Abstract
Interleukin-10 (IL-10) has been found to inhibit lipopolysaccharide (LPS)-induced tissue factor expression by monocytes in vitro. To determine the effects of IL-10 on LPS-induced activation of the hemostatic mechanisms in vivo, we performed a placebo-controlled, cross-over study of human endotoxemia. Two groups of eight volunteers were challenged with LPS (4 ng/kg) on two occasions: once in conjunction with placebo, and once with recombinant human IL-10 (rhIL-10; 25 μg/kg). In group 1, placebo or rhIL-10 was given 2 minutes before LPS challenge, group 2 received placebo or rhIL-10 1 hour after LPS administration. Pretreatment with rhIL-10 reduced both LPS-induced activation of the fibrinolytic system (plasma concentrations of tissue type plasminogen activator, plasmin-α2–antiplasmin complexes, and D-dimer), and inhibition of fibrinolysis (plasma levels of plasminogen activator inhibitor 1), whereas posttreatment only inhibited the latter response. Both IL-10 pre- and posttreatment attenuated activation of the coagulation system (plasma levels of prothrombin fragment F1 + 2 and thrombin–antithrombin complexes). These results indicate that rhIL-10, besides its well-described inhibitory effects on cytokine release, potently modulates the fibrinolytic system and inhibits the coagulant responses during endotoxemia.
SYSTEMIC inflammation is associated with activation of the coagulation and fibrinolytic system. Injection of endotoxin (lipopolysaccharide [LPS]) to humans is a widely applied model to study early host responses to acute systemic inflammation, resulting in the release of cytokines and activation of the coagulation and fibrinolytic pathways.1,2 Within 2 hours after administration of a low dose of LPS, a transient activation of fibrinolysis can be detected, starting with the release of tissue type plasminogen activator (t-PA), which leads to generation of plasmin, as indicated by an increase in plasmin-α2–antiplasmin (PAP) complexes. Rapidly after the onset of plasminogen activation, plasminogen activator inhibitor 1 (PAI-1) concentrations increase, resulting in suppression of the fibrinolytic system.2,3 Increases in D-dimer, reflecting the formation of cross-linked fibrin, can be observed up to 10 hours after LPS administration.4 After the activation and inhibition of the fibrinolytic system, tissue factor–mediated activation of the common pathway of the coagulation system occurs, as reflected in increases in plasma concentrations of prothrombin fragment F1 + 2 and thrombin–antithrombin (TAT) complexes, which are maximal at the time of completely suppressed fibrinolysis.
Interleukin-10 (IL-10) is a 35-kD, noncovalently linked, homodimeric cytokine, produced by various cell types, including Th0 and Th2 cells, monocytes, and macrophages. IL-10 inhibits the synthesis of the proinflammatory cytokines tumor necrosis factor (TNF ) and IL-1 in vitro5,6 and prevents LPS-induced lethality in mice.7,8 Whole blood of human volunteers treated with IL-10 exhibited lower TNF production upon stimulation with LPS.9 Furthermore, we have recently demonstrated that during human endotoxemia, IL-10 reduced the release of TNF and prevented LPS-induced granulocyte accumulation in the lungs.10
In addition to the effects of IL-10 on cytokine release, IL-10 can directly affect the coagulation pathway by inhibiting the expression of LPS-induced tissue factor by monocytes in vitro.11-13 Tissue factor is essential for activating the extrinsic pathway of the coagulation system, the main pathway of coagulation activation during infection and inflammation.14 15 To our knowledge, the effect of IL-10 on LPS-induced activation of coagulation and fibrinolysis in vivo has not been demonstrated. Therefore, we conducted a cross-over, placebo-controlled, randomized study in a model of human endotoxemia and compared the effects of recombinant human IL-10 (rhIL-10) on activation of the fibrinolytic and coagulation system, when rhIL-10 was given either 2 minutes before or 1 hour after LPS challenge.
MATERIALS AND METHODS
Study group and design.This study was performed simultaneously with investigations of the effects of rhIL-10 on endotoxin-induced cytokine production and leukocyte activation, the results of which are reported elsewhere.10 A total of 16 healthy male volunteers 20 to 35 years of age participated in this double-blind, cross-over, randomized, placebo-controlled study. Written informed consent was obtained from all study subjects. The study was approved by the research and ethical committees of the Academic Medical Center. The medical history and the results of physical and routine laboratory examination, chest x-ray, and electrocardiography of all volunteers were normal. Each participant was studied on two occasions, separated by a wash-out period of 6 weeks. On one occasion the subject was challenged with endotoxin in combination with placebo, on the other in combination with rhIL-10. The volunteers were randomized into two groups of eight subjects. Group 1 received placebo or rhIL-10 treatment 2 minutes before endotoxin challenge; group 2 received placebo or rhIL-10 treatment 1 hour after endotoxin administration.
The study was performed at a special research unit under continuous supervision of at least two physicians, with emergency and resuscitation equipment immediately available.
Blood pressure and heart rate were assessed continuously using a Dinamap device (Criticon, Tampa, FL) during the first 8 hours after LPS challenge; oral temperature and respiratory rate were assessed each 30 minutes during the first 8 hours after LPS challenge. Adverse events were registered throughout the confinement periods.
rhIL-10 (Schering-Plough Research Institute, Kenilworth, NJ) was supplied as a sterile powder. After reconstitution with sterile water, the appropriate volume of rhIL-10 was administered by direct intravenous injection at a dose of 25 μg/kg. The reconstituted placebo powder, containing excipients, was identical in appearance and was administered in an identical manner. The endotoxin preparation used in this study, endotoxin reference standard lot G (Escherichia coli; US Pharmacopeia Convention Inc, Rockville, MD) was infused over 1 minute into an antecubital vein at a dose of 4 ng/kg, contralateral to the administration site of rhIL-10. The endotoxin preparation lot G exerts similar endotoxin activity as endotoxin E coli lot EC-5 (US Food and Drug Administration, Bethesda, MD) when measured using the Limulus amebocyte lysate assay (data not shown).
Blood sampling and assays.Blood for measurement of coagulation and fibrinolysis activation was drawn from antecubital veins by separate venipunctures immediately before endotoxin administration and at 1 (in group 2 just before placebo/rhIL-10 treatment), 2, 3, 4, 5, 6, 7, 8, and 20 hours thereafter. For the measurement of coagulation and fibrinolysis activation. Blood was collected in tubes containing buffered citrate solution (3.2%; Becton Dickinson, Mountain View, CA). Activation and inhibition of fibrinolysis was measured using sensitive immunoassays; for measurement of plasminogen activation and inhibition of plasminogen activation, plasma levels of t-PA antigen and PAI-1 were measured (Innogenetics, Nijmegen, The Netherlands; normal ranges, 1.5 to 15 ng/mL and 10 to 70 ng/mL respectively). Resulting plasmin generation and fibrin degradation was quantitated via enzyme-linked immunosorbent assays (ELISAs) for the measurement of PAP complexes and D-dimer, respectively (Behringwercke AG, Marburg, Germany; normal ranges 120 to 700 ng/mL and less than 400 ng/mL, respectively). Activation of coagulation was assessed by measurement of markers for thrombin generation, prothrombin fragment F1 + 2 and TAT complexes via ELISAs (Behringwercke AG, Marburg, Germany; normal ranges, 0.3 to 1.6 nmol/L and less than 4.6 ng/mL, respectively).
Statistical analysis.Values are given as means ± SEM. Differences between the placebo and rhIL-10 treatment groups were tested by cross-over analysis of variance (two-way ANOVA) for repeated measures using SPSS for Windows statistical package; the P values thus indicate the total treatment effect (P value versus placebo). Changes of variables over time were analyzed using one-way ANOVA (P value over time). A two sided P ≤ .05 was considered to represent statistically significant differences.
RESULTS
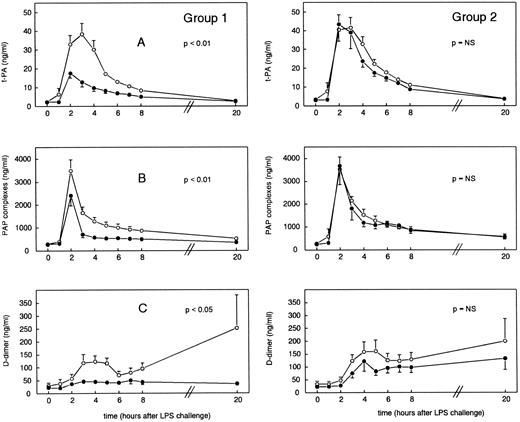
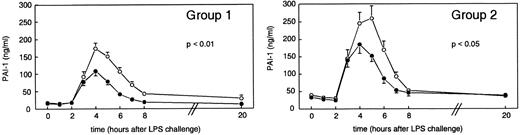
Activation and inhibition of fibrinolysis.Administration of LPS resulted in activation of fibrinolysis as measured by an increase in t-PA, PAP complexes, and D-dimer concentrations (Fig 1), which was subsequently suppressed owing to an increase in PAI-1 levels (Fig 2). After administration of LPS plus placebo, t-PA concentrations increased from baseline levels of 2.39 ± 0.30 ng/mL to peak mean levels of 38.46 ± 5.72 ng/mL (group 1) and from 3.46 ± 0.29 ng/mL at t = 0 hours to 41.63 ± 5.25 ng/mL (group 2) at t = 3 hours (P < .01 v time). As shown in Fig 1, pretreatment with rhIL-10 (group 1) significantly reduced peak mean t-PA concentrations to 17.53 ± 2.43 ng/mL at t = 2 hours (P < .01 v placebo), whereas delayed treatment with rhIL-10 (group 2) did not influence t-PA release (P = .2). In group 1, PAP complex concentrations increased from 298.13 ± 27.87 ng/mL at t = 0 hours to peak mean levels of 3,487.25 ± 484.44 ng/mL at t = 2 hours (P < .01 in time), a response that was reduced by rhIL-10 pretreatment to 2,409.75 ± 441.60 ng/mL (P < .01 v placebo). In group 2, PAP complexes increased from baseline levels of 283.88 ± 39.27 ng/mL to 3,529.63 ± 637.15 ng/mL at t = 2 hours (P < .01 in time); in this group, posttreatment with rhIL-10 had no inhibitory effect on the release of PAP complexes (P = .7). D-dimer concentrations were significantly increased after LPS administration from 30.75 ± 10.56 ng/mL at t = 0 hours to 123.50 ± 21.72 ng/mL at t = 4 hours, decreasing thereafter, followed by a second increase to 252.75 ± 126.75 ng/mL at t = 20 hours (group 1). Group 2 showed a similar pattern in D-dimer increase from 34.57 ± 11.28 ng/mL at t = 0 hours to 157.00 ± 45.80 ng/mL at t = 5 hours, followed by a second increase of 229.43 ± 87.01 ng/mL at t = 20 hours. rhIL-10 pretreatment blunted both the early and late increase in D-dimer levels, resulting in concentrations of 46.19 ± 9.91 ng/mL at t = 3 hours (P < .05 v placebo). rhIL-10 posttreatment did not affect LPS-induced changes in D-dimer levels (P = .2). LPS injection was associated with an increase in PAI-1 levels, which followed t-PA release by approximately 1 hour, increasing from 17.94 ± 2.01 ng/mL (baseline) to peak levels of 173.58 ± 16.26 ng/mL (group 1, t = 4 hours) and from 40.26 ± 6.80 ng/mL (baseline) to 259.96 ± 36.64 ng/mL (group 2, t = 5 hours) (P < .01 in time for each). Both pre- and posttreatment with rhIL-10 significantly attenuated the increase in PAI-1 concentrations, reaching peak levels of 108.37 ± 13.51 ng/mL (group 1, t = 4 hours, P < .05 v placebo) and 185.44 ± 26.26 ng/mL (group 2, t = 4 hours, P < .05 v placebo).
Mean (±SEM) plasma concentrations of t-PA (A), PAP complexes (B), and D-dimer (C) after endotoxin administration (4 ng/kg) in humans. Placebo (○) or rhIL-10 (25 μg/kg IV, •) was given just before endotoxin challenge (group 1) or 1 hour after endotoxin administration (group 2). P values indicate differences between treatment groups.
Mean (±SEM) plasma concentrations of t-PA (A), PAP complexes (B), and D-dimer (C) after endotoxin administration (4 ng/kg) in humans. Placebo (○) or rhIL-10 (25 μg/kg IV, •) was given just before endotoxin challenge (group 1) or 1 hour after endotoxin administration (group 2). P values indicate differences between treatment groups.
Mean (±SEM) PAI-1 plasma concentrations after endotoxin administration (4 ng/kg) in humans. Placebo (○) or rhIL-10 (25 μg/kg IV, •) was given just before endotoxin challenge (group 1) or 1 hour after endotoxin administration (group 2). P values indicate differences between treatment groups.
Mean (±SEM) PAI-1 plasma concentrations after endotoxin administration (4 ng/kg) in humans. Placebo (○) or rhIL-10 (25 μg/kg IV, •) was given just before endotoxin challenge (group 1) or 1 hour after endotoxin administration (group 2). P values indicate differences between treatment groups.
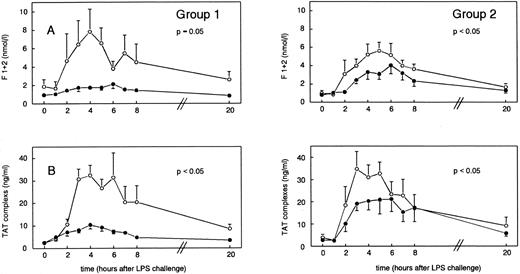
Coagulation activation.Injection of LPS induced increases in plasma levels of prothrombin fragment F1 + 2 from 1.88 ± 0.77 nmol/L (t = 0 hours) to peak concentrations of 7.82 ± 2.49 nmol/L (group 1 at t = 4 hours) and from 1.04 ± 0.31 nmol/L (t = 0 hours) to 5.63 ± 0.94 nmol/L (group 2 at t = 5 hours) (P < .05 in time). rhIL-10 treatment reduced F1 + 2 peak levels to 2.14 ± 0.47 nmol/L (group 1) and 3.65 ± 0.88 nmol/L (group 2) at t = 6 hours (P = .05 and P < .05 v placebo, respectively; Fig 3A). Plasma concentrations of TAT complexes increased from baseline levels of 2.47 ± 0.27 ng/mL to mean peak levels of 32.41 ± 4.41 ng/mL in group 1 (t = 4 hours) and from 4.01 ± 1.41 ng/mL (t = 0 hours) to peak concentrations of 34.81 ± 7.74 ng/mL in group 2 (t = 3 hours) (P < .05 v time). Pretreatment with rhIL-10 reduced the increase in TAT complexes (Fig 3B) to 10.41 ± 1.80 ng/mL (t = 4 hours, P < .05 v placebo), and when rhIL-10 was administered 1 hour after LPS challenge, plasma levels of TAT complexes were reduced with peak levels of 21.00 ± 6.46 ng/mL (t = 5 hours, P < .05 v placebo; Fig 3B).
Mean (±SEM) F1 + 2 (A) and TAT complex (B) concentrations after endotoxin administration (4 ng/kg) in humans. Placebo (○) or rhIL-10 (25 μg/kg IV, •) was given just before endotoxin challenge (group 1) or 1 hour after endotoxin administration (group 2). P values indicate differences between treatment groups.
Mean (±SEM) F1 + 2 (A) and TAT complex (B) concentrations after endotoxin administration (4 ng/kg) in humans. Placebo (○) or rhIL-10 (25 μg/kg IV, •) was given just before endotoxin challenge (group 1) or 1 hour after endotoxin administration (group 2). P values indicate differences between treatment groups.
DISCUSSION
LPS administration to humans elicits a systemic acute inflammatory response, characterized by release of cytokines and activation of the coagulation and fibrinolytic pathways. LPS administration first induces activation of the fibrinolytic pathway, which is followed by inhibition of fibrinolysis and activation of the coagulation system. It now seems evident that the induction of procoagulant and fibrinolytic activity during endotoxemia are two independent processes, of which the latter is mediated by TNF.16,17 Furthermore, endotoxin-induced coagulation activation is completely dependent on activation of the extrinsic pathway through tissue factor.15 18
IL-10 is a potent anti-inflammatory cytokine that inhibits the release of various proinflammatory cytokines in vitro and prevents endotoxin-induced lethality in mouse models.5-8 Increasing doses of IL-10 have been found to cause a dose-dependent inhibition of TNF and IL-1β release upon ex vivo stimulation with LPS.9 Based on these results, the concentration of 25 μg/kg was used in our study (the maximally tolerated dose). Independent from its immunosuppressive effects, IL-10 can influence coagulation activation by its capacity to inhibit LPS-induced production of tissue factor.11 12
The results of this study demonstrate that pretreatment with rhIL-10 reduces both activation and inhibition of fibrinolysis (t-PA, PAP complexes, D-dimer, and PAI-1) and activation of coagulation (F1 + 2 and TAT complexes) during human endotoxemia. Administration of rhIL-10 1 hour after LPS challenge significantly reduces inhibition of fibrinolysis and attenuates coagulation activation, but has no inhibitory effect on LPS-induced activation of fibrinolysis.
It has been shown that by blocking TNF activity with an anti-TNF monoclonal antibody, activation of fibrinolysis was almost completely prevented in endotoxemic chimpanzees, indicating that activation of fibrinolysis during endotoxemia is at least partially mediated by TNF.17 The differential effects of the two rhIL-10 treatment regimens in our study may be explained by differences in LPS-induced TNF release. As reported elsewhere,10 rhIL-10 pretreatment reduced LPS-induced TNF release (peak levels of 2.85 ± 0.77 ng/mL [LPS/placebo] v 0.67 ± 0.11 ng/mL [LPS/rhIL-10], P < .05), whereas posttreatment with rhIL-10 did not result in significantly reduced TNF release. The delayed rhIL-10 treatment (1 hour after LPS administration) might not have affected fibrinolysis activation because, at the time of administration of rhIL-10, TNF had already released to such an extent that activation of fibrinolysis could not be prevented. After activation of fibrinolysis, PAI-1 concentrations (reflecting inhibition of fibrinolysis) increased, starting 2 hours after LPS challenge, and peaking at 4 to 5 hours after LPS challenge. The increase in PAI-1 concentrations was blunted by both pre- and posttreatment with rhIL-10. In primate endotoxemia, the release of PAI-1 could be prevented using a TNF neutralizing antibody.3 The effects of rhIL-10 on PAI-1 release may have resulted in part from a reduction of TNF release. However, in view of the fact that rhIL-10 given 1 hour after LPS challenge still resulted in an important reduction of PAI-1 concentrations, other TNF-independent mechanisms may be involved in the rhIL-10–mediated inhibition of fibrinolysis during endotoxemia.
Activation of the coagulation system occurred together with the biphasic changes in fibrinolysis and remained detectable after suppression of LPS-induced fibrinolysis by PAI-1. Both pre- and posttreatment with rhIL-10 reduced LPS-induced increases in plasma concentrations of F1 + 2 and TAT complexes. Substantial evidence indicates that activation of the common pathway of coagulation is initiated via the extrinsic tissue factor–mediated pathway.14,19,20 Blockade of the tissue factor pathway in lethal sepsis in baboons completely prevented lethality.14,15 Because IL-10 is known to inhibit LPS-induced tissue factor in vitro,11 12 the reduction of LPS-induced thrombin formation could have resulted directly from rhIL-10–dependent prevention of tissue factor upregulation. This hypothesis is supported by the finding that posttreatment with rhIL-10 attenuated LPS-induced coagulation activation, although this treatment regimen had no significant effect on TNF release.
In conclusion, we have demonstrated that rhIL-10, when administered 2 minutes before LPS challenge, reduces the LPS-induced activation and inhibition of the coagulation and fibrinolytic pathways. rhIL-10, when given 1 hour after LPS challenge, does not influence LPS-induced activation of the fibrinolytic system, but the inhibition of fibrinolysis and the activation of coagulation is significantly reduced by rhIL-10. Clinically, this anticoagulant and profibrinolytic profile of IL-10 could result in an attractive modulation of coagulation and fibrinolysis in sepsis.
ACKNOWLEDGMENT
We thank D. Knol, M. Weijne, and H. Schipper for their technical assistance, as well as the nursing staff of the special research unit for their support in executing the study.
T.v.d.P. is a fellow of the Royal Netherlands Academy of Arts and Sciences.
Address reprint requests to Dasja Pajkrt, MD, Laboratory of Experimental Internal Medicine, Academic Medical Center, G2 - 105b, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal