No | 7.2 | 3 |
| 11 | BC | 47 | Yes† | No | 6.0 | 2 |
| 12 | BC | 53 | Yes† | No | 8.6 | 2 |
| 13 | BC | 41 | Yes† | No | 11.0 | 2 |
| 14 | BC | 51 | Yes† | No | 8.4 | 2 |
| Mean ± 1SD | 41 ± 13 | 8.4 ± 2.1 |
Sequential chemotherapy including CTX, MTX, VCR, EPI, VP-16.
Sequential chemotherapy including CTX, MTX, VCR, EPI.
Data analysis.Statistical comparisons were performed by Primer (McGraw Hill, New York, NY) and StatView (Abacus Concepts, Berkeley, CA) software using the paired t-test, the Bonferroni t-test, and analysis of variance (ANOVA) in paired studies and the nonparametric analyses of Mann-Whitney, Wilcoxon, and Kruskal-Wallis in nonpaired studies. Values of P less than .05 were considered to be statistically significant.
RESULTS
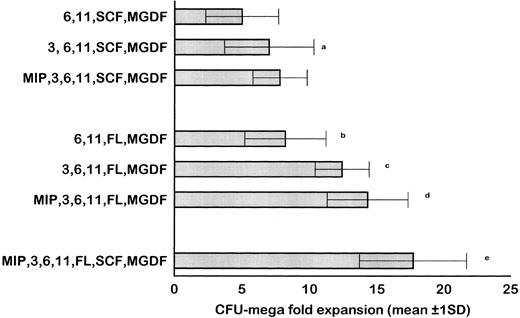
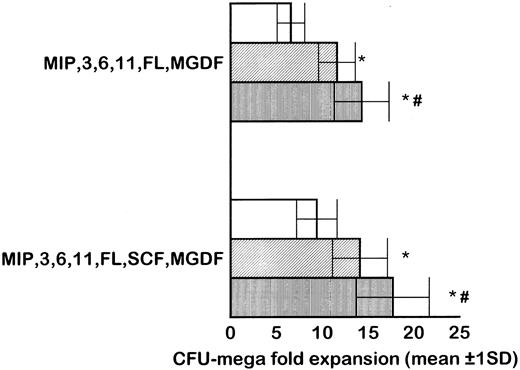
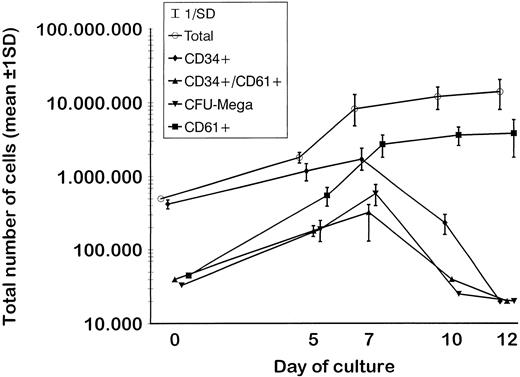
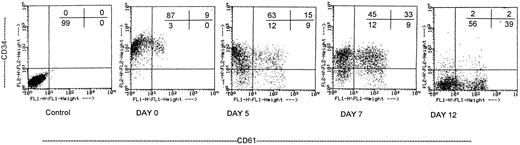
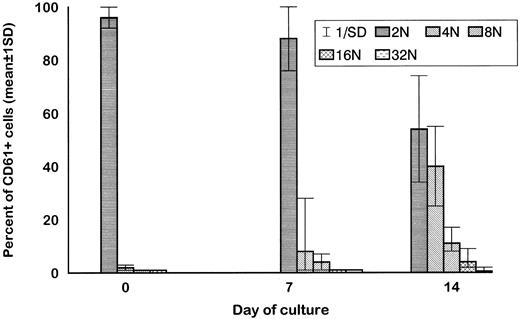
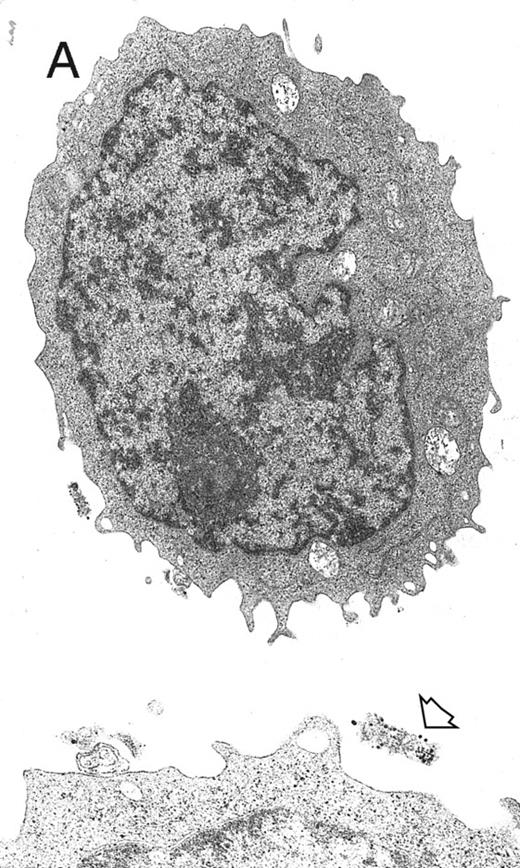
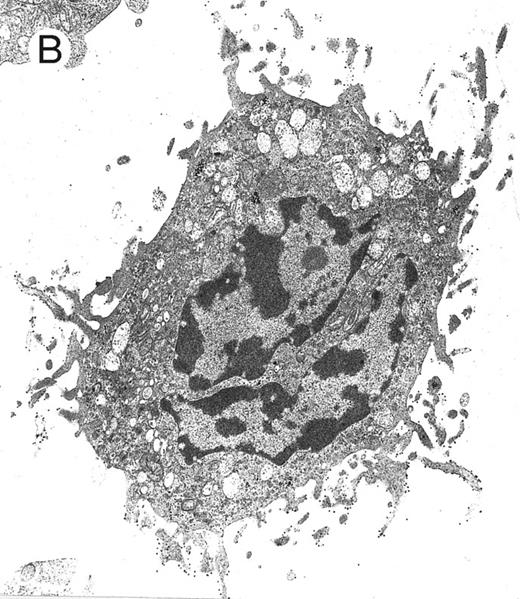
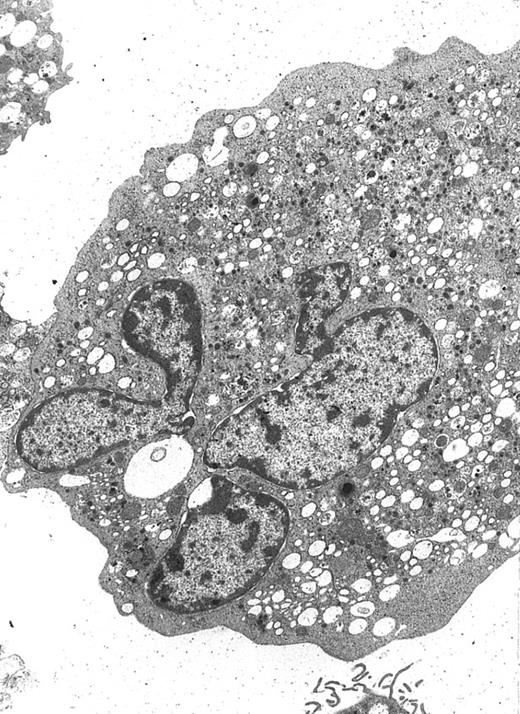
MP culture studies.We first determined in vitro in paired studies the best culture condition and cytokine combination for MP cell expansion. To this aim, cells from 5 different patients were enriched for hematopoietic progenitors and cultured in the presence of 7 different cytokine combinations. In our hands, serum-free culture was associated with significantly higher MP expansion compared with cultures in the presence of autologous or allogeneic platelet-free human serum (data not shown). As shown in Fig 1, the addition of IL-3 to the basic cytokine combination (including MGDF, IL-6, IL-11, and an early acting cytokine such as SCF or FL) boosted MP cell proliferation. Remarkably, when both FL and IL-3 were included in the cytokine combination, cell concentration had to be checked on days 4 and 5, because an increase greater than 150 × 103 cells/mL was highly detrimental for MP cell expansion. These cultures were therefore split to reduce cell concentration, and the concentration of cytokines was not further modified. FL was significantly more effective than SCF in MP cell proliferation (Fig 1), and the addition of MIP-1α to the IL-3, IL-6, IL-11, FL, and MGDF combination was also associated with an increase of MP expansion. Moreover, the presence of FL in the cytokine combination was associated with a lower incidence of apoptotic cells from day 7 onward (19% ± 14% apoptotic cells in the presence of FL v 35% ± 10% in the absence of FL, P < .05, paired t-test). Overall, in 5 paired studies (Fig 1), MIP-1α, IL-3, IL-6, IL-11, FL, SCF, and MGDF was found to be the most effective cytokine combination for MP proliferation. In 5 more paired studies, apheresis samples from the same patient were processed in 3 aliquots to determine the more appropriate procedure for cell purification. As shown in Fig 2, MP cell proliferation was significantly better in cultures of CD34+ purified cells than in LD cell cultures (P < .05, paired t-test). Remarkably, enrichment of CD34+ cells using negative depletion of lineage-positive cells produced significantly larger quantities of clonogenic MP than enrichment based on positive selection (P < .05, Fig 2). Figure 3 reports the pattern of MP cell proliferation in liquid cultures of CD34+ cells purified by negative depletion of lineage-positive cells (n = 4, cytokine combination MIP-1α, IL-3, IL-6, IL-11, FL, SCF, and MGDF ). MP expansion peaked on day 7, with a 1 log expansion of CD61+ cells between days 5 and 7. Using the cytokine combination including MIP-1α, IL-3, IL-6, IL-11, FL, SCF, and MGDF, we observed on day 7 an 8- ± 5-fold expansion of CD34+/CD61+ cells, a 17- ± 5-fold expansion of CFU-mega, and a 58- ± 14-fold expansion of the total number of CD61+ cells. Interestingly, CFU-GM and CFU-GEMM were also expanded (24- ± 18-fold and 6- ± 3-fold expansion, respectively), whereas the total number of BFU-E declined during the culture. As shown by culture studies (Fig 3) and flow cytometry (Fig 4), after day 7 the number of clonogeneic cells declined as the CD34 phenotype was progressively lost and 30% to 70% of cells acquired the CD61+ phenotype. Regarding ploidy, when the cytokine combination including MIP-1α, IL-3, IL-6, IL-11, FL, SCF, and MGDF was used, CD61+ MP with mature histology and also polyploid were increasingly seen after 7 days of culture (Figs 5, 6, and 7). This notwithstanding, as recently reported also by Dolzhanskiy et al,8 we saw that some MP become polyploid before the loss of CD34 expression. Distinct populations of 2N (36% to 76%), 4N (25% to 54%), 8N (8% to 17%), 16N (2% to 9%), and 32N (0% to 0.5%) CD61+ cells were seen in the second week of culture (Fig 5). As shown by electron microscopy, on day 7 about 30% of cells showed sparse chromatin, intracytoplasmic vescicles, microvilli, and variable degrees of CD61 labeling (Fig 6). Mature megakaryocytes were rare. In the second week of culture, electron microscopy (Fig 7) showed the generation of well-differentiated megakaryocytes with normal distribution of granules, cytoplasmic organelles, and demarcation membranes. Immunohistochemistry (Fig 8A and B) showed an intense CD61 staining and generation of proplatelets formation.9 Interestingly, expression of the erythroid (glycophorin A-positive) phenotype was negligible, whereas most of the non-megakaryocytic cells were CD15+ and expressed a myeloid phenotype. Using the CG5 BC line in limiting dilution assays, the sensitivity of the flow cytometry and immunohistochemistry procedures for BC cell enumeration were found to be of 1 cytokeratin-positive cell of 10 × 103 cells and of 1 cytokeratin-positive cell of 500 × 103 cells, respectively. Using these assays we did not find contaminating cytokeratin-positive cells at the beginning and at the end of MP cell cultures of BC patients (n = 8).
Results of 5 paired studies comparing the efficiency of different cytokine combinations for ex vivo expansion of megakaryocytic progenitors. In these experiments, CD34+ cells purified by negative depletion of lineage-positive cells were cultured in serum-free conditions. The basic cytokine combination included IL-6, IL-11, SCF, and MGDF. The addition of IL-3 and MIP-1α significantly increased the output of CFU-mega. When FL was included in the cytokine combination instead of (or together with) SCF, CFU-mega expansion was also significantly enhanced. Overall, MIP-1α, IL-3, IL-6, IL-11, FL, SCF, and MGDF was found to be the most effective cytokine combination. Results are reported as the average of the 3 clonogenic assays used. (a) P = .003 v IL-6, IL-11, SCF, MGDF; (b) P = .002 v IL-6, IL-11, SCF, MGDF; (c) P = .013 v IL-6, IL-11, FL, MGDF; (d) P = .003 v IL-3, IL-6, IL-11, FL, MGDF; (e) P = .001 v MIP, IL-3, IL-6, IL-11, FL, MGDF.
Results of 5 paired studies comparing the efficiency of different cytokine combinations for ex vivo expansion of megakaryocytic progenitors. In these experiments, CD34+ cells purified by negative depletion of lineage-positive cells were cultured in serum-free conditions. The basic cytokine combination included IL-6, IL-11, SCF, and MGDF. The addition of IL-3 and MIP-1α significantly increased the output of CFU-mega. When FL was included in the cytokine combination instead of (or together with) SCF, CFU-mega expansion was also significantly enhanced. Overall, MIP-1α, IL-3, IL-6, IL-11, FL, SCF, and MGDF was found to be the most effective cytokine combination. Results are reported as the average of the 3 clonogenic assays used. (a) P = .003 v IL-6, IL-11, SCF, MGDF; (b) P = .002 v IL-6, IL-11, SCF, MGDF; (c) P = .013 v IL-6, IL-11, FL, MGDF; (d) P = .003 v IL-3, IL-6, IL-11, FL, MGDF; (e) P = .001 v MIP, IL-3, IL-6, IL-11, FL, MGDF.
In 5 paired studies, apheresis samples from the same mobilized cancer patient were processed in 3 aliquots to determine the more appropriate cell purification procedure for MP cell proliferation, evaluated as CFU-mega expansion. Cells were cultured in serum-free medium in the presence of MIP-1α, IL-3, IL-6, IL-11, FL, and MGDF, and SCF was added in 5 more paired studies. Results of LD cell culture were significantly inferior than those of CD34+ purified cells (P < .05, paired t-test). Among different methods for CD34+ cell enrichment, negative depletion of lineage-positive cells was associated with the generation of significantly larger quantities of clonogenic MP than enrichment based on positive selection (P < .05). Bar indicates 1 standard deviation. (□) LD cells; (▨) positive selection of CD34+ cells; (▥) lineage-positive cell depletion *P < .05 v LD cells. #P < .05 v positive selection.
In 5 paired studies, apheresis samples from the same mobilized cancer patient were processed in 3 aliquots to determine the more appropriate cell purification procedure for MP cell proliferation, evaluated as CFU-mega expansion. Cells were cultured in serum-free medium in the presence of MIP-1α, IL-3, IL-6, IL-11, FL, and MGDF, and SCF was added in 5 more paired studies. Results of LD cell culture were significantly inferior than those of CD34+ purified cells (P < .05, paired t-test). Among different methods for CD34+ cell enrichment, negative depletion of lineage-positive cells was associated with the generation of significantly larger quantities of clonogenic MP than enrichment based on positive selection (P < .05). Bar indicates 1 standard deviation. (□) LD cells; (▨) positive selection of CD34+ cells; (▥) lineage-positive cell depletion *P < .05 v LD cells. #P < .05 v positive selection.
Proliferation of CD34+ cells purified by negative depletion of lineage-positive cells (n = 5) and cultured in serum-free medium in the presence of MIP-1α, IL-3, IL-6, IL-11, FL, SCF, and MGDF. As shown by the increase of CD34+/CD61+ cells and of CFU-mega, expansion of megakaryocytic progenitors peaked on day 7. Expansion of CD61+ cells was particularly evident between days 5 and 7 and reached a plateau in the second week of culture, when the number of clonogeneic cells declined. In fact, cell differentiation was shown by the striking decrease of the CD34 phenotype, whereas 30% to 50% of cells maintained the CD61+ phenotype.
Proliferation of CD34+ cells purified by negative depletion of lineage-positive cells (n = 5) and cultured in serum-free medium in the presence of MIP-1α, IL-3, IL-6, IL-11, FL, SCF, and MGDF. As shown by the increase of CD34+/CD61+ cells and of CFU-mega, expansion of megakaryocytic progenitors peaked on day 7. Expansion of CD61+ cells was particularly evident between days 5 and 7 and reached a plateau in the second week of culture, when the number of clonogeneic cells declined. In fact, cell differentiation was shown by the striking decrease of the CD34 phenotype, whereas 30% to 50% of cells maintained the CD61+ phenotype.
Proliferation and differentiation of purified CD34+ cells cultured in the presence of MIP-1α, IL-3, IL-6, IL-11, FL, SCF, and MGDF was evaluated by means of two-color staining with PE-labeled anti-CD34 (vertical axis) and FITC-labeled anti-CD61 (horizontal axis) antibodies. The first panel represents a control obtained with irrelevant antibodies, and the percentage of positive cells per each quadrant is indicated. The flow cytometry analysis shows the presence of a small subset of CD34+ cells that are also CD61+ among cells seeded on day 0. The number of CD34+/CD61+ cells increased on day 5, and expansion peaked on day 7. Cells expressing the CD34+ phenotype were still present during the first week of culture. Conversely, on day 12 most of the CD34+ cells were already differentiated, and 39% of the cells acquired the CD34−/CD61+ phenotype. The results of one representative experiment of five are shown.
Proliferation and differentiation of purified CD34+ cells cultured in the presence of MIP-1α, IL-3, IL-6, IL-11, FL, SCF, and MGDF was evaluated by means of two-color staining with PE-labeled anti-CD34 (vertical axis) and FITC-labeled anti-CD61 (horizontal axis) antibodies. The first panel represents a control obtained with irrelevant antibodies, and the percentage of positive cells per each quadrant is indicated. The flow cytometry analysis shows the presence of a small subset of CD34+ cells that are also CD61+ among cells seeded on day 0. The number of CD34+/CD61+ cells increased on day 5, and expansion peaked on day 7. Cells expressing the CD34+ phenotype were still present during the first week of culture. Conversely, on day 12 most of the CD34+ cells were already differentiated, and 39% of the cells acquired the CD34−/CD61+ phenotype. The results of one representative experiment of five are shown.
Ploidy of purified CD34+ cells cultured in the presence of MIP-1α, IL-3, IL-6, IL-11, FL, SCF, and MGDF was evaluated by means of flow cytometry bidimensional analysis (n = 10). Although some megakaryocyte progenitors become polyploid in the first 7 days of culture, more distinct polulations of 4N, 8N, 16N, and 32N CD61+ cells were evident at the end of the second week of culture.
Ploidy of purified CD34+ cells cultured in the presence of MIP-1α, IL-3, IL-6, IL-11, FL, SCF, and MGDF was evaluated by means of flow cytometry bidimensional analysis (n = 10). Although some megakaryocyte progenitors become polyploid in the first 7 days of culture, more distinct polulations of 4N, 8N, 16N, and 32N CD61+ cells were evident at the end of the second week of culture.
Electron microscopy evaluation of MP generated from purified CD34+ cells cultured for 7 days in the presence of MIP-1α, IL-3, IL-6, IL-11, FL, SCF, and MGDF. In (A) (original magnification × 11,230), an MP cell shows a sparse chromatin, a nucleolus, and few vacuoles and polyribosomes; the cell outline shows some microvilli and few gold particles indicating CD61 labeling (see arrow in the inset; original magnification × 25,120). A significantly more pronounced CD61 labeling is shown in the more differentiated MP in (B) (original magnification × 9,250).
Electron microscopy evaluation of MP generated from purified CD34+ cells cultured for 7 days in the presence of MIP-1α, IL-3, IL-6, IL-11, FL, SCF, and MGDF. In (A) (original magnification × 11,230), an MP cell shows a sparse chromatin, a nucleolus, and few vacuoles and polyribosomes; the cell outline shows some microvilli and few gold particles indicating CD61 labeling (see arrow in the inset; original magnification × 25,120). A significantly more pronounced CD61 labeling is shown in the more differentiated MP in (B) (original magnification × 9,250).
A well-differentiated megakaryocyte (original magnification × 3,080) generated from purified CD34+ cells cultured for 12 days in the presence of MIP-1α, IL-3, IL-6, IL-11, FL, SCF, and MGDF. A normal distribution of granules, mitochondria, and other cytoplasmic organelles is visible.
A well-differentiated megakaryocyte (original magnification × 3,080) generated from purified CD34+ cells cultured for 12 days in the presence of MIP-1α, IL-3, IL-6, IL-11, FL, SCF, and MGDF. A normal distribution of granules, mitochondria, and other cytoplasmic organelles is visible.
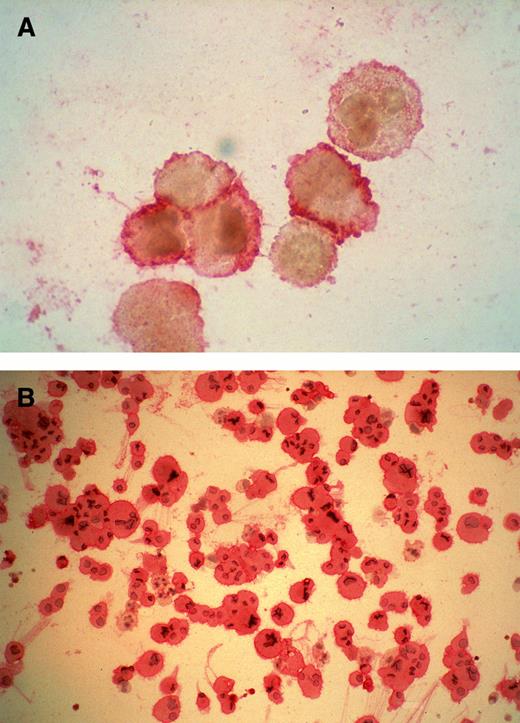
Cytospin preparations of megakaryocytes generated from purified CD34+ cells cultured for 14 days in the presence of MIP-1α, IL-3, IL-6, IL-11, FL, SCF, and MGDF. Most cells displayed a CD61+ phenotype detected by APAAP staining. In (A) cells were treated by BFA and staining was more intense on the cells' surface. Cells in (B) were permeabilized by acetone, and a number of proplatelet structures are visible, together with CD61+ cells with increased ploidy.
Cytospin preparations of megakaryocytes generated from purified CD34+ cells cultured for 14 days in the presence of MIP-1α, IL-3, IL-6, IL-11, FL, SCF, and MGDF. Most cells displayed a CD61+ phenotype detected by APAAP staining. In (A) cells were treated by BFA and staining was more intense on the cells' surface. Cells in (B) were permeabilized by acetone, and a number of proplatelet structures are visible, together with CD61+ cells with increased ploidy.
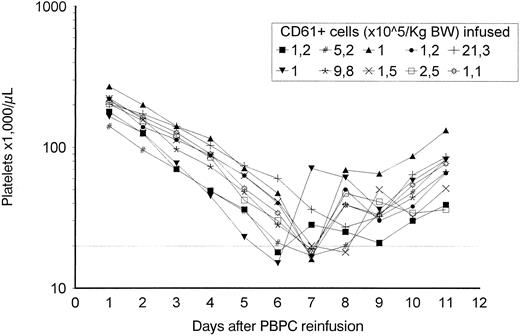
Absolute platelet recovery after high-dose chemotherapy for 10 patients that received cultured MP. Both cryopreserved, unmanipulated apheresis and cultured MP were infused on day 0. The dotted line represents 20,000 platelets/μL.
Absolute platelet recovery after high-dose chemotherapy for 10 patients that received cultured MP. Both cryopreserved, unmanipulated apheresis and cultured MP were infused on day 0. The dotted line represents 20,000 platelets/μL.
Clinical study.Adverse reactions associated with the reinfusion of cultured MP were not reported. Hematopoietic recovery was prompt in all 10 patients, with a median neutrophil recovery greater than 500/μL at day 9 (range, 8 to 11 days) and a median platelet recovery (>50,000/μL in the absence of platelet transfusion) on day 11 (median, 9 to 12 days; Fig 9). All patients experienced fever (>38°C) and mucositis >G2. Vaginal bleeding was observed in patient no. 9 from day 3 to day 6, when the platelet count was still greater than 30,000/μL. In 8 of 10 patients, the platelet count was less than 20,000/μL on two consecutive occasions, and these patients received one allogeneic platelet transfusion. Interestingly, platelet transfusions were not necessary in 2 of 4 patients receiving the highest doses of cultured MP (Table 2). In patient no. 8, who received 5.2 × 105/kg cultured CD61+ cells and unmanipulated PBPC containing 10.2 × 106/kg CD34+ cells, platelet transfusion was not required because the platelet nadir was at 17 × 103/μL for 12 hours without detectable bleeding. In patient no. 10, who received the higher dose of cultured MP (21.3 × 105/kg CD61+ cells) and unmanipulated PBPCs containing 5.7 × 106/kg CD34+ cells, platelet transfusion was not necessary because the platelet nadir was at 27 × 103/μL.
Characterization of Cultured CD34+ Cells Infused to Transplant Recipients and of HDCT
| Patient No. . | Cells Seeded on Day 0 (total CD34+ cells ×104/kg) . | Cells Infused After 7 Days of Culture . | HDCT . | ||||
|---|---|---|---|---|---|---|---|
| . | . | Total CD61+ Cells (×105/kg) . | % Viable . | CFU-mega (×104/kg) . | CFU-GM (×104/kg) . | CFU-GEMM (×104/kg) . | . |
| 1 | 2.2 | 1.0 | 82 | 1.8 | 2.8 | 0.1 | Mitox, L-PAM |
| 2 | 3.2 | 1.5 | 85 | 2.3 | 3.2 | 0.2 | Mitox, L-PAM |
| 3 | 2.8 | 1.2 | 79 | 2.3 | 3.9 | 0.2 | Thiotepa, L-PAM |
| 4 | 3.3 | 1.2 | 86 | 2.1 | 3.1 | 0.3 | Thiotepa, L-PAM |
| 5 | 3.0 | 1.0 | 86 | 2.7 | 3.0 | 0.2 | Thiotepa, L-PAM |
| 6 | 2.5 | 1.1 | 94 | 2.6 | 2.9 | 0.2 | Thiotepa, L-PAM |
| 7 | 6.1 | 2.5 | 75 | 4.9 | 7.5 | 0.5 | Thiotepa, L-PAM |
| 8 | 12.2 | 5.2 | 89 | 11.0 | 14.6 | 1.1 | Thiotepa, L-PAM |
| 9 | 23.0 | 9.8 | 92 | 23.7 | 27.6 | 1.6 | Thiotepa, L-PAM |
| 10 | 52.7 | 21.3 | 87 | 38.2 | 60.3 | 4.1 | Thiotepa, L-PAM |
| Patient No. . | Cells Seeded on Day 0 (total CD34+ cells ×104/kg) . | Cells Infused After 7 Days of Culture . | HDCT . | ||||
|---|---|---|---|---|---|---|---|
| . | . | Total CD61+ Cells (×105/kg) . | % Viable . | CFU-mega (×104/kg) . | CFU-GM (×104/kg) . | CFU-GEMM (×104/kg) . | . |
| 1 | 2.2 | 1.0 | 82 | 1.8 | 2.8 | 0.1 | Mitox, L-PAM |
| 2 | 3.2 | 1.5 | 85 | 2.3 | 3.2 | 0.2 | Mitox, L-PAM |
| 3 | 2.8 | 1.2 | 79 | 2.3 | 3.9 | 0.2 | Thiotepa, L-PAM |
| 4 | 3.3 | 1.2 | 86 | 2.1 | 3.1 | 0.3 | Thiotepa, L-PAM |
| 5 | 3.0 | 1.0 | 86 | 2.7 | 3.0 | 0.2 | Thiotepa, L-PAM |
| 6 | 2.5 | 1.1 | 94 | 2.6 | 2.9 | 0.2 | Thiotepa, L-PAM |
| 7 | 6.1 | 2.5 | 75 | 4.9 | 7.5 | 0.5 | Thiotepa, L-PAM |
| 8 | 12.2 | 5.2 | 89 | 11.0 | 14.6 | 1.1 | Thiotepa, L-PAM |
| 9 | 23.0 | 9.8 | 92 | 23.7 | 27.6 | 1.6 | Thiotepa, L-PAM |
| 10 | 52.7 | 21.3 | 87 | 38.2 | 60.3 | 4.1 | Thiotepa, L-PAM |
DISCUSSION
As suggested in a recent review by Emerson,10 in the near future a number of neoplastic and nonneoplastic patients may benefit from infusions of autologous or allogeneic hematopoietic precursors, progenitors, and stem cells generated ex vivo. The first aim of the present feasibility study was to evaluate whether clonogenic MP from cancer patients undergoing autologous transplantation could be induced to proliferate in vitro. Paired studies showed that the most effective culture condition was serum-free and included a number of early acting and late acting cytokines. The inhibitory effect of human serum over the growth of MP is well known11 and seems to be due to cytokines such as transforming growth factor β, β-thromboglobulin, or platelet factor 4, either present in the human plasma or released from activated platelets. Conversely to what has been recently reported by Schattner et al,12 in our studies the addition of platelet-free human serum did not increase MP proliferation in comparison to serum-free conditions. However, this might be due to subtle differences in the preparation of platelet-free serum. The role of MGDF, SCF, IL-3, IL-6, and IL-11 in megakaryocyte development has been the object of an intense study in the past and has been reviewed recently by Kaushansky13; in a previous preliminary study, we have already shown that IL-6 and IL-11 add to the MGDF effect.14 Recent work has also suggested the presence of two populations of MP, one responsive to IL-3 that can fully differentiate only in the presence of MGDF and a second that is dependent on MGDF for both proliferation and differentiation.15 In this context, it has been also recently suggested in a submitted abstract that IL-3 is an effective inducer of CD41 expression, but inhibits polyploidization and the differentiation of CD34+/CD41− progenitors.16 In our studies, the presence of IL-3 in the cytokine combination was beneficial for MP proliferation, but required adjustment of cell concentration to avoid the detrimental effect of increased cell concentration. Interestingly, our data about MP proliferation ex vivo indicate that the presence of FL in the cytokine combination is significantly more effective than the presence of SCF without FL. Remarkably, FL, a recently discovered member of class III tyrosine kinase receptor family,17 is able to induce proliferation of very early hematopoietic progenitors that are nonresponsive to other early acting cytokines and to improve the maintenance of progenitors in vitro.18 This is also supported by our finding that FL significantly reduced the number of cultured cells undergoing apoptosis. Another novel observation from our studies is the effect of MIP-1α over MP cells in culture. Although in certain conditions19 MIP-1α has an inhibitory effect on progenitor cell proliferation, it has been reported in the past that MIP-1α, in combination with IL-3, can efficiently support Dexter long-term hematopoietic cultures20 and that it exerts an enhancing effect (depending on other growth factors present) on the growth of defined subsets of progenitor/stem cells.19-21 The intriguing bidirectional regulator effect of MIP-1α is currently under further investigation in our laboratory.
Our data about MP proliferation in vitro indicate that CD34+ cell purification should be recommended, not only to reduce the cost of MP cell generation ex vivo (10 to 100 L of medium would be necessary to culture LD cells), but also to improve MP cell growth. As shown in a recent report,8 cells of the megakaryocytic lineage are 20-fold more common in the CD34+ progenitor pool (about 10% of total CD34+ cells, as in our data) than in the more mature CD34− population. In our studies, CD34+ cell enrichment by depletion of lineage-positive cells was associated with higher MP cell output when compared with results obtained with positive selection of CD34+ cells. This could be due to a detrimental effect of direct binding of anti-CD34 antibodies to the surface of CD34+ cells.
In the trial reported here, we evaluated the feasibility and safety of the clinical use of MP generated ex vivo. CD34+ cells from cryopreserved samples were enriched by clinically approved immunoaffinity procedures (positive selection). One might speculate that even better results might be possible by means of CD34+ cell enrichment obtained by negative delpetion of lineage-positive cells. For 10 of 10 cancer patients, it was possible to generate clonogenic MP from an aliquot of cryopreserved PBPC collected for autologous transplant after HDCT, and bacterial contamination was not observed. MP proliferation was observed also in a patient who received chemotherapy and radiotherapy before HDCT. Interestingly, in the present study, the culture conditions did not lead to a proliferation of detectable cytokeratin-positive cells in MP cultures from breast cancer patients. This is particularly important given that some fresh breast cancer cells appear to be CD34+.22
We have decided to culture MP for no more than 7 days for a number of different reasons, including the increased risk of bacterial proliferation during prolonged 37°C culture. In vitro data indicated that most of MP differentiation to mature megakaryocytes and signs of proplatelet formation occurred from day 10 to 12 onward (Fig 8). Moreover, previous clinical experience with autologous transplantation of PBPC indicated that the nadir of platelet count occurred 7 to 8 days after transplantation. We reasoned that 7 days of culture in the presence of a defined cytokine combination could be sufficient to induce a commitment of progenitor cells to the megakaryocytic lineage. Following this hypothesis, further differentiation and possibly platelet generation around the time of platelet nadir due to HDCT was expected to occur in vivo after the reinfusion of MP cultured for 7 days. Among the 10 patients enrolled in the clinical feasibility study, 2 of the group of 4 who received highest doses of cultured MP did not require platelet transfusion support. The patient showing the most efficient platelet recovery after HDCT received the highest MP dose. This prompt platelet recovery was observed despite the fact that the patient received unmanipulated PBPC containing a number of CD34+ cells (5.7 × 106/kg) at the lower limit of the range of CD34+ cells administered to the study group of 10 patients (5.2 to 16.5 × 106/kg). Although the database is not large enough to draw a final conclusion, present results compare favorably with retrospective data from our institution, in which 12 BC patients and 2 NHL patients received the same HDCT regimen in the past (Table 1). Control patients received a total number of CD34+ cells similar to the study group (9.1 ± 3.8 and 8.4 ± 2.1 × 106 CD34+ cells/kg in the study group and in the retrospective control, respectively), and all patients in the control group required platelet transfusion support (mean 1.2 ± 0.4 platelet transfusion per patient, P = .02). In the control group, the median tempo to 50,000 platelets/μL in the absence of platelet transfusion was 12 days (range, 9 to 12 days). In other studies, patients received CD34+ cells expanded in the absence of MGDF and no effect on the platelet recovery was seen.10 23-25
In conclusion, we have defined culture conditions that support the proliferation of MP, and our data confirm the feasibility and the safety of the clinical use of MP generated ex vivo. A larger clinical trial now seems necessary to define the appropriate dosing of MP and the reinfusion schedule able to consistently abrogate the need for platelet transfusion support in autologous transplantation. In addition, MP could also be used to reduce the need for platelet transfusion support in recipients of allogeneic transplantation, in which the greatest potential benefit may be for patients receiving cord blood, who have recently been reported to be at risk for delayed recovery from thrombocytopenia.26
ACKNOWLEDGMENT
We thank Amgen for SCF and MGDF used in the in vitro studies and Immunex for the availability of FLT-ligand used in the in vitro studies.
Supported in part by a US National Blood Foundation grant and by European Union Concerted Action Biomed 2 “Eurocord”.
Address reprint requests to Francesco Bertolini, MD, PhD, Division of Medical Oncology, IRCCS Maugeri Foundation, Pavia Medical Center, viale Boezio 26, 27100 Pavia, Italy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal