Abstract
Bcl-2 and its homologue, Bcl-xl, encode membrane-associated proteins that protect neoplastic cells from DNA damage-induced apoptosis, whereas Bax is a Bcl-2 antagonist that promotes cell death. In the present study, we examined the expression and regulation of these genes at both the mRNA and protein level in 22 pediatric acute lymphoblastic leukemia (ALL) cell lines, as well as their sensitivity to apoptosis after exposure to ionizing radiation (IR). Eleven of 22 lines expressed wild-type (wt) p53, 4 expressed mutant p53, and 7 did not express p53 (p53-null). Nine of 22 (41%) lines expressed Bcl-2; of these, 8 were wt-p53+ and 1 expressed mutant p53. Bcl-2 was not expressed in any p53-null lines. In contrast, all 22 lines were positive for Bcl-xl and Bax, although expression level varied. Treatment with IR (10 Gy) induced both downregulation of Bcl-2 and upregulation of Bax at 2 to 5 hours post-IR in 5 of 8 (63%) wt-p53+ lines, leading to apoptosis. Conversely, lines that failed to both downregulate Bcl-2 and upregulate Bax after IR were resistant to apoptosis. Although levels of Bcl-xl expression varied among the 22 lines, high levels of Bcl-xl were observed in 5 of 7 (71%) p53− lines. There were no obvious changes in the expression of Bcl-xl in these lines after IR. However, among the p53-null lines, resistance to IR was observed only in those expressing high levels of Bcl-xl. These results suggest that expression of Bcl-2 but not Bcl-xl is p53-dependent and that IR-induced downregulation of Bcl-2 and upregulation of Bax occur in most wt-p53+ lines and are associated with radiosensitivity. Furthermore, high-level expression of Bcl-xl occurs predominantly in p53-null lines and is associated with resistance to IR-induced apoptosis in these lines, indicating differential expression and regulation of Bcl-2 and Bcl-xl in pediatric ALL.
PROGRAMMED CELL death, also called apoptosis, is an active process that can be induced by DNA-damaging agents such as ionizing radiation (IR) and chemotherapeutic drugs. Studies have shown an association between increased resistance to chemotherapeutic agents and decreased capacity to undergo apoptosis.1-3 Thus, susceptibility to apoptosis is an important determinant of response to antineoplastic therapy.4 Central to this response are proteins that modulate apoptosis, including p53, Bcl-2, Bax, and the more recently identified Bcl-xl gene product.
The Bcl-2 protein, because of its antiapoptotic effects, is considered to be an important multidrug resistance molecule. Studies of certain types of tumor cell lines in vitro have shown that overexpression of Bcl-2 prevents cell death initiated by a variety of means, including growth factor withdrawal, IR, glucocorticoids, and multiple chemotherapeutic agents.5-10 Conversely, cytokine- or antisense-mediated reduction of Bcl-2 expression increases the sensitivity of these lines to several cytotoxic drugs.11,12 Moreover, in clinical studies, a correlation between high Bcl-2 expression and poor response to chemotherapy has been reported in acute myeloid leukemia (AML).13 14
In contrast to the studies mentioned above, higher levels of Bcl-2 in primary pediatric acute lymphoblastic leukemia (ALL) cells appear not to confer a poor response to therapy. Instead, cellular levels of Bcl-2 in this disease are not correlated with response to cytotoxic drugs either in vivo or in vitro.15 16 Furthermore, our own studies of the relationship between Bcl-2 expression by pediatric ALL cells and sensitivity to apoptosis indicated that high levels of Bcl-2 expression are not sufficient to protect these cells from apoptosis due to IR or adriamycin (author's unpublished data). Together, these results suggest that Bcl-2 alone is not predictive of resistance to therapy-induced apoptosis in pediatric ALL.
It is now apparent that Bcl-2 is a member of a family of Bcl-2 homologues that differentially regulate programmed cell death. One such protein with extensive homology to Bcl-2, termed Bax, is capable of binding to and counteracting the antiapoptotic effect of Bcl-2.17,18 A second protein, termed Bcl-x, has two alternatively spliced forms, long (Bcl-xl) and short (Bcl-xs). Bcl-xl resembles Bcl-2 in suppressing apoptosis, whereas Bcl-xs antagonizes the death-inhibiting activity of Bcl-2.19 Bcl-xl and Bcl-2 are both able to heterodimerize with Bax, inhibit p53-induced apoptosis, and, in Bcl-2/Bcl-xl transgenic mice, downregulate each other's expression, suggesting that they may function in a common pathway of death repression.20,21 However, other studies have shown that Bcl-xl can exert an antiapoptotic effect independently of Bax and that Bcl-xl but not Bcl-2 blocks immunosuppressant-induced apoptosis in B cells, suggesting that these proteins may act independently to prevent cell death.22 To further understand the interaction of these genes in regulating apoptosis, we have studied the expression at both the mRNA and protein level of Bcl-2, Bax, and Bcl-xl in ALL cell lines previously characterized for p53 phenotype. We have also evaluated the expression of these genes after exposure to IR and correlated these changes with sensitivity to IR-induced apoptosis.
MATERIALS AND METHODS
Cell lines and cell treatment.Twenty-two cell lines established from children with ALL were studied. Eleven of these lines (EU-1 through EU-9, EU-12, and EU-13) were established at Emory University and 9 (UoC-B1, UoC-B3, UoC-B4, UoC-B11, SUP-B2, SUP-B7, SUP-B13, SUP-B15, and KT) were obtained from S. D. Smith (University of Chicago Medical Center, Chicago, IL). The Reh ALL line was obtained from C. Rosenfeld (INSERM, Villejuif, France) and CCRF-CEM was obtained from the American Type Culture Collection (Rockville, MD). The phenotypes of these cell lines are summarized in Table 1 and in previous publications.23-25 All cell lines were grown at 37°C in 95% air and 5% CO2 in RPMI 1640 containing 10% fetal bovine serum, 2 mmol/L L-glutamine, 50 U penicillin, and 50 μg/mL streptomycin. Exponentially growing cells were irradiated with 10 Gy (1 Gy = 100 Rads) using a 137Cs γ-irradiator at a dose rate of 3.7 Gy/min. Cells were incubated for the indicated times at 37°C and then harvested for total RNA, DNA, and protein extraction.
Expression of Bcl-2 Family Genes, p53 Status, and Their Association With Sensitivity to DNA Damage Induced by IR in Pediatric ALL Cell Lines
| Cell Line . | Type . | Gene Expression . | p53 Status . | Apoptosis* . | ||
|---|---|---|---|---|---|---|
| . | . | Bcl-2 . | Bcl-xl . | Bax . | . | . |
| Reh | EPB | + | + | + | WT | − |
| EU-1 | EPB | + | + | + | WT | − |
| EU-3 | PB | + | + | + | WT | + |
| EU-13 | EPB | − | + | + | WT | + |
| Sup-B7 | EPB | − | + | + | WT | + |
| Sup-B13 | PB | + | + | + | WT | + |
| Sup-B15 | PB | + | + | + | WT | + |
| UoC-B1 | EPB | + | ++ | + | WT | − |
| UoC-B3 | PB | − | + | + | WT | + |
| UoC-B4 | EPB | + | + | + | WT | + |
| UoC-B11 | EPB | + | + | + | WT | + |
| EU-4 | AML† | − | +++ | + | − | − |
| EU-5 | EPB | − | ++ | + | − | − |
| EU-8 | EPB | − | +++ | + | − | − |
| EU-9 | T | − | +++ | + | − | − |
| EU-12 | EPB | − | + | + | − | + |
| Sup-B2 | EPB | − | + | + | − | + |
| KT | T | − | +++ | + | − | − |
| EU-2 | PB | − | + | + | MUT | + |
| EU-6 | EPB | + | + | + | MUT | − |
| EU-7 | T | − | + | + | MUT | + |
| CCRF-CEM | T | − | + | + | MUT | − |
| Cell Line . | Type . | Gene Expression . | p53 Status . | Apoptosis* . | ||
|---|---|---|---|---|---|---|
| . | . | Bcl-2 . | Bcl-xl . | Bax . | . | . |
| Reh | EPB | + | + | + | WT | − |
| EU-1 | EPB | + | + | + | WT | − |
| EU-3 | PB | + | + | + | WT | + |
| EU-13 | EPB | − | + | + | WT | + |
| Sup-B7 | EPB | − | + | + | WT | + |
| Sup-B13 | PB | + | + | + | WT | + |
| Sup-B15 | PB | + | + | + | WT | + |
| UoC-B1 | EPB | + | ++ | + | WT | − |
| UoC-B3 | PB | − | + | + | WT | + |
| UoC-B4 | EPB | + | + | + | WT | + |
| UoC-B11 | EPB | + | + | + | WT | + |
| EU-4 | AML† | − | +++ | + | − | − |
| EU-5 | EPB | − | ++ | + | − | − |
| EU-8 | EPB | − | +++ | + | − | − |
| EU-9 | T | − | +++ | + | − | − |
| EU-12 | EPB | − | + | + | − | + |
| Sup-B2 | EPB | − | + | + | − | + |
| KT | T | − | +++ | + | − | − |
| EU-2 | PB | − | + | + | MUT | + |
| EU-6 | EPB | + | + | + | MUT | − |
| EU-7 | T | − | + | + | MUT | + |
| CCRF-CEM | T | − | + | + | MUT | − |
Abbreviations: EPB, early pre-B ALL; PB, pre-B ALL; T, T-ALL; WT, wild type; MUT, mutant.
Measured by DNA fragmentation after treatment with IR (10 Gy) for 5 hours.
Established from a child with ALL but expressing predominantly myeloid antigens.
RNA preparation and Northern blot analysis.Total cellular RNA was extracted from cell lines before and after irradiation by the guanidine thiocyanate-cesium chloride method, as previously described.24 Ten micrograms of total RNA was electrophoresed per lane using a 1% agarose/6% formaldehyde gel and transferred to a nylon filter. To detect p53, Bcl-2, Bax, and Bcl-xl gene expression, we used cDNA probes for the corresponding genes that were kindly donated by Dr B. Vogelstein (Johns Hopkins University, Baltimore, MD; p53), Dr J. Reed (La Jolla Cancer Research Foundation, La Jolla, CA; Bcl-2 and Bax), and Dr G. Nunez (University of Michigan, Ann Arbor, MI; Bcl-xl). Probes were prepared by randomized labeling using 32P-dCTP. Hybridization was performed in 50% (vol/vol) formamide/5× SSC/1% sodium dodecyl sulfate (SDS)/5× Denhardt's/20% dextran sulfate/100 μg/mL sheared salmon sperm DNA at 42°C for 16 hours. A final wash was performed in 1× SSC/1% SDS at 65° for 30 minutes. The membrane was checked for equal loading by rehybridization with a human β-actin probe. After washing, the filter was autoradiographed for 24 hours.
Western blot assay.Cells were lysed in a buffer composed of 150 mmol/L NaCl, 50 mmol/L Tris (pH 8.0), 5 mmol/L EDTA, 1% (vol/vol) Nonidet p-40, 1 mmol/L phenylmethylsulfonyl fluoride, 20 μg/mL aprotinin, and 25 μg/mL leupeptin for 30 minutes at 4°C. After clarification, equal amounts of protein extracts were resolved by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose paper. After blocking with buffer containing 20 mmol/L Tris-HCl (pH 7.5), 500 mmol/L NaCl, and 5% nonfat milk for 1 hour at room temperature, filter was incubated with specific antibodies to p53 (Ab-2; Oncogene Science Inc, Uniondale, NY) as well as to Bcl-2 (Ab-3), Bax (Ab-1), and Bcl-x (Ab-1 Calbiochem, Cambridge, MA) for 1 hour at room temperature followed by horseradish peroxidase (HRP) labeled secondary antibody. Blots were developed using a chemiluminescent detection system (ECL; Amersham Life Science, Buckinghamshire, UK). Levels of protein expression before and after treatment were compared using densitometry.
DNA fragmentation analysis.Apoptosis was determined by DNA fragmentation assay. Cells were washed in phosphate-buffered saline and lysed by incubation in 45 mmol/L Tris-borate/EDTA buffer, pH 8, containing 0.25% NP40 and 0.1% RNase A at a concentration of 5 × 106/mL at 37°C for 30 minutes, followed by 1 mg/mL proteinase K for an additional 30 minutes. Lysates from 5 × 105 cells were resolved by electrophoresis on a 1.5% agarose gel containing ethidium bromide, and DNA fragments were visualized under UV light.
RESULTS
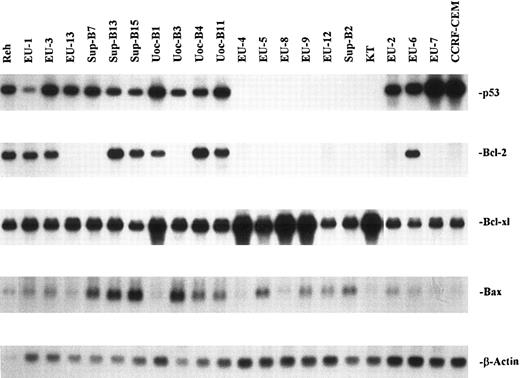
Expression of p53, Bcl-2, Bcl-xl, and Bax in ALL cell lines.The results of Northern blot analysis for Bcl-2, Bcl-x1, and Bax gene expression in the 22 cell lines studied are summarized in Table 1, together with their p53 phenotype and sensitivity to IR-induced apoptosis. mRNA for p53 was expressed in 15 of the 22 lines. As previously reported, 4 of the lines (EU-2, EU-6, EU-7, and CCRF-CEM) expressed mutant p53, and the remaining 18 lines contained the wild-type (wt)-p53 gene as detected by single-strand conformation polymorphism (SSCP) assay.25 Eleven of these lines expressed wt-p53 mRNA, whereas 7 lines contained the wt-p53 gene but failed to express the mRNA (p53-null phenotype). Bcl-2 mRNA was detected by Northern blot assay in 9 of the 22 lines, including 8 of the 11 lines with wt-p53 expression. In contrast, Bcl-2 was expressed by only 1 of 4 mut-p53+ lines (EU-6) and not by any p53-null lines.
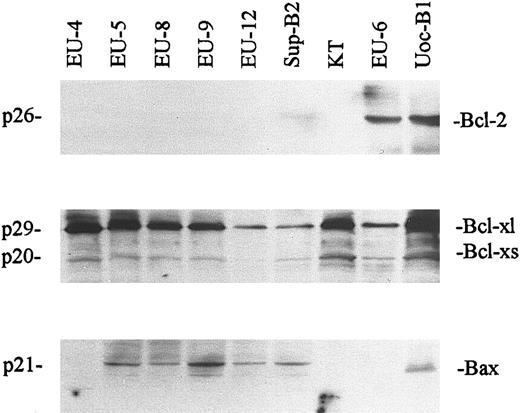
All 22 lines were positive for Bax and Bcl-xl mRNA expression. Constitutive expression of Bax varied considerably, but tended to be low in mut-p53+ and p53-null lines and higher in wt-p53+ lines (Fig 1). Bcl-xl expression was also variable, with marked overexpression in 5 of the 7 p53-null cell lines. Figure 2 shows representative Western blotting results for Bcl-2, Bcl-x, and Bax protein expression by all 7 p53-null lines, the mutant p53+ line EU-6, and the wt-p53+ line UoC-B1. In each case, expression of mRNA as detected by Northern blotting was correlated with expression of the corresponding protein. For example, high levels of Bcl-xl protein were detected in those lines with overexpression of Bcl-xl mRNA. Conversely, Bcl-2 protein was not detected in any of the 7 p53-null lines, consistent with their lack of the corresponding mRNA.
Northern blot analysis of p53, Bcl-2, Bcl-xl, and Bax mRNA from 22 ALL cell lines. Total RNA was extracted with the GITC-cesium chloride centrifugation method. Total RNA (10 μg per lane) was electrophoresed on a 1% agarose/6% formaldehyde gel. The p53, Bcl-2, Bcl-xl, and Bax probes were prepared by 32P randomized labeling using cDNAs. Hybridization and wash were performed as described in the Materials and Methods. Membranes were rehybridized with a β-actin probe as a control for equal RNA loading and RNA integrity.
Northern blot analysis of p53, Bcl-2, Bcl-xl, and Bax mRNA from 22 ALL cell lines. Total RNA was extracted with the GITC-cesium chloride centrifugation method. Total RNA (10 μg per lane) was electrophoresed on a 1% agarose/6% formaldehyde gel. The p53, Bcl-2, Bcl-xl, and Bax probes were prepared by 32P randomized labeling using cDNAs. Hybridization and wash were performed as described in the Materials and Methods. Membranes were rehybridized with a β-actin probe as a control for equal RNA loading and RNA integrity.
Western blot assay of Bcl-2, Bcl-xl, and Bax protein expression from representative ALL cell lines (EU-4 → KT, p53-null lines; EU-6, mutant p53+ line; UoC-B1, wt-p53+ line). Protein (20 μg) from cell lysates was electrophoresed in SDS-PAGE gels, transferred to nitrocellulose, and probed with anti-Bcl-2, anti-Bcl-x, and anti-Bax as described in the Materials and Methods. Anti-Bcl-x antibody recognizes both Bcl-xl and Bcl-xs forms.
Western blot assay of Bcl-2, Bcl-xl, and Bax protein expression from representative ALL cell lines (EU-4 → KT, p53-null lines; EU-6, mutant p53+ line; UoC-B1, wt-p53+ line). Protein (20 μg) from cell lysates was electrophoresed in SDS-PAGE gels, transferred to nitrocellulose, and probed with anti-Bcl-2, anti-Bcl-x, and anti-Bax as described in the Materials and Methods. Anti-Bcl-x antibody recognizes both Bcl-xl and Bcl-xs forms.
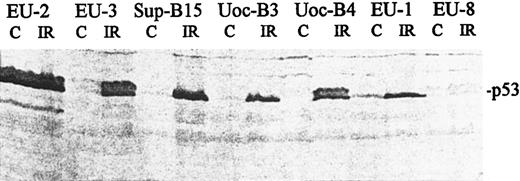
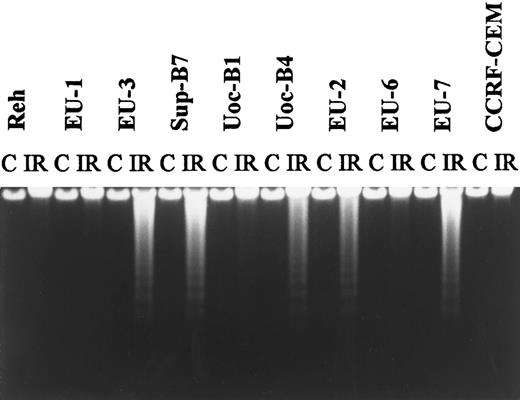
Regulation of p53 expression and sensitivity to apoptosis after irradiation.Consistent with previous reports showing that regulation of p53 occurs at the protein level, we found that p53 mRNA expression was unchanged after IR (data not shown), whereas p53 protein levels increased in wt-p53+ lines but not in mut-p53+ or p53-null lines, as determined by immunoblotting (representative data shown in Fig 3). Characteristic DNA ladder formation was observed by 5 hours postirradiation in 12 of the 22 cell lines. Sensitive lines included 8 of 11 lines with wt-p53 expression, 2 of 4 lines with mutant p53 expression, and 2 of 7 p53-null lines (Table 1). Figure 4 is a representative agarose gel showing DNA fragmentation at 5 hours post-IR.
Western blot showing expression and accumulation of p53 protein in ALL cell lines. Protein was harvested from irradiated cells (18 hours post-IR) and from untreated control cells (C). Blots were analyzed with an anti-p53 antibody (Ab2; Oncogene Science) that recognizes both wt and mutant p53. p53 phenotypes: mutant-p53+, EU-2; wt-p53+, EU-3, Sup-B15, UoC-B3, UoC-B4, and EU-1; and p53-null, EU-8.
Western blot showing expression and accumulation of p53 protein in ALL cell lines. Protein was harvested from irradiated cells (18 hours post-IR) and from untreated control cells (C). Blots were analyzed with an anti-p53 antibody (Ab2; Oncogene Science) that recognizes both wt and mutant p53. p53 phenotypes: mutant-p53+, EU-2; wt-p53+, EU-3, Sup-B15, UoC-B3, UoC-B4, and EU-1; and p53-null, EU-8.
A representative agarose-gel electrophoresis pattern obtained with DNA extracted from ALL lines. Cells were incubated for 5 hours after exposure to 10 Gy IR; untreated cells served as a control (C).
A representative agarose-gel electrophoresis pattern obtained with DNA extracted from ALL lines. Cells were incubated for 5 hours after exposure to 10 Gy IR; untreated cells served as a control (C).
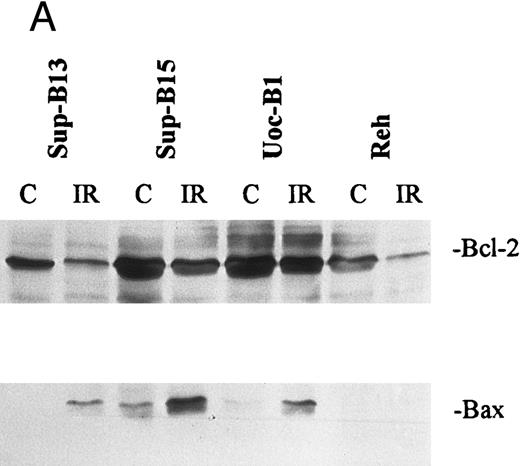
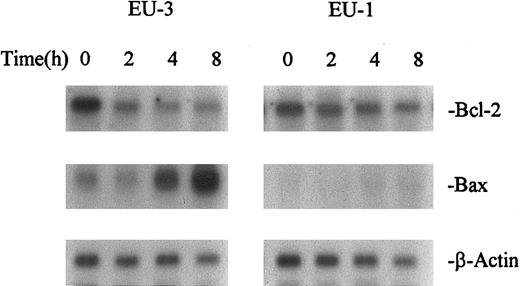
Regulation of Bcl-2, Bcl-xl, and Bax after irradiation.Changes in the expression of Bcl-2 and Bax after IR were analyzed by both Western and Northern blot. Two to 8 hours after exposure to IR (10 Gy), downregulation of Bcl-2 and upregulation of Bax were observed in 5 of 8 wt-p53+/Bcl-2+ lines (EU-3, SUP-B13, SUP-B15, UoC-B4, and UoC-B11; Table 2 and Fig 5). These lines underwent apoptosis as shown by DNA fragmentation. Although downregulation of Bcl-2 was observed in 2 other wt-p53+/Bcl-2+ ALL lines (Reh and EU-1), no upregulation of Bax was induced and these lines were resistant to IR. Conversely, downregulation of Bcl-2 was not significantly induced in a third wt-p53+/Bcl-2+ line (UoC-B1), and this line was resistant to IR; this line also expresses high levels of Bcl-xl. Figure 6 shows the kinetics of Bcl-2 and Bax regulation in 2 wt-p53+ lines with differential response and sensitivity to irradiation. EU-3 cells showed marked downregulation of Bcl-2 and upregulation of Bax by 4 hours post-IR, leading to apoptosis. However, EU-1 cells did not show upregulation of Bax or apoptosis after treatment, even though Bcl-2 expression decreased.
Regulation of Bcl-2 and Bax in Bcl-2+ ALL Cell Lines After Treatment With IR
| Cell Line . | Downregulation of Bcl-2 . | Upregulation of Bax . | Apoptosis . |
|---|---|---|---|
| Reh | + | − | − |
| EU-1 | + | − | − |
| EU-3 | + | + | + |
| Sup-B13 | + | + | + |
| Sup-B15 | + | + | + |
| Uoc-B1 | − | + | − |
| Uoc-B4 | + | + | + |
| Uoc-B11 | + | + | + |
| EU-6* | − | − | − |
| Cell Line . | Downregulation of Bcl-2 . | Upregulation of Bax . | Apoptosis . |
|---|---|---|---|
| Reh | + | − | − |
| EU-1 | + | − | − |
| EU-3 | + | + | + |
| Sup-B13 | + | + | + |
| Sup-B15 | + | + | + |
| Uoc-B1 | − | + | − |
| Uoc-B4 | + | + | + |
| Uoc-B11 | + | + | + |
| EU-6* | − | − | − |
Expression of Bcl-2 and Bax mRNA was detected by Northern blot analysis after treatment with IR (10 Gy) for 2 to 8 hours. Apoptosis was measured by DNA fragmentation after IR for 5 hours.
EU-6 expressed mutant p53.
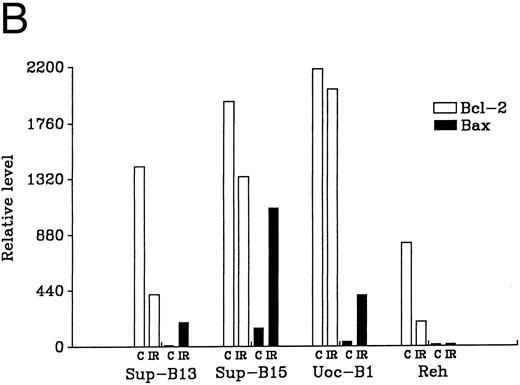
Regulation of Bcl-2 and Bax in wt-p53+ ALL lines either sensitive (Sup-B13 and Sup-B15) or resistant (UoC-B1 and Reh) to IR (10 Gy). (A) shows Western blot analysis of Bcl-2 and Bax protein that was extracted either pre-IR (C) or at 7 hours post-IR (IR). Densitometric scan of bands in (A) shows relative levels of protein post-IR compared with pre-IR (B).
Regulation of Bcl-2 and Bax in wt-p53+ ALL lines either sensitive (Sup-B13 and Sup-B15) or resistant (UoC-B1 and Reh) to IR (10 Gy). (A) shows Western blot analysis of Bcl-2 and Bax protein that was extracted either pre-IR (C) or at 7 hours post-IR (IR). Densitometric scan of bands in (A) shows relative levels of protein post-IR compared with pre-IR (B).
Kinetics of Bcl-2 and Bax expression after IR in ALL cell lines EU-3 (IR-sensitive) and EU-1 (IR-resistant). RNA was extracted from cells at different times after treatment with IR (10 Gy).
Kinetics of Bcl-2 and Bax expression after IR in ALL cell lines EU-3 (IR-sensitive) and EU-1 (IR-resistant). RNA was extracted from cells at different times after treatment with IR (10 Gy).
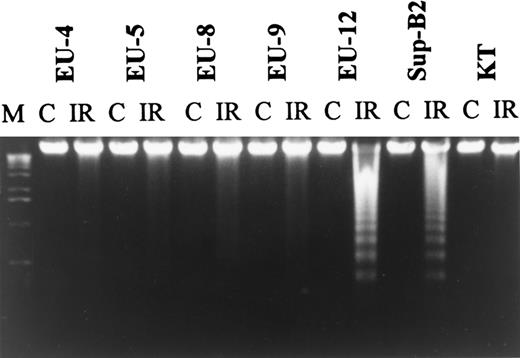
Changes in Bcl-xl expression post-IR were not observed in any cell lines (data not shown). However, resistance of p53−/Bcl-2− lines to IR was strongly correlated with high levels of Bcl-xl expression before IR. Apoptosis was not induced after treatment with IR (10 Gy) in any of the 5 p53−/Bcl-2− ALL lines with constitutively high-level expression of Bcl-xl, whereas 2 p53-null lines with lower levels of Bcl-xl were sensitive to IR (Fig 7). Besides being Bcl-2−, all p53-null lines were characterized by low levels of Bax that failed to undergo upregulation after IR.
Agarose gel electrophoresis of DNA from 7 wt-p53−/Bcl-2− ALL lines. DNA fragmentation was not observed in lines EU-4, EU-5, EU-8, EU-9, and KT with Bcl-xl overexpression after treatment with IR.
Agarose gel electrophoresis of DNA from 7 wt-p53−/Bcl-2− ALL lines. DNA fragmentation was not observed in lines EU-4, EU-5, EU-8, EU-9, and KT with Bcl-xl overexpression after treatment with IR.
Three of four lines expressing mutant-p53 showed no constitutive expression of Bcl-2, although all expressed Bcl-xl and low levels of Bax. Although 2 lines were sensitive to IR, sensitivity was not correlated with differential regulation of Bcl-2, Bax, or Bcl-xl, because no changes in the expression of these genes were observed post-IR.
DISCUSSION
Expression of an exogenous Bcl-2 gene in Bcl-2− cells can suppress apoptosis induced by various cytotoxic treatments, and Bcl-2 is therefore thought to be a multidrug resistance molecule.8,26 Several studies have shown a correlation between high Bcl-2 expression and poor response to therapy in certain tumors; however, other studies have concluded that low Bcl-2 expression is related to poor response and shortened survival in non–small-cell lung cancer,27,28 childhood ALL,14,15 and myelodysplastic syndrome.29 One possible explanation for this discrepancy is that low Bcl-2 expression might be associated with expression of a mutant p53 gene, thereby conferring the resistant phenotype.30 31 Additionally, expression of other Bcl-2–related genes, including Bax and Bcl-xl, might modulate the effect of Bcl-2 itself.
To further evaluate the relationship between Bcl-2 expression and resistance to apoptosis in pediatric ALL, we examined 22 pediatric ALL cell lines for Bcl-2 expression and analyzed the correlation of Bcl-2 expression with p53 status and sensitivity to IR. Bcl-2 was expressed in 41% of these lines and significantly correlated with wt-p53 expression: 73% of wt-p53+ lines were Bcl-2+, compared with 25% of mutant p53+ lines and none of the p53-null lines. These results are similar to those obtained for gliomas, in which Bcl-2 was positive in seven of seven tumors with the wt-p53 phenotype but in only one of seven with mutant p53.31 Furthermore, in studies of breast cancer and non-Hodgkin's lymphoma, respectively, higher levels of mutant p53 protein were correlated with lower levels of Bcl-2.32,33 Thus, Bcl-2 expression in ALL cells may be p53-dependent, with the wt-p53 conformation being more effective than the mutant conformation in eliciting Bcl-2 gene transcription.34 35 Our finding of an absence of Bcl-2 expression in p53-null lines is consistent with this idea.
In addition to the association between high levels of Bcl-2 expression and the wt-p53 phenotype, our study found that most (5/8) of the ALL lines with Bcl-2+/wt-p53+ underwent apoptosis after IR treatment. This suggests that high levels of Bcl-2 expression alone are not sufficient to protect ALL cells from apoptosis. These results agree with those of Coustan-Smith et al16 who found that high levels of Bcl-2 in pediatric ALL are not correlated with resistance to cytotoxic drugs.
An unexpected finding in the present study was that 3 of 8 Bcl-2+/wt-53+ lines were resistant to apoptosis, although the remaining 5 lines were sensitive. To examine this variability further, we evaluated expression of the Bax gene, which encodes a 21-kD protein sharing considerable amino-acid homology with Bcl-2 and capable of binding to the latter protein.17 18 Thus, Bax can form Bcl-2:Bax heterodimers as well as Bax:Bax homodimers, the latter being preferentially formed when the level of Bax exceeds that of Bcl-2. Formation of Bcl-2:Bax heterodimers appears to inhibit the antiapoptotic function of Bcl-2, whereas the Bax:Bax homodimers contribute to initiation of apoptosis. Thus, higher levels of Bax relative to Bcl-2 after DNA damage may increase sensitivity to apoptosis.
In our study, Bax was expressed in all 22 cell lines, suggesting that Bax expression, unlike Bcl-2, is not dependent on expression of p53. Significantly, changes in the level of Bcl-2 and Bax expression in wt-p53+ lines after exposure to IR were highly correlated with sensitivity to apoptosis. Every wt-p53+ line showing both downregulation of Bcl-2 and upregulation of Bax after IR underwent apoptosis, and only those wt-p53+ lines that show this pattern of response to IR were sensitive. Of the 11 wt-p53+ lines studied, 3 were resistant to IR due either to a failure to downregulate Bcl-2 (UoC-B1) or to absence of Bax upregulation (Reh and EU-1). This suggests that a decrease in the Bcl-2:Bax ratio due to reciprocal changes in the expression of these genes after irradiation is necessary for induction of apoptosis in wt-p53+ ALL lines. These results are consistent with the mechanism proposed by Oltvai et al,17 in which changes in the Bcl-2:Bax ratio regulate apoptosis. Furthermore, a previous study of other human leukemia lines has shown that induction of Bax occurs only in wt-p53+ lines and only in association with apoptosis.36 We have further found that simultaneous downregulation of Bcl-2 and upregulation of Bax in wt-p53+ ALL lines appears to be required for induction of apoptosis after IR.
Although both Bcl-2 and Bax are regulated by p53, p53 upregulation protein alone was not predictive of sensitivity to apoptosis. Thus, p53 upregulation occurred in IR-resistant as well as IR-sensitive lines, suggesting that induction of p53 is not in itself sufficient to trigger apoptosis. Apparently, the relative changes in the expression of Bcl-2 and Bax posttreatment are more directly linked to, and predictive of, apoptosis than are changes in the level of p53 expression.
In contrast to the situation with wt-p53+ lines, the Bcl-2:Bax regulatory paradigm did not extend to lines expressing mutant p53 or to p53-null lines in our study. Thus, two lines expressing mutant p53 (EU-2 and EU-7) were sensitive to IR-induced apoptosis even though these lines did not show any upregulation of Bax. Because cells expressing mutant p53 are generally resistant to apoptosis, the reason for this sensitivity is not clear. Although the absence of Bcl-2 expression in these lines might explain their sensitivity, a third line with a similar mutant-p53+/Bcl-2− phenotype was resistant to apoptosis. Both sensitive and resistant lines showed no induction of Bax after IR. The sensitivity of some mut-p53+ lines to IR-induced apoptosis may therefore involve a p53-independent, Bcl-2/Bax-independent mechanism of apoptosis.
To investigate p53-independent apoptosis, we examined the expression of Bcl-xl in our cell lines. Bcl-xl is a Bcl-2 homologue encoded by the Bcl-x gene that resembles Bcl-2 in its ability to inhibit apoptosis induced by growth factor withdrawal and chemotherapeutic agents.19-21,37,38 Functionally, Bcl-xl resembles Bcl-2 in that it is able to suppress apoptosis in growth factor-dependent cell lines after factor withdrawal.19 However, certain differences between Bcl-2 and Bcl-xl have become apparent. Bcl-xl and Bcl-2 are expressed independently during embryonic development as well as in hematopoietic stem cells and lymphoid progenitor cells.39-41 Bcl-xl but not Bcl-2 protects from apoptosis induced by protein synthesis inhibitors and immunosuppressants.22 In certain myeloid leukemia cell lines, exposure to cytokines such as interleukin-6 and granulocyte-macrophage colony-stimulating factor strongly upregulates Bcl-xl but not Bcl-2, resulting in reduced susceptibility to induction of apoptosis by adriamycin or cyclohexamide.42 Furthermore, although Bcl-xl is capable of forming heterodimers with Bax, interaction with Bax is apparently not required for protection against apoptotic stimuli.43
We have found additional differences in the expression and regulation of Bcl-2 and Bcl-xl in pediatric ALL cells. Unlike Bcl-2, the expression of Bcl-xl in our lines was independent of p53, because Bcl-xl was expressed by all 7 p53-null lines as well as by wt-p53+ and mutant-p53+ lines. Furthermore, there was no downregulation of Bcl-xl in any of the ALL lines (either p53+ or p53-null) after IR, whereas Bcl-2 was downregulated in the majority of wt-p53+ lines by IR, further suggesting that regulation of Bcl-xl is independent of p53 and Bcl-2.
Although high levels of Bcl-2 are common in pediatric ALL, no correlation between elevated Bcl-2 expression and poor prognosis has been detected in this disease.15,16 Our results suggest that high levels of Bcl-xl expression by p53-null lines is associated with resistance to DNA damage induced by IR. Thus, constitutively high levels of Bcl-xl may protect p53−/Bcl-2− cells from apoptosis due to DNA damage. Preliminary studies with primary ALL cells from diagnosis and relapse patients show higher expression of Bcl-xl in drug-resistant relapse cells than in drug-sensitive cells at diagnosis, suggesting that increased expression of Bcl-xl is associated with drug resistance in vivo (unpublished data). Although definitive demonstration of a Bcl-xl protective effect in ALL will require gene expression studies, our observations are consistent with studies showing that murine cells and human histiocytic lymphoma cells overexpressing the human Bcl-xl gene have increased resistance to chemotherapeutic agents in vitro.37 44
In conclusion, we have found that the Bcl-2 expression in pediatric ALL cells is p53-dependent and that the response of wt-p53+ cells (but not of mutant-p53+ cells) to IR-induced DNA damage is determined by the regulation of Bcl-2 and Bax posttreatment. In contrast, Bcl-xl is expressed in pediatric ALL cells in a p53-independent manner, and the response of p53-null cells to IR depends partly on the pretreatment level of Bcl-xl expression. Further investigation of the regulation of these genes in primary ALL cells should be important in elucidating mechanisms of drug resistance in cells with different p53 phenotypes and patterns of Bcl-2 family gene regulation.
Supported by CURE of Georgia, in part by a grant from the American Cancer Society, Inc, and by the Winship Cancer Center of Emory University.
Address reprint requests to Muxiang Zhou, MD, Division of Pediatric Hematology/Oncology/BMT, Emory University School of Medicine, 2040 Ridgewood Dr NE, Atlanta, GA 30322.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal