Abstract
We report the results of intravenous anti-D (WinRho, WinRho SD) therapy in 261 non-splenectomized patients treated at the New York Hospital-Cornell Medical Center over the period from 1987 to 1994. Children (n = 124) and adult patients (n = 137) with classic immune thrombocytopenic purpura (ITP; n = 156) or human immunodeficiency virus (HIV) related thrombocytopenia (n = 105) and acute (n = 75) or chronic (n = 186) disease at the time of the initial anti-D treatment were studied. In addition, 11 previously splenectomized patients were treated as a separate group. Our objectives were to evaluate the following. (1) Efficacy of anti-D: The response after the initial infusion was analyzed according to clinical parameters, such as patient's age, HIV status, gender, disease duration, pretreatment platelet count, and hemoglobin value, as well as treatment-related factors, including the dose of anti-D, the solvent detergent treatment of the preparation, and the type of administration. (2) Use of anti-D as maintenance therapy: The duration of response after the initial infusion and the results of subsequent treatments were evaluated. (3) Safety/toxicity of anti-D: Postinfusion reactions and hemoglobin decrease after treatment were studied. Anti-D is a safe treatment providing a hemostatic platelet increase in greater than 70% of the Rh+ non-splenectomized patients. The group with the best results is HIV− children, but all patient groups respond and the effect lasts more than 21 days in 50% of the responders. Duration of response is not influenced by HIV status; furthermore, HIV+ patients show no adverse effects on hemoglobin decrease or HIV disease progression. Patients with chronic ITP after splenectomy have minimal or no response to intravenous anti-D.
IMMUNE thrombocytopenic purpura (ITP) is a relatively common entity in which antibody-coated platelets undergo accelerated destruction by the mononuclear phagocytic system.1,2 Thrombocytopenia is also a manifestation of human immunodeficiency virus (HIV) infection, occuring in greater than 10% of affected patients.3-5 Severe thrombocytopenia (platelet count <50 × 103/μL) is seen in 1% to 5% of them.6
Treatment of ITP7 and HIV-ITP has included conventional modalities, such as steroids,8-10 intravenous gammaglobulin (IVIG),11-13 and splenectomy.8,14 Antiretroviral agents, primarily zidovudine (AZT), have been used for the treatment of HIV-related thrombocytopenia.15,16 Infusions of IVIG result in substantial platelet increases in more than 70% to 80% of patients with ITP12 as well as in HIV-ITP.13 The dominant mechanism responsible for the acute effect of IVIG in ITP appears to be the Fc receptor blockade.17
In 1983, Salama et al18 hypothesized that the Fc blocking activity of IVIG might be a result of the anti-red blood cell (RBC) antibodies, ie, isohemagglutinins, contained in low titers in IVIG preparations. Immune-mediated clearance of the antibody-coated RBCs would spare the sensitized platelets because of preferential destruction of the RBCs by the MPS.18 The initial clinical trials of Rho (D) Ig in patients with ITP showed platelet increases in the majority of them.18-20 As expected, mild hemolysis was seen after treatment.
Subsequent studies have described the usefulness of anti-D in the treatment of patients with ITP,21-31 as well as those with HIV-ITP.30,32,33 Although the mechanism of effect has not been entirely clarified, antibody-coating of RBCs is unequivocally required for efficacy, because Rh− patients consistently failed to respond.29,33 34 Splenectomized patients responded less well than patients with an intact spleen20,29,35,36; a phenomenon consistent with studies showing that the spleen is the primary site of removal of the antibody-coated RBCs.
This report summarizes the results of intravenous anti-D therapy in 261 patients with ITP and HIV-ITP who had not had a splenectomy and were treated at a single institution over a period of 7.5 years. Patient and treatment variables were evaluated to define the efficacy and toxicity of anti-D treatment and identify the patient groups that would benefit from this therapeutic modality. In addition, 11 splenectomized patients were treated with higher doses of anti-D to evaluate efficacy in this less-responsive group.
MATERIALS AND METHODS
Patients
The study included all patients who received their initial anti-D treatment at the New York Hospital-Cornell Medical Center during the period from June 1987 to December 1994. A total of 261 non-splenectomized patients were evaluated, including the 43 patients reported in the first publication by our group.29
Entry criteria in this single-arm trial included the clinical diagnosis of ITP (isolated thrombocytopenia without an alternative explanation, classic ITP) or HIV-related thrombocytopenia (HIV-ITP) and a need for treatment, usually a platelet count less than 30 × 103/μL. All patients were Rh+ (D+). There were 124 children and 137 adults; 156 patients had classic ITP and 105 had HIV-ITP. Five patients had secondary ITP not related to HIV: 2 with systemic lupus erythematosus and 3 with chronic lymphocytic leukemia. Seventy-five patients had acute and 186 patients had chronic thrombocytopenia at the time of the initial anti-D treatment.
Eleven adult patients with chronic ITP who had undergone splenectomy (3 reported in 1991)29 were treated as a separate group and are not included in the overall analyses.
Many patients had received other treatments. Patients receiving concomitant medications at the time of the initial IV anti-D treatment, ie, steroids, had these tapered after the anti-D infusion, if a response was seen.
Approval for the study was obtained from the New York Hospital Institutional Review Board (IRB), and patients or their parents gave informed consent before initiation of treatment. Separate IRB approvals and consents were obtained for the specific studies: WinRho-WinRho SD, treatment of splenectomized patients, and Heavy-Light Sensitization of RBCs (see details below). The entire study was performed under BB-IND-2053 of the Food and Drug Administration.
Treatment
The first patients enrolled in the trial were treated with daily infusions of anti-D for 4 to 5 days (as previously reported).29 Subsequently, treatment consisted of a single administration. Anti-D was infused IV over 2 to 5 minutes.
The 11 splenectomized patients were treated with anti-D doses escalating from 100 to 200 μg/kg. In addition, a single dose of 80 μg/kg was administered subcutaneously in 5 of them.
Criteria for repeat infusions were the same as those for study entry, ie, a platelet count less than 20 to 30 × 103/μL. Patients who had suffered reactions with the initial infusion received premedication (acetaminophen and/or diphenhydramine) with the subsequent treatments.
Preparation of Anti-D
The only preparation of anti-D37,38 used in this study was WinRho (Cangene [Winnipeg, Manitoba, Canada] and NABI [Boca Raton, FL]). Lots incorporated solvent detergent methodology as of January 1993 and were known as WinRho SD.
Laboratory Evaluation
The initial complete blood counts (CBCs) were performed by the New York Hospital Hematology Laboratory; follow-up counts were often obtained at local laboratories. Platelet, hemoglobin, and reticulocyte counts were observed as frequently as needed. Response was defined as a platelet increase ≥20 × 103/μL.
Patients were not required to undergo HIV testing. For those known to be HIV+, the use of antiviral agents was noted. AZT therapy was not discontinued during anti-D treatment.
To evaluate the effect of anti-D infusions on the progression of HIV disease, CD3, CD4, and CD8 counts were observed at 3- to 6-month intervals and were analyzed retrospectively. The 18 HIV+ patients studied were all those that had received more than two anti-D infusions per year, were observed for a minimum of 2 years after initiation of anti-D treatment, and had a minimum of two CD4 measurements per year. Lymphocyte subset determination was performed by flow cytometry using a FACscalibur (Becton Dickinson, Mansfield, MA) and appropriate monoclonal antibodies.
Statistical Analysis
Analysis of variance (ANOVA) was used to evaluate platelet and hemoglobin differences among the various study groups. The level of significance was set at .05. (P values ranging from 0.05 to 0.1 were considered a trend; P values ≤.2 are shown in the tables, with the higher ones reported as not significant [NS].) For the HIV+ patients, the change of CD4 percentage per year was estimated by least square analysis.
Analysis of Data
Efficacy
The primary measure of efficacy was the platelet increase within 10 days after the initial anti-D treatment. Mean platelet increases (±SD) with the number of patients in each group are shown. Platelet increases and response rates were analyzed according to independent patient variables, including age, gender, chronicity of ITP, and HIV status, as well as pretreatment platelet and hemoglobin values. Treatment parameters, including dose of anti-D, preparation (WinRho or WinRho SD), and type of administration (heavy v light coating of the RBCs), were also evaluated. Dose was analyzed retrospectively; patients that received multiple daily infusions were not included in this analysis.
The comparison of WinRho to WinRho SD was performed in two ways. First, all patients were analyzed according to the preparation they received for their initial (single) infusion. A prospective comparison was also performed by alternating infusions of WinRho and WinRho SD in 10 patients (1 to 3 pairs of infusions per patient).
To evaluate if coating a smaller number of RBCs with a greater number of antibodies per cell (heavy sensitization) would accelerate their destruction and improve the rapidity of the platelet response, 19 pairs of infusions were studied in 9 patients. The same dose was administered either by IV infusion (light) over 15 minutes or by preincubating the patient's blood with the anti-D and then infusing it (heavy).
The patients who responded to the initial anti-D infusion (n = 189) were evaluated for the duration of effect. Response to subsequent treatments was analyzed in all 79 patients that received three infusions and in the 20 patients who received 10 or more infusions.
The duration of effect after all anti-D treatments was retrospectively compared with the response duration after all IVIG infusions in two groups: (1) in 23 HIV− patients with chronic ITP and (2) in 10 patients with HIV-related thrombocytopenia. All patients, except 1, had received IVIG first and subsequently received anti-D.
Safety
The primary toxicity was the decrease in hemoglobin values (hemolysis). The results were analyzed similarly to the platelet changes. The time interval to hemoglobin recovery was evaluated.
Infusion reactions were identified by reviewing case report forms and listing all adverse events that were considered to be probably or definitely related to the infusion of anti-D. A focused analysis of a 6-month period (July through December 1994) was also used because patients were contacted the day after their infusion. Serial CD4 percentage was monitored in HIV-ITP patients.
Review of the Medical Literature
All studies published by March 1, 1996 were reviewed.
RESULTS
Efficacy
Response to Initial IV Anti-D Treatment
Overall results.The mean platelet increase for all 261 non-splenectomized patients was 76 × 103/μL (SD, 97.3). A total of 189 patients (72%) responded to anti-D with an increase in the platelet count ≥20 × 103/μL (Table 1), and 119 patients (46%) had platelet increases greater than 50 × 103/μL (Table 2).
Response to Initial Anti-D Treatment: Platelet Increase and Hemoglobin Decrease
| . | All Patients . | Age . | Gender . | Disease Duration . | HIV Status . | ||||
|---|---|---|---|---|---|---|---|---|---|
| . | . | <18 yr . | >18 yr . | Male . | Female . | Acute . | Chronic . | HIV− . | HIV+ . |
| Patients (N) | (261) | (124) | (137) | (152) | (109) | (75) | (186) | (156) | (105) |
| Mean platelet increase (×1,000/μL) | 76 | 110 | 45 | 72 | 84 | 82 | 74 | 95 | 49 |
| SD | 97.3 | 122.9 | 49.5 | 83.0 | 114.4 | 117.3 | 88.3 | 114.3 | 54.0 |
| Response rate (%) | 72 | 81 | 65 | 71 | 74 | 73 | 72 | 78 | 64 |
| Patients (N) | (253) | (119) | (134) | (149) | (104) | (73) | (180) | (150) | (103) |
| Mean hemoglobin decrease (g/dL) | 0.8 | 0.9 | 0.8 | 0.7 | 1.0 | 0.7 | 0.9 | 0.9 | 0.7 |
| SD | 1.1 | 1.1 | 1.1 | 1.2 | 1.0 | 1.0 | 1.2 | 1.1 | 1.2 |
| P value (platelet increase) | P < .001 | P = NS | P = NS | P < .001 | |||||
| . | All Patients . | Age . | Gender . | Disease Duration . | HIV Status . | ||||
|---|---|---|---|---|---|---|---|---|---|
| . | . | <18 yr . | >18 yr . | Male . | Female . | Acute . | Chronic . | HIV− . | HIV+ . |
| Patients (N) | (261) | (124) | (137) | (152) | (109) | (75) | (186) | (156) | (105) |
| Mean platelet increase (×1,000/μL) | 76 | 110 | 45 | 72 | 84 | 82 | 74 | 95 | 49 |
| SD | 97.3 | 122.9 | 49.5 | 83.0 | 114.4 | 117.3 | 88.3 | 114.3 | 54.0 |
| Response rate (%) | 72 | 81 | 65 | 71 | 74 | 73 | 72 | 78 | 64 |
| Patients (N) | (253) | (119) | (134) | (149) | (104) | (73) | (180) | (150) | (103) |
| Mean hemoglobin decrease (g/dL) | 0.8 | 0.9 | 0.8 | 0.7 | 1.0 | 0.7 | 0.9 | 0.9 | 0.7 |
| SD | 1.1 | 1.1 | 1.1 | 1.2 | 1.0 | 1.0 | 1.2 | 1.1 | 1.2 |
| P value (platelet increase) | P < .001 | P = NS | P = NS | P < .001 | |||||
Platelet increase and hemoglobin decrease were calculated as the difference between the pretreatment and the day 7 posttreatment value. Response was defined as a platelet increase ≥20.
Response to Initial Anti-D Treatment: Interaction of Age and HIV Status — Distribution of Responses
| . | All Patients (N = 261) . | Age <18 yr . | Age >18 yr . | ||||
|---|---|---|---|---|---|---|---|
| . | . | All Children (N = 124) . | HIV− (N = 104) . | HIV+ (N = 20) . | All Adults (N = 137) . | HIV− (N = 52) . | HIV+ (N = 85) . |
| Platelet increases | |||||||
| <20 | 27% (72) | 19% (24) | 17% (18) | 30% (6) | 35% (48) | 31% (16) | 38% (32) |
| 20-50 | 27% (70) | 22% (27) | 21% (22) | 25% (5) | 31% (43) | 37% (19) | 28% (24) |
| 51-99 | 20% (52) | 18% (22) | 19% (20) | 10% (2) | 22% (30) | 21% (11) | 22% (19) |
| >100 | 26% (67) | 41% (51) | 42% (44) | 35% (7) | 12% (16) | 12% (6) | 12% (10) |
| Platelet increase ≥20 | 72% (189) | 81% (100) | 83% (86) | 70% (14) | 65% (89) | 69% (36) | 62% (53) |
| Platelet increase >50 | 45% (119) | 59% (73) | 62% (64) | 45% (9) | 34% (46) | 33% (17) | 34% (29) |
| Posttreatment platelet count | |||||||
| 2× baseline and >50 | 54% (140) | 66% (82) | 70% (72) | 50% (10) | 42% (58) | 40% (21) | 44% (37) |
| . | All Patients (N = 261) . | Age <18 yr . | Age >18 yr . | ||||
|---|---|---|---|---|---|---|---|
| . | . | All Children (N = 124) . | HIV− (N = 104) . | HIV+ (N = 20) . | All Adults (N = 137) . | HIV− (N = 52) . | HIV+ (N = 85) . |
| Platelet increases | |||||||
| <20 | 27% (72) | 19% (24) | 17% (18) | 30% (6) | 35% (48) | 31% (16) | 38% (32) |
| 20-50 | 27% (70) | 22% (27) | 21% (22) | 25% (5) | 31% (43) | 37% (19) | 28% (24) |
| 51-99 | 20% (52) | 18% (22) | 19% (20) | 10% (2) | 22% (30) | 21% (11) | 22% (19) |
| >100 | 26% (67) | 41% (51) | 42% (44) | 35% (7) | 12% (16) | 12% (6) | 12% (10) |
| Platelet increase ≥20 | 72% (189) | 81% (100) | 83% (86) | 70% (14) | 65% (89) | 69% (36) | 62% (53) |
| Platelet increase >50 | 45% (119) | 59% (73) | 62% (64) | 45% (9) | 34% (46) | 33% (17) | 34% (29) |
| Posttreatment platelet count | |||||||
| 2× baseline and >50 | 54% (140) | 66% (82) | 70% (72) | 50% (10) | 42% (58) | 40% (21) | 44% (37) |
The values shown are percentages with the number of patients (N) in parentheses. Platelet increase was calculated as the difference between the pretreatment and the day 7 posttreatment platelet count. Response was defined as a platelet increase ≥20. There was a significant difference (P = .005) in the mean platelet increases between HIV− and HIV+ children. No difference between the two pediatric groups was seen for platelet increases greater than 50. There was no difference in the mean platelet increases and responses between HIV− and HIV+ adults. Patients who achieved a certain posttreatment count (doubling of the pretreatment value and >50) are presented.
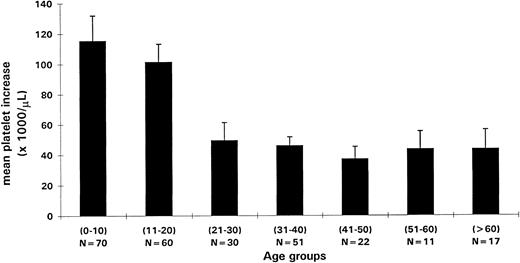
Clinical variables influencing response to anti-D: age, HIV infection (children), pretreatment hemoglobin (adults), and dose (trend).Age was the dominant factor influencing the acute platelet increase (Table 1). Platelet increases in children were greater than two-fold higher than those in adults (P < .001). When platelet increase was analyzed according to patient age by decade, there was a dramatic difference for age less than 20 years compared with age greater than 20 years (Fig 1).
Mean platelet increases (y-axis) for the study population divided in age groups by decade (x-axis) are shown. The number of patients per group (N) and SEs are shown. Young patients (<20 years) had significantly higher platelet increases (P < .001). Patients greater than 60 years of age had similar platelet increases to those 20 to 60 years of age.
Mean platelet increases (y-axis) for the study population divided in age groups by decade (x-axis) are shown. The number of patients per group (N) and SEs are shown. Young patients (<20 years) had significantly higher platelet increases (P < .001). Patients greater than 60 years of age had similar platelet increases to those 20 to 60 years of age.
The response rate in the HIV− children was higher (83%) than in the HIV− adults (69%; P = .05; Table 2).
The only clinical factor affecting the platelet increase in the pediatric population was HIV infection. HIV− children had a significantly higher mean platelet increase (119 × 103/μL) than HIV+ children (P = .005). Nonetheless, the mean platelet increase in the HIV-infected pediatric patients was 65 × 103/μL, with 70% responding to anti-D treatment, and 45% had a platelet increase greater than 50 × 103/μL (Table 2).
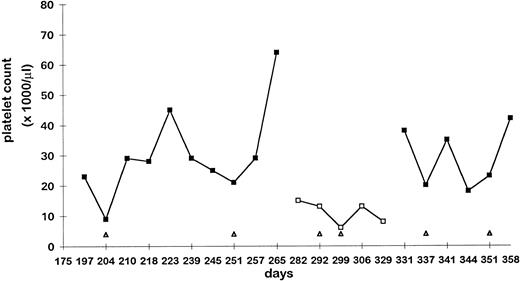
In adults, HIV infection did not influence the platelet responses to anti-D. Mean platelet increases were 46 × 103/μL for the HIV− patients and 45 × 103/μL for the HIV+ ones; the response distributions are shown in Table 2. Concomitant AZT treatment did not affect the platelet increase within the HIV-infected group. Mean platelet increases for the 56 patients receiving AZT and the 30 patients off AZT were similar (47 × 103/μL and 60 × 103/μL, respectively). In contrast to the group results, in 1 patient who started, stopped, and resumed receiving AZT while on anti-D therapy, AZT had an important additive effect (Fig 2).
Patient with HIV-related thrombocytopenia. The y-axis depicts platelet counts (×1,000/μL), whereas the x-axis depicts time (days). (▪) Platelet counts while the patient was receiving AZT; (□) platelet counts off AZT. Anti-D treatments are indicated (▵) along the x-axis. Good responses to anti-D were only seen with concomitant AZT treatment, despite the fact that AZT alone did not improve the platelet count. No difference in hemoglobin decrease after anti-D was seen with the use of AZT (data not shown).
Patient with HIV-related thrombocytopenia. The y-axis depicts platelet counts (×1,000/μL), whereas the x-axis depicts time (days). (▪) Platelet counts while the patient was receiving AZT; (□) platelet counts off AZT. Anti-D treatments are indicated (▵) along the x-axis. Good responses to anti-D were only seen with concomitant AZT treatment, despite the fact that AZT alone did not improve the platelet count. No difference in hemoglobin decrease after anti-D was seen with the use of AZT (data not shown).
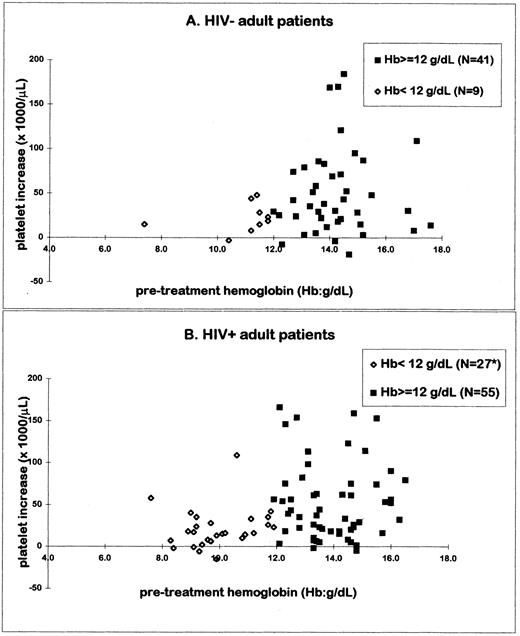
Baseline hemoglobin had a significant impact on platelet response to anti-D in adults (Fig 3). The mean platelet increase (51 × 103/μL) and the response rate (72%) in adults with pretreatment hemoglobin values ≥12 g/dL were significantly higher than the increase (21 × 103/μL) and response (42%) seen in adults with low baseline hemoglobins (P = .0002 and P < .01, respectively). Only 3 of 37 patients with a hemoglobin level less than 12 g/dL responded with a platelet increase greater than 50 × 103/μL, compared with 41 of 95 patients with a hemoglobin level ≥12 g/dL. The influence of the pretreatment hemoglobin was stronger in HIV+ adults (P < .01; Fig 3B) than in HIV− adults (P = .084; Fig 3A). In contrast, in 93 HIV− children greater than 3 years of age, no relationship between the baseline hemoglobin and the platelet increase or the response rate could be shown.
The relationship between the pretreatment hemoglobin and the platelet increase after anti-D was evaluated in HIV− (A) and HIV+ (B) adults. Patients with low baseline hemoglobin (<12 g/dL) were compared with those with normal values (≥12 g/dL). The x-axis depicts baseline hemoglobin values; the y-axis shows the platelet increases after anti-D treatment. Significantly higher platelet increases were seen in the adult patients with normal pretreatment hemoglobin (P = .0002). The influence was stronger in the HIV+ patients (P < .01) than in the HIV− ones (P = .084). *One HIV+ adult patient was excluded from the analysis; the baseline hemoglobin was 9.2 g/dL, but the platelet increase after anti-D (327 × 103/μL) was out of the range of the entire adult group.
The relationship between the pretreatment hemoglobin and the platelet increase after anti-D was evaluated in HIV− (A) and HIV+ (B) adults. Patients with low baseline hemoglobin (<12 g/dL) were compared with those with normal values (≥12 g/dL). The x-axis depicts baseline hemoglobin values; the y-axis shows the platelet increases after anti-D treatment. Significantly higher platelet increases were seen in the adult patients with normal pretreatment hemoglobin (P = .0002). The influence was stronger in the HIV+ patients (P < .01) than in the HIV− ones (P = .084). *One HIV+ adult patient was excluded from the analysis; the baseline hemoglobin was 9.2 g/dL, but the platelet increase after anti-D (327 × 103/μL) was out of the range of the entire adult group.
In 220 patients (84% of the study population), platelet increases and response rates did not differ significantly among five different dose levels ranging from less than 20 μg/kg to 60 μg/kg (P = .37). A trend towards a higher platelet increase was seen in patients who received anti-D doses of 40.1 to 60.0 μg/kg, compared with those who received ≤40.0 μg/kg (P = .085).
Clinical variables not influencing response to anti-D: gender, duration of ITP, and pretreatment platelet count.Patient gender had no effect on platelet increases or response rates (Table 1). When only HIV-infected patients were considered, male (n = 86) and female (n = 19) patients also had similar platelet increases and response rates. Duration of ITP did not affect platelet increase (Table 1). The response rates in children were the same whether they had acute (n = 43) or chronic (n = 61) disease (81% and 84%, respectively); mean platelet increases were 110 × 103/μL and 125 × 103/μL, respectively (P = .66).
No difference (P = .76) in platelet increases or response rates was found related to the pretreatment platelet count. The mean platelet increase and the response rate tended to be lower when the baseline platelet count was ≤10 × 103/μL (67 × 103/μL and 58%, respectively) compared with those of patients with pretreatment platelet counts greater than 10 × 103/μL (81 × 103/μL and 77%, respectively). These differences were not significant (P = .33 and P = .1, respectively).
Variables related to the administration of anti-D: solvent detergent treatment of the anti-D preparation and heavy or light sensitization of RBCs.Solvent detergent treatment of the WinRho preparation did not alter the platelet increase or response to initial treatment (Table 3). In the prospective cross-over trial, similar platelet increases were seen after the administration of each preparation. Nine patients received a total of 19 pairs of infusions alternating between heavy and light administration of anti-D. The platelet increases at 1, 7, and 14 days after treatment were not significantly different.
Preparation of Anti-D (WinRho or WinRho SD): Effect on Platelet Response and Hemoglobin Change
| Preparation . | Patients (N) . | <18 yr, HIV− . | Dose3-150 (mean) . | Mean Platelet Increase (SD) (×1,000/μL) . | Mean Hemoglobin Decrease (g/dL) (SD) . | |
|---|---|---|---|---|---|---|
| . | . | N . | % . | . | . | . |
| WinRho | 139 | 57 | 41 | 31.0 | 72 (99.9) | 0.9 (1.0) |
| WinRho SD | 92 | 38 | 41 | 42.4 | 93 (100.6) | 0.8 (1.1) |
| P = .12 | P = NS | |||||
| Preparation . | Patients (N) . | <18 yr, HIV− . | Dose3-150 (mean) . | Mean Platelet Increase (SD) (×1,000/μL) . | Mean Hemoglobin Decrease (g/dL) (SD) . | |
|---|---|---|---|---|---|---|
| . | . | N . | % . | . | . | . |
| WinRho | 139 | 57 | 41 | 31.0 | 72 (99.9) | 0.9 (1.0) |
| WinRho SD | 92 | 38 | 41 | 42.4 | 93 (100.6) | 0.8 (1.1) |
| P = .12 | P = NS | |||||
All patients were analyzed according to the anti-D preparation they received for their initial treatment. The distribution of patients with the best responses (HIV− children) was similar in the two groups. Platelet increase and hemoglobin decrease were calculated as the difference between the pretreatment and the day 7 posttreatment values.
There was a significant difference in the dose of anti-D between the two groups (dose in micrograms per kilogram).
Duration of response after the initial anti-D treatment.A total of 189 patients responded to the initial treatment. The effect of anti-D lasted more than 21 days in 50% of the responders. Significantly longer duration of response was seen in patients with platelet increases greater than 50 × 103/μL (P < .01). No difference in the response duration was found related to age or HIV status (Table 4).
Duration of Effect of Anti-D Treatment: Length of Platelet Response After the Initial Infusion
| . | . | Response Duration (d) . | |||||
|---|---|---|---|---|---|---|---|
| . | . | <7 . | >7 . | >14 . | >21 . | >28 . | >42 . |
| All responding patients | (N = 189) | 4 | 98% (185) | 75% (141) | 50% (94) | 37% (69) | 15% (29) |
| Responding patients with platelet increase >50 | (N = 120) | 100% (120) | 78% (94)4-150 | 57% (69)4-150 | 43% (52)4-150 | 17% (20) | |
| Responding patients with platelet increase 20-50 | (N = 69) | 1 | 98% (68) | 52% (36)4-150 | 30% (21)4-150 | 20% (14)4-150 | 10% (7) |
| HIV− children | (N = 86) | 3 | 97% (83) | 73% (63) | 45% (39) | 35% (30) | 15% (13) |
| HIV+ children | (N = 14) | 100% (14) | 93% (13) | 71% (10) | 57% (8) | 7% (1) | |
| HIV− adults | (N = 36) | 1 | 97% (35) | 75% (27) | 53% (19) | 36% (13) | 19% (7) |
| HIV+ adults | (N = 53) | 100% (53) | 72% (38) | 51% (27) | 36% (19) | 15% (8) | |
| . | . | Response Duration (d) . | |||||
|---|---|---|---|---|---|---|---|
| . | . | <7 . | >7 . | >14 . | >21 . | >28 . | >42 . |
| All responding patients | (N = 189) | 4 | 98% (185) | 75% (141) | 50% (94) | 37% (69) | 15% (29) |
| Responding patients with platelet increase >50 | (N = 120) | 100% (120) | 78% (94)4-150 | 57% (69)4-150 | 43% (52)4-150 | 17% (20) | |
| Responding patients with platelet increase 20-50 | (N = 69) | 1 | 98% (68) | 52% (36)4-150 | 30% (21)4-150 | 20% (14)4-150 | 10% (7) |
| HIV− children | (N = 86) | 3 | 97% (83) | 73% (63) | 45% (39) | 35% (30) | 15% (13) |
| HIV+ children | (N = 14) | 100% (14) | 93% (13) | 71% (10) | 57% (8) | 7% (1) | |
| HIV− adults | (N = 36) | 1 | 97% (35) | 75% (27) | 53% (19) | 36% (13) | 19% (7) |
| HIV+ adults | (N = 53) | 100% (53) | 72% (38) | 51% (27) | 36% (19) | 15% (8) | |
The values shown are percentages with the number of patients (N) in parentheses. Response was defined as a platelet increase ≥20. Duration of the anti-D effect was evaluated as follows: time for the platelet count to return to less than 20 (if the pretreatment count was <20) or to return to baseline (if the pretreatment count was >20) or time interval to the next infusion, whichever occurred first. Age and HIV status did not influence the response duration.
The platelet increase had a significant effect on the duration of response after anti-D (P < .01).
Platelet Response to Subsequent Anti-D Infusions
Response to the first three infusions.Seventy-nine patients received at least three infusions. Mean platelet increases (100 × 103/μL, 84 × 103/μL, and 83 × 103/μL, respectively) and response rates (72%, 67%, and 72%, respectively) were similar.
Response to 10 or more infusions.Among the 58 responders to all three infusions, 20 patients received an average of 18 infusions (range, 10 to 39), with an overall response rate of 86%. This group included a number of patients with marginal platelet increases (15 to 25 × 103/μL), for whom response or no response varied from infusion to infusion, depending on the exact platelet increase.
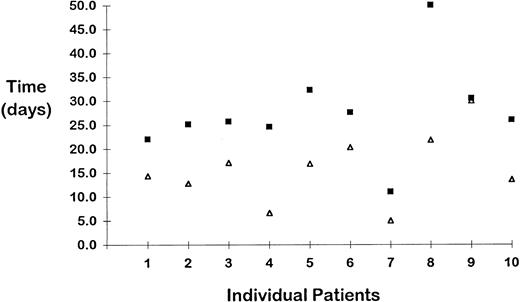
Retrospective comparison of the duration of response after IV anti-D to the duration of response after IVIG treatment.In 23 patients with classic ITP, no difference was seen in the duration of response after IVIG or IV anti-D treatment. In contrast, in 9 of the 10 patients with HIV-related thrombocytopenia, the effect of the anti-D lasted substantially longer than that of the IVIG (mean difference, 11.6 days; P = .007; Fig 4).
The duration of treatment effect was evaluated in 10 patients with HIV-related thrombocytopenia who received an average of 10 IVIG infusions (range, 1 to 26) and 15 anti-D infusions (range, 2 to 40), retrospectively. The y-axis depicts the mean duration (days) of effect for IVIG and anti-D treatments for each patient. The anti-D effect lasted longer in 9 of the 10 patients (mean difference, 11.6 days; P = .007). The mean platelet increase after anti-D infusions was significantly higher than the mean platelet increase after IVIG treatments (P = .02; data not shown). (▵) IVIG; (▪) anti-D.
The duration of treatment effect was evaluated in 10 patients with HIV-related thrombocytopenia who received an average of 10 IVIG infusions (range, 1 to 26) and 15 anti-D infusions (range, 2 to 40), retrospectively. The y-axis depicts the mean duration (days) of effect for IVIG and anti-D treatments for each patient. The anti-D effect lasted longer in 9 of the 10 patients (mean difference, 11.6 days; P = .007). The mean platelet increase after anti-D infusions was significantly higher than the mean platelet increase after IVIG treatments (P = .02; data not shown). (▵) IVIG; (▪) anti-D.
Safety
Infusion Reactions
A total of 59 adverse events were reported for 1,842 infusions (3.2%). The reactions that occurred in greater than 2 infusions were headache, nausea, chills, fever, and dizziness.39 During the 6-month period with the follow-up interviews, 179 infusions administered to 42 patients were evaluated. Twenty adverse reactions (11%) were reported, following 14 infusions in 11 patients. Only five reactions were reported as severe (2.8%), consisting of headache, chills, vomiting, hematuria, and change in skin color.
Hemolysis
Severity of hemolysis after the initial anti-D treatment.The mean hemoglobin decrease was 0.8 g/dL (SD, 1.5) 7 days after the first treatment (Table 1). Mean hemoglobin values at 7, 14, 21, and 28 days after the initial anti-D infusion are shown in Fig 5A. No patient required an RBC transfusion only as a result of anti-D treatment. Twenty-nine patients (16%) manifested hemoglobin decreases greater than 2.1 g/dL. Of those patients, 10 had received multiple infusions as their initial treatment with a cumulative total dose greater than 60 μg/kg in the first week.
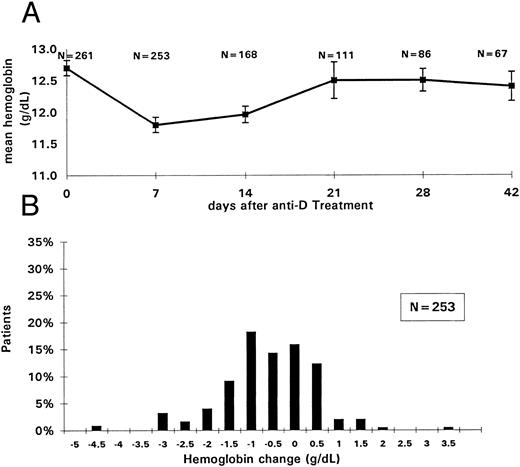
Changes in hemoglobin values after anti-D infusion. (A) Mean hemoglobin values for all patients treated with anti-D. The mean pretreatment (day 0) and the mean posttreatment hemoglobin values at 7, 14, 21, 28, and 42 days after the initial anti-D infusion are shown on y-axis; the days after treatment are shown on the x-axis. SEs and number of patients (N) evaluated at each time point are indicated. (B) Distribution of the hemoglobin changes for the entire study population. The differences between the pretreatment and the day 7 posttreatment hemoglobin values after the initial anti-D infusion are depicted on the x-axis; the percentage of patients within each group is shown on the y-axis.
Changes in hemoglobin values after anti-D infusion. (A) Mean hemoglobin values for all patients treated with anti-D. The mean pretreatment (day 0) and the mean posttreatment hemoglobin values at 7, 14, 21, 28, and 42 days after the initial anti-D infusion are shown on y-axis; the days after treatment are shown on the x-axis. SEs and number of patients (N) evaluated at each time point are indicated. (B) Distribution of the hemoglobin changes for the entire study population. The differences between the pretreatment and the day 7 posttreatment hemoglobin values after the initial anti-D infusion are depicted on the x-axis; the percentage of patients within each group is shown on the y-axis.
Clinical variables.No correlation of hemoglobin decrease was seen with age, gender, duration of ITP (Table 1), dose, solvent detergent treatment of the anti-D (Table 3), or heavy sensitization. Specifically, although hemolysis was more pronounced 1 day after the heavy administration (P = .07), the hemoglobin decreases at 7 and 14 days were not different from those seen after light RBC sensitization. Surprisingly, there was not a tendency for more pronounced hemoglobin decrease in the HIV+ patients (P = .2), not even in those with concomitant AZT treatment (mean hemoglobin decrease was 0.8 g/dL for the patients not on AZT and 0.7 g/dL for the patients on AZT). AZT also did not prolong the time to hemoglobin recovery. The distribution of hemoglobin changes for the study population is shown in Fig 5B. No correlation was found between the hemoglobin decrease and the platelet increase for all patients (n = 261, r = −.007), for responders only (n = 189, r = −0.1), or for HIV− children (n = 104, r = −.14).
Duration of the hemoglobin decrease.Of the 253 patients with hemoglobin data available on days 1 and 7 after treatment, 28.5% had no change or had complete hemoglobin recovery by day 7 (Table 5). Almost 50% of the evaluable patients had returned to baseline hemoglobin by day 14 and 65% by day 21.
Hemoglobin Recovery: Duration of Hemoglobin Decrease After the Initial Anti-D Treatment
| All Patients (N = 253) . | Complete Recovery (N = 133) . | Second Treatment Before Recovery (N = 65) . | Last Follow-Up Visit (N = 55) . | Recovery . |
|---|---|---|---|---|
| . | . | . | . | % (N)5-151 . |
| Day 7 | 725-150 | 23 | 28 | 28% (72/253) |
| Day 14 | 24 | 17 | 18 | 47% (96/202) |
| Day 21 | 13 | 12 | — | 65% (109/167) |
| Day 28 | 14 | 13 | — | 79% (123/155) |
| Day 42 | 10 | — | 9 | 93% (133/142) |
| All Patients (N = 253) . | Complete Recovery (N = 133) . | Second Treatment Before Recovery (N = 65) . | Last Follow-Up Visit (N = 55) . | Recovery . |
|---|---|---|---|---|
| . | . | . | . | % (N)5-151 . |
| Day 7 | 725-150 | 23 | 28 | 28% (72/253) |
| Day 14 | 24 | 17 | 18 | 47% (96/202) |
| Day 21 | 13 | 12 | — | 65% (109/167) |
| Day 28 | 14 | 13 | — | 79% (123/155) |
| Day 42 | 10 | — | 9 | 93% (133/142) |
Decrease was defined as a decrease greater than 0.2 g/dL from the pretreatment hemoglobin value. Recovery was defined as a return to the pretreatment hemoglobin value ±0.2 g/dL.
A total of 72 patients had no hemoglobin decrease 7 days after the anti-D treatment.
Patients with complete Hb recovery at a given day/evaluable patients at the same day (subtracting the ones who received a second infusion or were lost to follow-up).
Effect of Anti-D Treatment on HIV Disease Progression
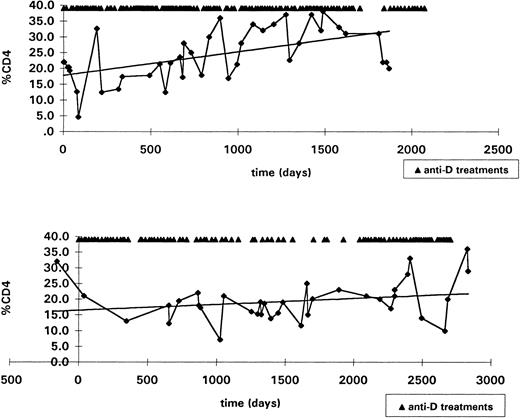
Eighteen HIV+ patients who received an average of 30 anti-D infusions over their entire course of treatment (median, 16.5; range, 6 to 145) had serial CD4 counts evaluated over a median period of 4.8 years. The average decline of the percentage of CD4 was 1.3 per year. Figure 6 shows the change of CD4 percentage over time for the two most intensively treated HIV+ patients.
To assess the effect of long-term anti-D treatment on HIV disease progression, serial CD4 counts were retrospectively evaluated in all 18 HIV+ patients who received more than two anti-D infusions per year, had two or more CD4 measurements per year, and were observed for a minimum of 2 years after starting anti-D therapy. The two most heavily treated patients are shown. The y-axis represents the percentage of CD4 and the x-axis the time (days). The arrows illustrate the anti-D infusions. Least square analysis of the change of CD4 percentage over time is presented. (Upper panel) A 33-year-old woman with HIV-related thrombocytopenia, who received 145 infusions of anti-D over 5 years and had a change of +2.7/yr in her CD4 percentage, ie, a total increase of 13.5%. (Lower panel) An HIV+ child with hemophilia who started anti-D treatment at 9 years of age and received 95 infusions over 8 years showed a change of +0.65/yr in the CD4 percentage, ie, a total increase of 5.2%.
To assess the effect of long-term anti-D treatment on HIV disease progression, serial CD4 counts were retrospectively evaluated in all 18 HIV+ patients who received more than two anti-D infusions per year, had two or more CD4 measurements per year, and were observed for a minimum of 2 years after starting anti-D therapy. The two most heavily treated patients are shown. The y-axis represents the percentage of CD4 and the x-axis the time (days). The arrows illustrate the anti-D infusions. Least square analysis of the change of CD4 percentage over time is presented. (Upper panel) A 33-year-old woman with HIV-related thrombocytopenia, who received 145 infusions of anti-D over 5 years and had a change of +2.7/yr in her CD4 percentage, ie, a total increase of 13.5%. (Lower panel) An HIV+ child with hemophilia who started anti-D treatment at 9 years of age and received 95 infusions over 8 years showed a change of +0.65/yr in the CD4 percentage, ie, a total increase of 5.2%.
Splenectomized Patients
Dose of Anti-D
The 11 splenectomized patients were treated with 100 μg/kg (n = 3),29 150 μg/kg (n = 2), and 200 μg/kg (n = 6) as a total weekly dose (Table 6). Responses were seen in none of the three patients who received 100 μg/kg, one of the two patients who received 150 μg/kg, and four of the six patients who received 200 μg/kg dose (2 responded by day 7, 2 others only at day 14 after treatment). The mean platelet increase for the 6 patients at the highest dose was 38 × 103/μL (median, 15 × 103/μL) by day 7 and 48 × 103/μL by day 14.
Splenectomized Patients: Treatment With High Doses of Anti-D
| Patient No. . | Dose (μg/kg) . | Platelet Increase (×1,000/μL) . | Hemoglobin Decrease (g/dL) . |
|---|---|---|---|
| (A) IV administration of anti-D | |||
| 1 | 90 | 4 | 0.7 |
| 2 | 110 | 3 | 0.4 |
| 3 | 130 | −13 | 2.8 |
| 4 | 150 | 25 | 1.1 |
| 5 | 150 | −5 | 1.7 |
| Median | 3 | 1.1 | |
| 6 | 200 | 17/526-150 | 0.6 |
| 7 | 200 | 3 | 0.4 |
| 8 | 200 | 59 | 0.4 |
| 9 | 200 | 13/306-150 | 0.5 |
| 10 | 200 | 7 | 2.2 |
| 11 | 200 | 135 | 1.6 |
| Median | 15 | 0.55 | |
| (B) Subcutaneous administration of anti-D | |||
| 6 | 80 | 3 | +1.2 |
| 7 | 80 | −11 | +0.8 |
| 9 | 80 | −10 | 0.5 |
| 106-151 | 80 | 3 | 0.1 |
| 11 | 80 | 34 | 0.3 |
| Median | 3 | +0.5 |
| Patient No. . | Dose (μg/kg) . | Platelet Increase (×1,000/μL) . | Hemoglobin Decrease (g/dL) . |
|---|---|---|---|
| (A) IV administration of anti-D | |||
| 1 | 90 | 4 | 0.7 |
| 2 | 110 | 3 | 0.4 |
| 3 | 130 | −13 | 2.8 |
| 4 | 150 | 25 | 1.1 |
| 5 | 150 | −5 | 1.7 |
| Median | 3 | 1.1 | |
| 6 | 200 | 17/526-150 | 0.6 |
| 7 | 200 | 3 | 0.4 |
| 8 | 200 | 59 | 0.4 |
| 9 | 200 | 13/306-150 | 0.5 |
| 10 | 200 | 7 | 2.2 |
| 11 | 200 | 135 | 1.6 |
| Median | 15 | 0.55 | |
| (B) Subcutaneous administration of anti-D | |||
| 6 | 80 | 3 | +1.2 |
| 7 | 80 | −11 | +0.8 |
| 9 | 80 | −10 | 0.5 |
| 106-151 | 80 | 3 | 0.1 |
| 11 | 80 | 34 | 0.3 |
| Median | 3 | +0.5 |
Platelet increase and hemoglobin decrease were evaluated 7 to 14 days after treatment. Median values for day 7 platelet and hemoglobin changes are shown.
Two patients responded only on day 14.
Patient no. 10 was also treated with 80 μg/kg Rhogam, an intramuscular form of anti-D, with no response.
One patient was maintained on regular anti-D treatments for a period of 6 months and received a total of 7 infusions at an average interval of 18 days, resulting in a mean platelet increase of 31 × 103/μL and a mean hemoglobin decrease of 1.2 g/dL.
Administration of Anti-D: Subcutaneous Versus IV Route
Five of the six patients treated with 200 μg/kg IV also received subcutaneous anti-D at 80 μg/kg (Table 6). Only one patient had a platelet increase (34 × 103/μL) 7 to 14 days after the subcutaneous treatment (patient no. 11, who also had the best response to IV anti-D).
Safety
Mean hemoglobin decreases were 1.3 g/dL for the doses of 100 to 150 μg/kg and 0.9 g/dL for the 200 μg/kg dose. The highest dose administered as a single infusion without side effects was 130 μg/kg. Both patients who received a single dose of 200 μg/kg experienced severe bone pain, despite premedication (acetaminophen and diphenhydramine) and slow infusion (30 to 60 minutes). Subsequently, the 200 μg/kg dose was divided into two infusions 2 to 4 days apart.
Comparison of the Current Report to the Previously Published Studies
All clinical trials of anti-D were summarized in Table 7.
Anti-D Treatment in ITP: Review of Published Studies
| Study . | Patients . | Mean Platelet Increase (×1,000/μL) . | Mean Hemoglobin Decrease (g/dL) . | Comments . | ||
|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . |
| Children | 1 | 11 | No change | |||
| (1) Acute ITP, HIV− | ||||||
| Panzer21 (1986) | ||||||
| Becker22 (1986) | 5 | 26 | Stable | Platelet increase at day 10 (figures) | ||
| Smith23 (1989) | 1 | 110 | NR | |||
| Blanchette24 (1994) | 38 | 239 | 1.52 | Data provided by author, patients treated at diagnosis | ||
| Current study | 47 | 108 | 0.6 | Platelet and hemoglobin changes at day 7, 2 patients treated at diagnosis | ||
| (2) Chronic ITP, HIV− | 1 | 22 | No change | |||
| Panzer21 (1986) | ||||||
| Becker22 (1986) | 10 | 118 | Stable | Platelet increase at day 10 (figures) | ||
| Andrew25 (1991) | 25 | 200 | 1.4 | Platelet increase at day 7 (figure) | ||
| Borgna-Pignatti26 (1994) | 7 | 119 | 1.6 | Intramuscular administration, max/min counts | ||
| Current study | 61 | 111 | 1.0 | Platelet and hemoglobin changes at day 7 | ||
| (3) Acute/chronic, HIV+ | ||||||
| No previous study | ||||||
| Current study | 20 | 65 | 0.7 | Platelet and hemoglobin changes at day 7 | ||
| Adults | ||||||
| (1) Acute ITP, HIV− | ||||||
| No previous study | ||||||
| Current study | 10 | 33 | 0.9 | Platelet and hemoglobin changes at day 7 | ||
| (2) Chronic ITP, HIV− | ||||||
| Salama19 (1984/1986) | 6 | 52 | 0.3 | Peak values | ||
| Salama20 (1986) | 13 | 71 | 0.7 | Peak values | ||
| Baglin35 (1986) | 3 | 115 | NR | |||
| Panzer21 (1986) | 3 | 71 | NR | |||
| Boughton27 (1988) | 7 | 107 | 0.9 | |||
| Smith28 (1990) | 4 | 94 | NR | |||
| Gringeri30 (1991) | 27 | 76 | 1.2 | Median values | ||
| Rodeghiero31 (1992) | 8 | 118 | 2.4 | |||
| Current study | 42 | 49 | 1.0 | Platelet and hemoglobin changes at day 7 | ||
| (3) Acute ITP, HIV+ | ||||||
| Rossi32 (1987) | 9 | 132 | 1.7 | Peak post-Tx platelet count, most patients had no prior Tx | ||
| Current study | 18 | 41 | 0.7 | Platelet and hemoglobin changes at day 7 | ||
| (4) Chronic ITP, HIV+ | ||||||
| Rossi32 (1987) | 4 | 107 | 1.0 | Peak Post-Tx platelet count, patients had prior Tx | ||
| Oskenhendler33 (1988) | 14 | 44 | 0.4-2.2 | Platelet increase at days 3-12, patients had prior Tx | ||
| Gringeri30 (1991) | 24 | 92 | 1.2 | Median values (IV induction), no prior Tx | ||
| Current study | 67 | 47 | 1.0 | Platelet and hemoglobin changes at day 7 | ||
| Splenectomized patients | Dose | Preparation | Administration | |||
| Baglin35 (1986) | 2 | 31 | NR | 100 μg × 5 | Anti-Rh(D) | IV |
| Salama20 (1986) | 4 | 19 | 1.7 | 300-3,600 μg | Anti-Rh(D) | IV |
| Cardo36 (1991) | 2 | 42 | NR | 1800 μg × 3 | Rhogam | SC |
| Current study | 11 | 27 | 1.1 | 100-200 μg/kg | WinRho SD | IV/SC |
| Study . | Patients . | Mean Platelet Increase (×1,000/μL) . | Mean Hemoglobin Decrease (g/dL) . | Comments . | ||
|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . |
| Children | 1 | 11 | No change | |||
| (1) Acute ITP, HIV− | ||||||
| Panzer21 (1986) | ||||||
| Becker22 (1986) | 5 | 26 | Stable | Platelet increase at day 10 (figures) | ||
| Smith23 (1989) | 1 | 110 | NR | |||
| Blanchette24 (1994) | 38 | 239 | 1.52 | Data provided by author, patients treated at diagnosis | ||
| Current study | 47 | 108 | 0.6 | Platelet and hemoglobin changes at day 7, 2 patients treated at diagnosis | ||
| (2) Chronic ITP, HIV− | 1 | 22 | No change | |||
| Panzer21 (1986) | ||||||
| Becker22 (1986) | 10 | 118 | Stable | Platelet increase at day 10 (figures) | ||
| Andrew25 (1991) | 25 | 200 | 1.4 | Platelet increase at day 7 (figure) | ||
| Borgna-Pignatti26 (1994) | 7 | 119 | 1.6 | Intramuscular administration, max/min counts | ||
| Current study | 61 | 111 | 1.0 | Platelet and hemoglobin changes at day 7 | ||
| (3) Acute/chronic, HIV+ | ||||||
| No previous study | ||||||
| Current study | 20 | 65 | 0.7 | Platelet and hemoglobin changes at day 7 | ||
| Adults | ||||||
| (1) Acute ITP, HIV− | ||||||
| No previous study | ||||||
| Current study | 10 | 33 | 0.9 | Platelet and hemoglobin changes at day 7 | ||
| (2) Chronic ITP, HIV− | ||||||
| Salama19 (1984/1986) | 6 | 52 | 0.3 | Peak values | ||
| Salama20 (1986) | 13 | 71 | 0.7 | Peak values | ||
| Baglin35 (1986) | 3 | 115 | NR | |||
| Panzer21 (1986) | 3 | 71 | NR | |||
| Boughton27 (1988) | 7 | 107 | 0.9 | |||
| Smith28 (1990) | 4 | 94 | NR | |||
| Gringeri30 (1991) | 27 | 76 | 1.2 | Median values | ||
| Rodeghiero31 (1992) | 8 | 118 | 2.4 | |||
| Current study | 42 | 49 | 1.0 | Platelet and hemoglobin changes at day 7 | ||
| (3) Acute ITP, HIV+ | ||||||
| Rossi32 (1987) | 9 | 132 | 1.7 | Peak post-Tx platelet count, most patients had no prior Tx | ||
| Current study | 18 | 41 | 0.7 | Platelet and hemoglobin changes at day 7 | ||
| (4) Chronic ITP, HIV+ | ||||||
| Rossi32 (1987) | 4 | 107 | 1.0 | Peak Post-Tx platelet count, patients had prior Tx | ||
| Oskenhendler33 (1988) | 14 | 44 | 0.4-2.2 | Platelet increase at days 3-12, patients had prior Tx | ||
| Gringeri30 (1991) | 24 | 92 | 1.2 | Median values (IV induction), no prior Tx | ||
| Current study | 67 | 47 | 1.0 | Platelet and hemoglobin changes at day 7 | ||
| Splenectomized patients | Dose | Preparation | Administration | |||
| Baglin35 (1986) | 2 | 31 | NR | 100 μg × 5 | Anti-Rh(D) | IV |
| Salama20 (1986) | 4 | 19 | 1.7 | 300-3,600 μg | Anti-Rh(D) | IV |
| Cardo36 (1991) | 2 | 42 | NR | 1800 μg × 3 | Rhogam | SC |
| Current study | 11 | 27 | 1.1 | 100-200 μg/kg | WinRho SD | IV/SC |
Platelet and hemoglobin changes were calculated as the difference between the pretreatment and the peak posttreatment counts; mean values are shown.
Abbreviations: Figure, data obtained from graphs; Tx, treatment; NR, not reported.
The previous pediatric studies using the same preparation of anti-D in HIV− children with acute24 or chronic ITP25 showed greater platelet increases than the ones reported here. Limited data exist for the treatment of thrombocytopenia in HIV+ children.40 This is the first study evaluating the effect of anti-D in this patient group.
There was considerable variation in the platelet increases seen in non-splenectomized adult patients with chronic ITP. The previous studies reported overall higher platelet increases, although the time of the peak platelet count was not always defined. No data were available for adult patients with acute ITP. For adults with HIV-related thrombocytopenia, three studies reported good responses to anti-D in both newly diagnosed and previously treated patients.30,32,33 Notwithstanding the higher doses of anti-D used, the splenectomized patients in the current study had similar responses to the 8 reported patients.20,35 36
DISCUSSION
The aim of this study was to define the therapeutic role of anti-D in ITP. Response rates for different patient groups are presented and several clinical variables are evaluated (Table 8), allowing comparison of anti-D to other treatments.
Hematologic Changes After the Initial Anti-D Treatment: Evaluation of Clinical Variables
| Significant Positive Effect . | Trend . | No Significant Effect . |
|---|---|---|
| PLATELET INCREASE | ||
| Blood type Rh+ 8-150 | Dose of anti-D | Gender |
| Duration of ITP | ||
| Prescence of spleen | HIV infection (adults) | |
| Baseline hemoglobin ≥12 g/dL (adults) | Pretreatment platelet count | |
| Age <20 yr | Solvent detergent treatment | |
| HIV− (children) | Administration (heavy or light) | |
| Hemoglobin decrease after treatment | ||
| RESPONSE DURATION | ||
| Peak platelet increase | Age | |
| HIV infection | ||
| HEMOGLOBIN DECREASE No variables with significant effect or trend were identified | ||
| Significant Positive Effect . | Trend . | No Significant Effect . |
|---|---|---|
| PLATELET INCREASE | ||
| Blood type Rh+ 8-150 | Dose of anti-D | Gender |
| Duration of ITP | ||
| Prescence of spleen | HIV infection (adults) | |
| Baseline hemoglobin ≥12 g/dL (adults) | Pretreatment platelet count | |
| Age <20 yr | Solvent detergent treatment | |
| HIV− (children) | Administration (heavy or light) | |
| Hemoglobin decrease after treatment | ||
| RESPONSE DURATION | ||
| Peak platelet increase | Age | |
| HIV infection | ||
| HEMOGLOBIN DECREASE No variables with significant effect or trend were identified | ||
The level of significance was set at .05.
Who are the Appropriate Candidates for Anti-D Treatment?
All subgroups of patients with ITP were represented in this series. Overall, IV anti-D treatment provided a hemostatic platelet increase for almost three quarters of the patients without prior splenectomy (Table 1). Moreover, approximately 50% of the treated patients had a platelet increase greater than 50 × 103/μL. The effect of anti-D lasted for more than 3 weeks in 50% of the responding patients and development of tachyphylaxis was infrequent.
Children had substantially greater platelet increases than adults. We observed an abrupt change in the level of platelet increase at approximately 20 years, rather than a more gradual decrease with advancing age (Fig 1). No such dramatic difference in the platelet responses has been delineated between children and adults with other treatment modalities, although IVIG41 and splenectomy42 have been suggested to be more efficacious in children. The greater efficacy of anti-D in children as well as the tendency of ITP of childhood to remit spontaneously or improve even after months of disease42 make anti-D an ideal maintenance treatment for these patients. It is also encouraging that the most elderly patients had good responses to anti-D, because they may be least able to tolerate splenectomy, have increased toxicity from steroids, and may be at greater risk of hemorrhagic complications.43
Treatment of HIV-related thrombocytopenia remains controversial. Steroids lead to short-lasting responses9 and may result in opportunistic infections. AZT increases the platelet count by stimulating thrombopoiesis,15,16 but some patients do not respond and others refuse it. Splenectomy increases the platelet count in the majority of patients and apparently does not influence the progression to acquired immunodeficiency syndrome.14 However, it increases the risk for fulminant infections, places the surgeon at risk, and seems most useful in patients with a reasonable life expectancy. Anti-D therapy offers advantages for HIV-ITP, because the duration of response for the HIV-infected patients was significantly longer than the duration of effect after IVIG treatment (Fig 4). This finding requires confirmation by prospective studies. Consistent with their separate mechanisms of action, AZT and anti-D appear to have additive effects in some individuals (Fig 2), surprisingly without aggravating anemia. Long-term follow-up of CD4 counts in the heavily treated patients showed no accelerated decline in CD4 numbers.44-46 More precise estimation of the effect on HIV disease progression would also require serial measurements of viral load.47 48 The relationship between the baseline hemoglobin and the platelet response after anti-D treatment was significant only in the HIV+ patients (Fig 3B). Lower hemoglobin levels in HIV-infected patients may indicate more advanced disease with bone marrow suppression; these patients respond less to all therapeutic interventions.
Anti-D was not an efficacious therapy for patients with chronic ITP after splenectomy (Table 8). Even with a fourfold increase in dose or subcutaneous administration, response was clearly limited and slower than in patients with an intact spleen. Furthermore, the need for more infusions per course of treatment and the cost associated with the higher dose are additional factors weighing against the use of anti-D in this group. Most adult patients with ITP undergo splenectomy. Anti-D may potentially have a role in postponing splenectomy, if this is proven by ongoing studies. However, given the low rate of spontaneous remission of adult ITP, we would not recommend its routine long-term use for adult patients at this time.
All patients treated were Rh+; anti-D has not proven efficacious for Rh− patients.33,34 In one study, the three Rh− patients who had not responded to anti-D responded to plasma containing anti-c.33
The severity of the thrombocytopenia did not affect the platelet increase or the response to anti-D significantly. However, as shown in our previous report,29 and confirmed by the randomized trial of Blanchette et al,24 posttreatment platelet counts in the responders did not increase as rapidly as those after IVIG infusion. Therefore, it has not been our practice to use IV anti-D as the initial treatment for patients presenting with very low platelet counts, such as children with acute ITP, or where there is medical urgency for hemostasis. In these cases, we would treat with IVIG and institute anti-D as maintenance therapy, if further treatment was required.
Hemolysis was the main adverse event of anti-D, but the decrease in hemoglobin values averaged less than 1 g/dL (Fig 5A). The anemia was transient and never severe enough to require a transfusion. No patient group was particularly susceptible to therapy-induced hemolysis and no clinical variable predicted for excessive hemolysis. Specifically, anemia was not a problem in the HIV-infected patients. Moreover, there was no correlation between the dose of anti-D, ranging from 20 to 60 μg/kg, and the degree of hemolysis. An unpublished study that evaluated the hemoglobin changes in healthy controls treated with different doses of IV anti-D (range, 25 to 75 μg/kg) also showed no relationship between dose and degree of hemolysis (B.M.R. Woloski, personal communication, 1994).
Can We Predict Response?
The current study as well as other smaller reports29 31 showed no relationship between the decrease in hemoglobin and the platelet increase, despite the fact that opsonization of RBCs is required for a platelet increase to occur.
The fact that escalating doses did not clearly result in greater platelet increases or more severe hemolysis highlights the complexity of the response to anti-D. Although a trend for the dose-response effect was seen in this series, a smaller study of 8 patients had indicated that dose escalations in individual patients resulted in higher peak platelet counts in seven of eight and longer duration of effect in six of eight patients.29 The lack of dose-response relationship in groups of patients in contrast to the dose-response effect seen in individual cases suggests that other clinical variables dominate the response to anti-D, such as the rate of platelet production and the patient's susceptibility to hemolysis.
As a result of the variability of the platelet increase and the hemoglobin decrease, there is a wide range of the optimally effective and maximally tolerated doses of anti-D. Management with anti-D initially requires weekly platelet counts and hemoglobin levels and probably reticulocyte counts. Standard doses may not apply to individual patients; dose titration is indicated in responders as well as in nonresponders with small hemoglobin decrease. Efficacy and duration of response may be optimized by increasing the dose, with anemia being the dose-limiting toxicity. This approach increases both the peak platelet count and the interval between infusions.
Speculations on the Mechanism of Action
HIV infection influenced the results of anti-D treatment in the pediatric patients only. HIV can infect megakaryocytes49,50 and can decrease platelet production15; the latter may be responsible for the poorer platelet responses seen in HIV+ compared with HIV− children. Some adults with chronic ITP apparently have decreased platelet production.51 The presence of HIV infection did not change the response to anti-D in this population. Furthermore, AZT may augment the response to anti-D by increasing platelet production,15 allowing for a greater platelet increase after FcR blockade.
The finding that the platelet increase and, presumably, the FcR blockade generated by anti-D last longer than those produced by IVIG in HIV-ITP requires confirmation. HIV-infected patients have high levels of serum IgG; therefore, the effect of IVIG treatment may be less than the effect seen in HIV− patients. In addition, HIV can alter FcR expression.52 Prolonged FcR-mediated clearance of antibody-coated RBCs has been shown in HIV-infected patients53; this mechanism could contribute to the longer effect of anti-D and decreased hemolysis.
The heavy-light study addressed the FcR blockade. The hypothesis was that the heavily-coated RBCs would result in a more rapid clearance54 and would provide a more instantaneous Fc blockade leading to a faster platelet increase. Although there was a trend to a more pronounced hemolysis at 24 hours on the heavy arm of the study, no substantial platelet effect was seen.
The relative failure of splenectomized patients to respond underlines the importance of spleen-mediated clearance of the antibody-coated RBCs. Given the limited number of D sites per RBC and their relative distance, anti-D antibodies generally do not fix complement. In the absence of spleen, it is surprising that anti-D has any effect at all. Possibly, polyclonal preparations of anti-D (such as WinRho) contain anti-idiotypic antibodies, which may result in immune complexes that can fix complement, explaining some degree of efficacy in splenectomized patients. Only one of seven ITP patients treated with a monoclonal preparation of anti-D had a substantial platelet increase.55
Practical Issues
Administration of anti-D is easy, requiring an IV infusion over 3 to 5 minutes during an outpatient visit. Most of the posttransfusion reactions are mild and can be minimized with premedication during future infusions.56 The preparation is IgA-depleted, and the solvent detergent treatment eliminates lipid-envelope viruses.57 Finally, the cost of anti-D is substantially lower than the cost of IVIG treatment.56
Concern has been raised regarding a potential shortage of the anti-D supply for prophylaxis in Rh− pregnant women as a result of the overuse in the treatment of ITP.58 If monoclonal anti-D preparations were to prove effective for either indication, their use may circumvent the problem. On the other hand, the use of immunization protocols allows for collection of increased quantities of anti-D plasma. It is, therefore, especially important that the solvent detergent treatment of the WinRho preparation of anti-D had no adverse effect on its efficacy.
Anti-D is an attractive alternative for long-term medical treatment of patients with immune thrombocytopenia. The patient population that would benefit the most from this treatment are children with persistent ITP and patients with HIV-related thrombocytopenia. Anti-D has a very limited, if any, role in the treatment of chronic ITP after splenectomy. Ongoing studies, including our own, are focusing on HIV− adults with acute ITP, evaluating the role of anti-D in avoiding splenectomy and on the effects of anti-D infusions on HIV.
ACKNOWLEDGMENT
The authors acknowledge the many physicians who referred patients for study; F. Malinoski and C. Wolf for review of the manuscript; and M. Novoa, G. Lanoix, and K. Jones for technical assistance.
Supported in part by Cangene Corp, NABI, and the Children's Blood Foundation.
Presented in part at the American Society of Hematology Meeting, Seattle, WA, December 1995 and at the Society of Pediatric Research, Washington, DC, May 1996.
Address reprint requests to James B. Bussel, MD, N740, Pediatric Hematology, The New York Hospital-Cornell Medical Center, 525 E 68th St, New York, NY 10021.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal