Abstract
Myasthenia gravis (MG) is a human autoimmune disease mediated by anti-acetylcholine receptor (AChR) antibodies. The thymus is probably the site where the autoimmune response is triggered and maintained. Recent reports have linked various autoimmune disease with defective Fas expression. We thus analyzed Fas expression in thymocytes and peripheral blood lymphocytes (PBL) from MG patients. The proportion of a thymocyte subpopulation with strong Fas expression (Fashi) was markedly enhanced in MG patients with anti-AChR antibodies (P < .0003, compared with controls). In this group of patients, the proportion of CD4+Fashi and CD4+CD8+Fashi thymocytes were significantly increased (P < .002 for both subsets). Fashi thymocytes were enriched in activated cells and showed intermediate CD3 expression. They were preferentially Vβ5.1-expressing cells, previously shown to be enriched in potentially autoreactive cells. The proliferative response of thymocytes from MG patients to peptides from the AChR was abolished after depletion of Fashi cells. Fashi thymocytes were sensitive to an agonistic anti-Fas antibody. In peripheral blood, Fashi lymphocytes proportion was not significantly modified in MG patients whatever their anti-AChR antibody titer, compared with controls. Altogether, these results indicate that Fashi thymocytes, which accumulate in MG patients with anti-AChR antibodies, could be involved in the autoimmune response that targets the AChR.
MYASTHENIA GRAVIS (MG) is an autoimmune disease characterized by antibodies that target the acetylcholine receptor (AChR) at the neuromuscular junction and that are present in more than 85% of patients.1 These autoantibodies are produced under the control of major histocompatibility class (MHC) II-restricted CD4+ helper T lymphocytes. Several arguments suggest a relationship between MG and the thymus: (1) MG is often associated with morphologic thymus abnormalities.2 10% to 15% of patients have thymomas and 50% to 60% have hyperplasia; these are characterized by the presence of lymphoid follicles with germinal centers and are found in young patients with high titers of anti-AChR antibodies3; (2) thymectomy has a beneficial effect4,5; (3) after thymectomy of MG patients, both anti-AChR antibody titers6 and in vitro production of anti-AChR antibodies from stimulated peripheral blood lymphocyte (PBL)7 decrease; (4) isolated thymocytes from MG patients can produce anti-AChR antibodies spontaneously8,9; (5) AChR-reactive T cells are present in the thymus of MG patients10,11; and (6) T lymphocytes12 and B lymphocytes13 from MG thymuses are activated. Altogether these arguments indicate that the thymus is probably the site where the autoimmune response is triggered and maintained.
During intrathymic development, programmed cell death appears to be an important option at several stages. A large number of immature thymocytes die because they fail to express a T-cell receptor (TCR) with sufficient affinity for thymic MHC molecules. Cells that are not positively selected may die from “neglect”14 and may constitute the bulk of cells that die of apoptosis in the thymus.15 The same mechanism of cell death underlies the deletion of autoreactive immature thymocytes.16 Thymocytes bearing autoreactive TCR with a high affinity for MHC molecules and specific peptide presented by stromal cells in the thymus undergo negative selection by apoptosis, whereas cells with lower affinity can further differentiate into mature CD4+ or CD8+ cells strongly expressing TCR. In addition, some autoreactive single-positive CD4+ or CD8+ cells are deleted in the periphery.17
It has been suggested that autoimmune disease is caused by a failure to eliminate self-reactive lymphocytes, essentially in the periphery, and also by defective apoptosis. In three different strains of mice that develop a disease analogous to human lupus, abnormalities have been detected in the Fas and Fas ligand genes, whose protein products interact to mediate apoptosis.18 Elevated serum concentrations of a soluble form of the Fas receptor that is able to inhibit Fas-mediated apoptosis have been found in human systemic lupus erythematosus19 and Fas antigen expression is increased in peripheral lymphocytes.20-22 Recent studies have linked defective Fas-mediated T-lymphocyte apoptosis to various Fas gene mutations in a human autoimmune lymphoproliferative syndrome.23 24
In the immune system, the Fas/Fas ligand system is involved in two mechanisms: T-cell cytotoxicity25 and activation-induced cell death.26-28 The involvement of Fas in thymic apoptosis (and particularly in negative selection) is controversial. In lpr mice, in which systemic autoimmune disease is related to a Fas gene defect,29 and in Fas-null mice, negative selection is normal in the thymus while activation-induced cell death of activated peripheral lymphocytes is impaired.30,31 However, an accumulation of immature cells (CD4+CD8+TCRlow) resistant to apoptosis has been described in the thymus of lpr mice.32 Mariani et al33 have reported that Fas expression is 10 times lower in the lpr mouse thymus than in control animals; this low expression could be sufficient for adequate programmed cell death of autoreactive cells. Furthermore, agonistic anti-Fas antibody was able to induce mouse thymocyte apoptosis in the presence of additional signals: metabolic inhibitors34 or TCR stimulation.35
MG is a human autoimmune disease in which the autoantigen (AChR) is well characterized. Recent reports have linked various autoimmune diseases to defective Fas-mediated apoptosis or Fas expression. This raises the possibility that such abnormalities could be involved in the pathogenesis of MG. Because the thymus is clearly involved in MG, we analyzed Fas expression in thymocytes and also in PBL from patients with MG.
MATERIALS AND METHODS
Patients.We studied 31 patients with myasthenia gravis (28 females and 3 males, ages 8 to 48 years) who were undergoing thymectomy at Marie-Lannelongue Hospital. Table 1 summarizes the following clinical characteristics: age, sex, disease severity, thymic histology, anti-AChR antibody titer, time since onset, and concomitant treatments. Disease severity was evaluated using Osserman's classification (I: ocular symptoms; IIA: generalized without bulbar symptoms; IIB: generalized with bulbar symptoms). Fresh thymic tissue was obtained in aseptic conditions. No thymocytes could be obtained from 5 patients because the thymus was too involuted. Patients with thymomas were excluded from the study. Thymic hyperplasia was defined according to the classification of Levine and Rosai36 as thymuses containing germinal centers, and was found in 19 patients (6 with few germinal centers, 13 with a large number of germinal centers). Twelve patients had a normal or involuted thymus. All the patients were on anticholinesterase treatment and 4 were on steroids and immunosuppressive drugs. Three groups of patients were distinguished according to the anti-AChR antibody titer: high titer > 10 nmol/L (10 patients); intermediate titer, 1 to 10 nmol/L (11 patients), and negative or borderline titer, <1 nmol/L (10 patients). Anti-AChR antibody titers were determined in serum as previously described.37 38
Characteristics of the Patients Studied
| . | No. of Patients . | Frequency (%) . |
|---|---|---|
| Sex | ||
| Female | 28 | 90 |
| Male | 3 | 10 |
| Age | ||
| ≤35 yr | 22 | 71 |
| >35 yr | 9 | 29 |
| Onset | ||
| ≤1 yr | 13 | 42 |
| >1 yr | 18 | 58 |
| Severity grade | ||
| I | 2 | 6 |
| IIA | 4 | 13 |
| IIB | 25 | 81 |
| Thymus histology | ||
| Normal or involuted | 12 | 39 |
| Hyperplasia + | 6 | 19 |
| Hyperplasia ++ | 13 | 42 |
| Steroid treatment | ||
| No | 27 | 88 |
| Yes | 4 | 12 |
| Anti-AChR Ab titers (nmol/L) | ||
| <1 | 10 | 32 |
| 1-10 | 11 | 36 |
| >10 | 10 | 32 |
| . | No. of Patients . | Frequency (%) . |
|---|---|---|
| Sex | ||
| Female | 28 | 90 |
| Male | 3 | 10 |
| Age | ||
| ≤35 yr | 22 | 71 |
| >35 yr | 9 | 29 |
| Onset | ||
| ≤1 yr | 13 | 42 |
| >1 yr | 18 | 58 |
| Severity grade | ||
| I | 2 | 6 |
| IIA | 4 | 13 |
| IIB | 25 | 81 |
| Thymus histology | ||
| Normal or involuted | 12 | 39 |
| Hyperplasia + | 6 | 19 |
| Hyperplasia ++ | 13 | 42 |
| Steroid treatment | ||
| No | 27 | 88 |
| Yes | 4 | 12 |
| Anti-AChR Ab titers (nmol/L) | ||
| <1 | 10 | 32 |
| 1-10 | 11 | 36 |
| >10 | 10 | 32 |
Blood from the same patients (except for 8 patients) was collected just before thymectomy, and PBL were isolated over Ficoll gradients.
Normal thymuses were obtained from young adults (age range, from 10 to 25 years) undergoing heart surgery. Blood from healthy adult volunteers was also used in control experiments.
Thymocyte isolation and selection.Thymocytes were mechanically isolated by gently scraping fresh thymic tissue, filtering the cells through sterile gauze, and washing them once in Hanks' balanced salt solution (HBSS). When the quantity of cells (counted by using the Trypan blue dye exclusion method) was sufficient, aliquots of 20 million cells were stored in liquid nitrogen.
In some experiments, cells were thawed and CD8-depleted thymocytes were isolated. After washing, total thymocytes were mixed with anti-CD8 magnetic beads (Immunotech, Marseille, France) in phosphate-buffered saline (PBS)-30% human AB serum to separate CD8+ cells. Anti-Fas antibody (UB2) was fixed on goat-antimouse–coated magnetic beads (Immunotech) following the manufacturer's instructions. Using these beads CD8-depleted thymocytes were depleted in Fashi cells. CD8-depleted cells and CD8- and Fashi-depleted cells were cultured (0.2 × 106 cells/200 μL/well) in RPMI 1640 medium supplemented with 2 mmol/L L-glutamine, 25 mmol/L HEPES, 100 IU/mL penicillin, 100 μg/mL streptomycin, and 2% human AB serum in the absence or in the presence of peptides (5 μg/mL) from the AChR α-subunit, p168-181 (1H) or p351-368 (3H). After a 6-day culture period, 1 μCi of 3H-thymidine (NEN, Les Ulis, France; 6.7 Cie/mmol) was added to each well. After 20 hours, cells were obtained and 3H-thymidine incorporation was determined by counting the radioactivity on filters. Cultures were performed in 4 to 8 samples.
Immunofluorescence studies.Thymocytes and PBL were labeled with the following monoclonal fluorochrome-coupled antibodies (Immunotech): anti-CD4, anti-CD8, anti-CD3, anti-CD25, and anti–HLA-DR. FITC-anti Vβ5.1 (LC4 clone) and FITC-anti Vβ6.7 (OT145 clone) were obtained from T Cell Sciences Inc (Cambridge, MA). Three-color flow cytometry was used to examine the relationship between Fas and other markers. PBL and thymocytes (106) were first incubated with anti-Fas (anti-CD95) monoclonal antibody (clone UB2; Immunotech) for 30 minutes at 4°C, then washed twice in HBSS supplemented with 5% fetal calf serum (FCS), stained with goat-antimouse IgG antibody, washed twice, and incubated with Cy-chrome-labeled streptavidin (Pharmingen, San Diego, CA) and membrane fluorescein (FITC)- or phycoerythrin (PE)-coupled antibodies.
Cell labeling was analyzed on a FACScan flow cytometer (Becton Dickinson, Grenoble, France) using Lysis II software that allows the analysis of data obtained with a Becton Dickinson flow cytometer. A gate was set on intact cells using forward- and side-scatter analysis; 104 cells were analyzed in this gate.
Thymocyte culture and anti-Fas antibody assay.After the isolation of thymic cells, 0.5 × 106 were cultured in the presence of 5 μg/mL anti-Fas antibody (clone CH-11; Immunotech) immobilized on 96-well plates, in RPMI 1640 medium supplemented with 10% FCS, 2 mmol/L L-glutamine, 25 mmol/L HEPES, 100 IU/mL penicillin, and 100 μg/mL streptomycin. After 18 hours the cells were harvested and labeled; Fas expression was analyzed as previously described.
Statistical analysis.Differences between groups were compared by using the Mann-Whitney or Wilcoxon test (Instat; Graph Pad Software, San Diego, CA). A difference was considered significant if the P value was below .05.
RESULTS
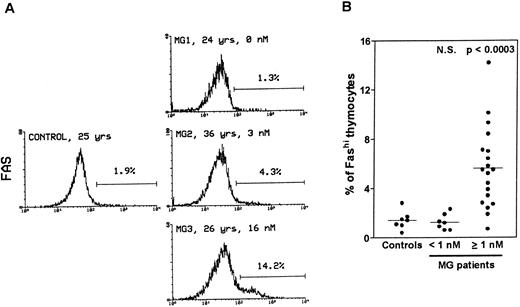
Thymocytes with strong Fas expression (Fashi) accumulate in MG patients with positive anti-AChR antibody titers.Fas expression was compared between thymuses from control subjects and MG patients by using immunofluorescence on freshly isolated thymocytes (Fig 1). In all the thymuses (Fig 1A) about 90% of thymocytes displayed low Fas expression (Faslo). In age-matched control thymuses a small proportion of thymocytes (0.4% to 2.8%) showed strong Fas expression (Fashi). The major peak of fluorescence was composed of Faslo cells. A second peak, at the end of the major peak, was composed of Fashi cells; its fluorescence level was above 102. In thymuses from patients with negative or borderline anti-AChR antibody titers (<1 nmol/L), the proportion of Fashi thymocytes was not significantly different from control values (1.3% ± 0.2% v 1.5% ± 0.3% in controls). By contrast, MG patients with positive titers (≥1 nmol/L) had far higher proportions of Fashi thymocytes (0.7% to 14.2% of total thymocytes; mean 5.6% ± 0.7%; P < .0003 compared to controls and P < .0001 compared to patients with negative or borderline titers (Fig 1B). Furthermore, this increase in the proportion of Fashi cells correlated with the autoantibody titer (4.1% ± 0.7% in thymuses from MG patients with an intermediate titer, P < .006 compared to controls; and 7.0% ± 1.1% in thymuses from MG patients with a high titer, P < .0004 compared to controls).
Comparison of Fas expression in thymocytes from controls and MG patients. Freshly isolated thymocytes were stained with anti-Fas antibody, then with goat-antimouse IgG antibody, and finally with Cy-chrome-labeled streptavidin. Using the Lysis II program, a marker was set to define the proportion of Fashi thymocytes. (A) Representative analysis of one control and three MG thymuses. The percentage of Fashi thymocytes is indicated, as well as age and the anti-AChR antibody titer (nmol/L). Fas expression is clearly increased in patients with positive anti-AChR antibody titers (MG2 and MG3) but not in the patient with a negative titer (MG1). (B) The proportion of Fashi thymocytes was determined in 26 MG patients and 7 control subjects. Two groups of patients (anti-AChR antibody titer < 1 nmol/L and ≥1 nmol/L) are distinguished and compared with controls by using the Mann-Whitney test. The bar represents the mean value. Only the group of patients with positive anti-AChR antibody titers differed from the controls.
Comparison of Fas expression in thymocytes from controls and MG patients. Freshly isolated thymocytes were stained with anti-Fas antibody, then with goat-antimouse IgG antibody, and finally with Cy-chrome-labeled streptavidin. Using the Lysis II program, a marker was set to define the proportion of Fashi thymocytes. (A) Representative analysis of one control and three MG thymuses. The percentage of Fashi thymocytes is indicated, as well as age and the anti-AChR antibody titer (nmol/L). Fas expression is clearly increased in patients with positive anti-AChR antibody titers (MG2 and MG3) but not in the patient with a negative titer (MG1). (B) The proportion of Fashi thymocytes was determined in 26 MG patients and 7 control subjects. Two groups of patients (anti-AChR antibody titer < 1 nmol/L and ≥1 nmol/L) are distinguished and compared with controls by using the Mann-Whitney test. The bar represents the mean value. Only the group of patients with positive anti-AChR antibody titers differed from the controls.
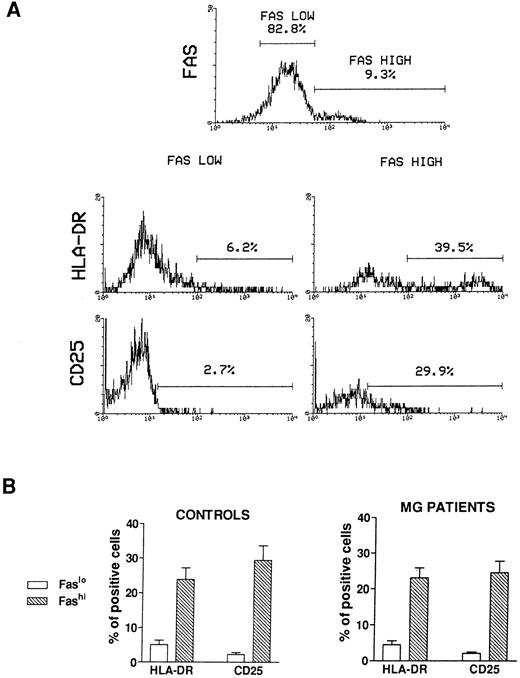
Activation state of Fashi thymocytes.We first compared the activation state of Fashi and Faslo cells by means of two-color immunofluorescence with anti-Fas and anti–interleukin-2 (IL-2) receptor α (anti-CD25) antibodies or anti-Fas and anti–HLA-DR antibodies on freshly isolated thymocytes from 6 MG patients and 6 control subjects. A representative analysis of 1 MG patient is presented in Fig 2A. In MG patients (Fig 2B), a higher proportion of Fashi thymocytes expressed the IL-2 receptor α and HLA-DR, relative to Faslo thymocytes. Similar results were obtained in controls (Fig 2B).
Activation state of Faslo and Fashi thymocytes. Freshly isolated thymocytes were stained with anti-Fas antibody and PE-labeled anti-CD25 or FITC-labeled anti-HLA-DR. (A) In Fas expression analysis, gates were set to define Faslo and Fashi thymocytes. During the acquisition step, events were accumulated in these gates. Analysis of one representative MG patient (anti-AChR antibody titer 33 nmol/L) is shown. In this representative analysis (one of six experiments) the percentage of HLA-DR–expressing or CD25-expressing cells is strikingly higher in Fashi thymocytes than in Faslo thymocytes. (B) Such analyses were performed on 6 controls and 6 MG patients. Data presented are mean ± SEM. The increase in HLA-DR or CD25+ cells in Fashi thymocytes compared with Faslo thymocytes were similar in controls and in MG patients.
Activation state of Faslo and Fashi thymocytes. Freshly isolated thymocytes were stained with anti-Fas antibody and PE-labeled anti-CD25 or FITC-labeled anti-HLA-DR. (A) In Fas expression analysis, gates were set to define Faslo and Fashi thymocytes. During the acquisition step, events were accumulated in these gates. Analysis of one representative MG patient (anti-AChR antibody titer 33 nmol/L) is shown. In this representative analysis (one of six experiments) the percentage of HLA-DR–expressing or CD25-expressing cells is strikingly higher in Fashi thymocytes than in Faslo thymocytes. (B) Such analyses were performed on 6 controls and 6 MG patients. Data presented are mean ± SEM. The increase in HLA-DR or CD25+ cells in Fashi thymocytes compared with Faslo thymocytes were similar in controls and in MG patients.
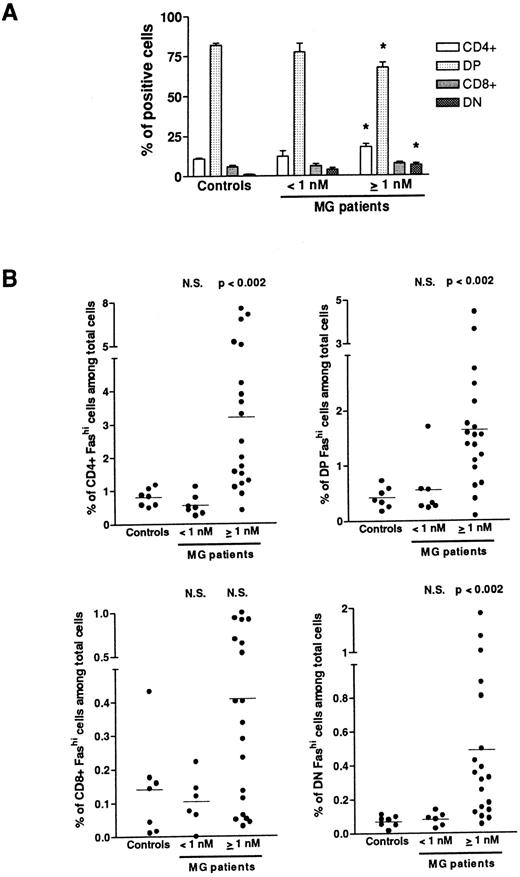
Fashi thymocytes in intrathymic differentiation.The three-color immunofluorescence technique was used to investigate the maturation step at which the proportion of Fashi thymocytes was enhanced. We first analyzed CD4 and CD8 expression in the whole thymocyte population. In MG patients with negative or borderline anti-AChR antibody titers, the proportion of single-positive CD4+ and CD8+, of double-positive CD4+CD8+, and double-negative CD4−CD8− cells were not modified (Fig 3A). In patients with positive anti-AChR antibody titers, the proportion of CD4+ and double-positive cells were significantly enhanced and decreased, respectively, as previously described.39 The significative increase in the proportion of double-negative thymocytes was associated with an increase in percentage of B cells in hyperplastic thymuses as previously shown.13 39 We then analyzed Fas expression in single-positive CD4+ and CD8+, doublepositive CD4+CD8+, and double-negative CD4−CD8− cells from all the patients' thymuses and calculated the proportion of CD4+Fashi, CD4+CD8+Fashi, CD8+Fashi, and CD4−CD8−Fashi thymocytes among the whole cell population (Fig 3B). In patients with positive anti-AChR antibody titer, but not in patients with negative or borderline titer, the proportion of CD4+Fashi and CD4+CD8+Fashi thymocytes were significantly increased, relative to the control subjects (P < .002 for both subsets). No significant increase was observed in CD8+Fashi cells, whatever the anti-AChR antibody titer. In patients with positive anti-AChR antibody titers, the significative increase in double-negative Fashi thymocytes was related to the presence of B cells in this population; indeed, the proportion of Fashi cells was about 30% of total thymic B cells (data not shown).
Proportion of FashiCD4+, FashiCD8+, FashiCD4+ CD8, and FashiCD4−CD8− thymocytes among the whole population. (A) CD4 and CD8 expression was first analyzed in 7 controls and 26 MG patients. Data are mean ± SEM. As in Fig 1, two groups were distinguished according to their anti-AChR antibody titer: negative or borderline (<1 nmol/L) and positive (≥1 nmol/L). In this last group, CD4+ and double-negative cell proportions were significantly increased whereas the proportion of double-positive cells was significantly decreased, compared with controls. Using three-color immunofluorescence, Fas expression was analyzed in each population (CD4+, CD8+, CD4+CD8+, and CD4−CD8−); it allowed us to calculate the proportions of FashiCD4+, FashiCD8+, FashiCD4+CD8+, and FashiCD4−CD8− thymocytes among the whole population. Differences were compared using the Mann-Whitney test. In the group of patients with positive anti-AChR antibody titers, the percentages of FashiCD4 and Fashi CD4+CD8+ were significantly increased relative to controls.
Proportion of FashiCD4+, FashiCD8+, FashiCD4+ CD8, and FashiCD4−CD8− thymocytes among the whole population. (A) CD4 and CD8 expression was first analyzed in 7 controls and 26 MG patients. Data are mean ± SEM. As in Fig 1, two groups were distinguished according to their anti-AChR antibody titer: negative or borderline (<1 nmol/L) and positive (≥1 nmol/L). In this last group, CD4+ and double-negative cell proportions were significantly increased whereas the proportion of double-positive cells was significantly decreased, compared with controls. Using three-color immunofluorescence, Fas expression was analyzed in each population (CD4+, CD8+, CD4+CD8+, and CD4−CD8−); it allowed us to calculate the proportions of FashiCD4+, FashiCD8+, FashiCD4+CD8+, and FashiCD4−CD8− thymocytes among the whole population. Differences were compared using the Mann-Whitney test. In the group of patients with positive anti-AChR antibody titers, the percentages of FashiCD4 and Fashi CD4+CD8+ were significantly increased relative to controls.
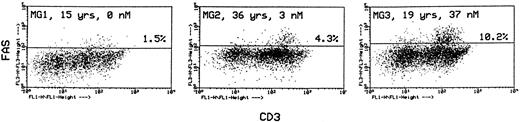
In the same labeling experiments we examined CD4 and CD8 markers in Fashi thymocytes from MG patients and controls to characterize their maturation state. As previously described by Debatin et al,40 Faslo cells had a CD4/CD8 profile similar to that of the total thymocyte population. By contrast, Fashi cells were mainly in the CD4 lineage, ie, CD4+ and CD4+CD8+ cells in MG patients and in control subjects (data not shown). Thymocytes were also simultaneously labeled with anti-Fas and anti-CD3 antibodies. Although Faslo thymocytes showed a wide range of CD3 expression, Fashi thymocytes uniformly showed intermediate CD3 (CD3int) expression (ie, between low and high). The same characteristics were found in control thymuses. Three representative analyses from three MG patients with no anti-AChR antibody, with an intermediate anti-AChR antibody titer, and with a high titer are shown (Fig 4). CD3intFashi thymocyte proportion was all the more increased as the patients' antibody titer was high.
Phenotypic characterization of Fashi thymocytes in three representative analyses of MG patients (anti-AChR antibody titer: 0, 3, and 37 nmol/L). Cells were stained with anti-Fas and FITC-labeled CD3. Fashi thymocytes uniformly display an intermediate level of CD3 expression.
Phenotypic characterization of Fashi thymocytes in three representative analyses of MG patients (anti-AChR antibody titer: 0, 3, and 37 nmol/L). Cells were stained with anti-Fas and FITC-labeled CD3. Fashi thymocytes uniformly display an intermediate level of CD3 expression.
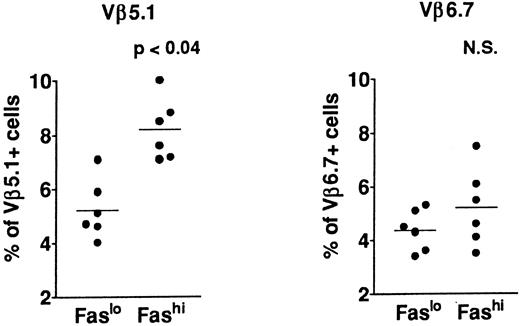
T-cell repertoire in Fashi thymocytes.In previous analyses of TCR Vβ gene segments in thymic cells, we found that the percentage of Vβ5.1-expressing cells, but not that of cells expressing Vβ6.7, was increased in MG patients39,41; this increase involved both mature single-positive thymocytes and their CD3++CD4+CD8+ late precursors. This study suggested a bias in intrathymic selection. In addition, expansion of Vβ5.1-expressing cells was observed in the presence of selected peptides from the AChR α subunit in CD4+ mature thymocytes from MG patients; Vβ5.1-expressing cells were thus enriched in potentially autoreactive cells (Cohen-Kaminsky et al, manuscript submitted, January 1997). We examined the expression of Vβ5.1 and Vβ6.7 in Fashi thymocytes in the present study (Fig 5) in isolated CD4+ thymocytes. This analysis was done on thawed thymocytes from six MG patients, after checking that the Fas expression level was not modified in CD4+ cells by cryopreservation. CD8-depleted thymocytes were selected after separation with magnetic CD8-coated beads. Three-color immunofluorescence analysis showed that the proportion of cells expressing Vβ5.1 was significantly enhanced among CD4+Fashi thymocytes relative to CD4+Faslo cells (P < .04). By contrast, Vβ6.7 expression was not significantly increased among CD4+Fashi thymocytes from MG patients.
Comparison of Vβ5.1 and Vβ6.7 expression in CD4+Faslo and CD4+Fashi thymocytes from six MG patients. CD8-depleted thymocytes were labeled with anti-Fas, PE-coupled anti-CD4, FITC-coupled anti-Vβ5.1 or -Vβ6.7. After setting gates to define Faslo and Fashi cells, Vβ5.1 and Vβ6.7 expression was analyzed in these gates and compared by using the Wilcoxon test. Vβ5.1-expressing cells were enriched in Fashi cells compared with Faslo cells, whereas Vβ6.7-expressing cells were equally represented in both populations.
Comparison of Vβ5.1 and Vβ6.7 expression in CD4+Faslo and CD4+Fashi thymocytes from six MG patients. CD8-depleted thymocytes were labeled with anti-Fas, PE-coupled anti-CD4, FITC-coupled anti-Vβ5.1 or -Vβ6.7. After setting gates to define Faslo and Fashi cells, Vβ5.1 and Vβ6.7 expression was analyzed in these gates and compared by using the Wilcoxon test. Vβ5.1-expressing cells were enriched in Fashi cells compared with Faslo cells, whereas Vβ6.7-expressing cells were equally represented in both populations.
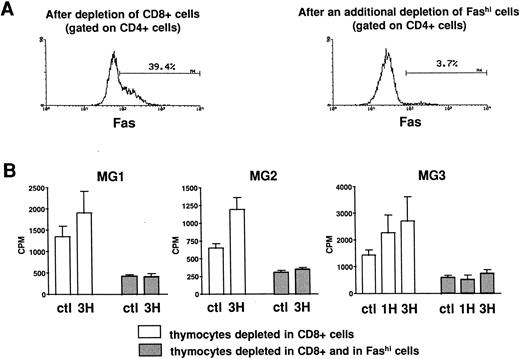
Involvement of Fashi thymocytes in the proliferative response to peptides from the AChR.CD8-depleted and CD8- and Fashi-depleted cells were obtained as previously described. The depletion of Fashi cells targeted around 90% of total CD4+Fashi cells (Fig 6A). CD8-depleted and CD8- and Fashi-depleted cells were cultured in the absence or in the presence of peptides from the AChR. CD4−CD8− cells constitute a source of antigen-presenting cells. 1H (169-181) and 3H (351-368) peptides were previously shown to stimulate the proliferative response in MG patient but not in control subject lymphocytes.42 Firstly, thymocytes depleted in Fashi cells spontaneously proliferate less efficiently than CD8-depleted cells in three MG patients (Fig 6B). Secondly, in these patients 1H and/or 3H peptides induce a significant proliferation response in CD8-depleted cells. This response was abrogated when Fashi cells were depleted (Fig 6B). These experiments indicate that cells involved in the proliferative response to peptides from the AChR are Fashi cells.
Involvement of Fashi thymocytes in the proliferative response to peptides from the AChR in three MG patients. (A) CD8-depleted thymocytes were labeled with anti-Fas (left). CD8-depleted thymocytes undergoing an additional depletion in Fashi cells were similarly labeled; about 90% of total Fashi cells were depleted (right). (B) After a 6-day period culture in the absence or in the presence of peptides from the AChR (1H and/or 3H, 5 μg/mL) the incorporation of 3H-thymidine (1 μCi for 0.2 × 106 cells during 20 hours) was analyzed and is expressed in counts per minute (means ± SEM from 4 to 8 determinations). When Fashi cells were depleted, the spontaneous proliferation of CD8-depleted cells was abrogated and the proliferative response to AChR peptides is abolished.
Involvement of Fashi thymocytes in the proliferative response to peptides from the AChR in three MG patients. (A) CD8-depleted thymocytes were labeled with anti-Fas (left). CD8-depleted thymocytes undergoing an additional depletion in Fashi cells were similarly labeled; about 90% of total Fashi cells were depleted (right). (B) After a 6-day period culture in the absence or in the presence of peptides from the AChR (1H and/or 3H, 5 μg/mL) the incorporation of 3H-thymidine (1 μCi for 0.2 × 106 cells during 20 hours) was analyzed and is expressed in counts per minute (means ± SEM from 4 to 8 determinations). When Fashi cells were depleted, the spontaneous proliferation of CD8-depleted cells was abrogated and the proliferative response to AChR peptides is abolished.
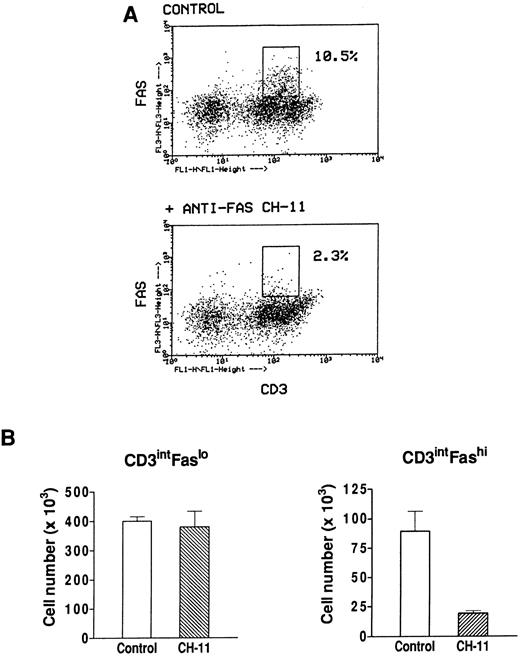
Effect of an agonistic anti-Fas antibody on Fashi thymocytes.We have previously found that Fashi thymocytes accumulate in the thymus of MG patients with positive anti-AChR antibody titers. Here we investigated whether the Fas receptor of Fashi cells was functional and whether Fashi cells were eliminated by an agonistic anti-Fas antibody. Immobilized CH-11 anti-Fas (5 μg/mL) was used on freshly isolated thymocytes from three MG patients. Fas and CD3 expression were analyzed after 20 hours of culture. We first checked that Fas staining with UB2 anti-Fas antibody was not blocked or dysregulated in the presence of CH-11 anti-Fas antibody on PBL which were previously shown to be resistant to an agonist antibody during a 1-day culture.43 Human PBL were cultured during 20 hours in the presence of immobilized CH-11 anti-Fas antibody and Fas expression was not significantly modified (data not shown). In MG patients, the proportion of Fashi cells was similar on freshly isolated and cultured thymocytes in control conditions. In the presence of CH-11 anti-Fas antibody the proportion of Fashi thymocytes was reduced by about 80% (Fig 7A). We compared living cell numbers (measured by Trypan blue assay) obtained with and without CH-11 anti-Fas antibody. The total cell number obtained in the presence of CH-11 anti-Fas antibody was not significantly modified compared with control conditions. Cell numbers in CD3intFaslo and CD3intFashi thymocyte populations were calculated from percentages obtained by immunofluorescence analysis. Although CD3intFaslo were not sensitive to an agonist anti-Fas antibody, most CD3intFashi were eliminated in these conditions (Fig 7B).
Effect of an agonistic anti-Fas antibody on MG Fashi thymocytes. (A) Fas and CD3 expression was analyzed after 20 hours of culture in the presence of immobilized CH-11 anti-Fas antibody (5 μg/mL) and in control conditions. A representative analysis of an MG patient is shown. After anti-Fas treatment a decrease in the proportion of Fashi thymocytes with intermediate CD3 expression was observed. (B) Living cell numbers (measured by Trypan blue assay) obtained with and without CH-11 anti-Fas antibody were compared in three MG patients. Cell numbers in CD3intFaslo and CD3intFashi thymocytes were calculated from percentages obtained by immunofluorescence analysis. Although CD3intFaslo were not sensitive to CH-11 anti-Fas antibody, most CD3intFashi were eliminated in this condition. Data are the mean ± SEM from three experiments.
Effect of an agonistic anti-Fas antibody on MG Fashi thymocytes. (A) Fas and CD3 expression was analyzed after 20 hours of culture in the presence of immobilized CH-11 anti-Fas antibody (5 μg/mL) and in control conditions. A representative analysis of an MG patient is shown. After anti-Fas treatment a decrease in the proportion of Fashi thymocytes with intermediate CD3 expression was observed. (B) Living cell numbers (measured by Trypan blue assay) obtained with and without CH-11 anti-Fas antibody were compared in three MG patients. Cell numbers in CD3intFaslo and CD3intFashi thymocytes were calculated from percentages obtained by immunofluorescence analysis. Although CD3intFaslo were not sensitive to CH-11 anti-Fas antibody, most CD3intFashi were eliminated in this condition. Data are the mean ± SEM from three experiments.
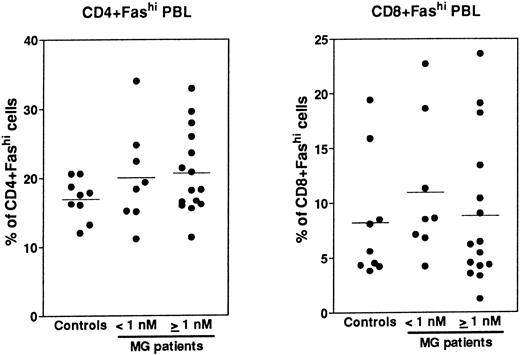
Expression of Fas in PBL.Fas expression was analyzed, as described above for thymocytes, on PBL isolated from the same MG patients at the time of thymectomy and from healthy volunteers (Fig 8). We examined the proportion of CD4+ and CD8+ peripheral cells and Fas expression in these subsets, then we calculated the proportion of CD4+Fashi and CD8+Fashi cells among total lymphocytes. CD4+ and CD8+ peripheral cells from controls contained 37.4% ± 0.9% and 32.1% ± 4.8% of Fashi lymphocytes, respectively, in keeping with previous results.44 Whatever the anti-AChR antibody titer of MG patients, the proportion of CD4+Fashi or CD8+Fashi lymphocytes was not significantly modified compared with control subjects. In both groups of MG patients, some individuals (25%) had significant increases (higher than the mean + 2 SD of control values) in the proportion of CD4+Fashi peripheral lymphocytes. A similar result has been obtained with lymphocytes from patients with systemic lupus erythematosus.20 Therefore, there was no major modification in the Fashi cell proportion among peripheral lymphocytes, contrary to thymocytes.
Comparison of the proportion of CD4+Fashi and CD8+Fashi cells in control and MG PBL. PBL freshly isolated over Ficoll gradients were stained with anti-Fas, anti-CD4, and anti-CD8 antibodies. The percentage of CD4+ and CD8+ peripheral lymphocytes and the proportion of Fashi cells among these subsets was determined. Two groups of patients (anti-AChR antibody titer <1 nmol/L, ≥1 nmol/L) are distinguished and compared to controls by using the Mann-Whitney test. No significant modification in the proportion of Fashi was observed in MG patient peripheral T-cell subsets.
Comparison of the proportion of CD4+Fashi and CD8+Fashi cells in control and MG PBL. PBL freshly isolated over Ficoll gradients were stained with anti-Fas, anti-CD4, and anti-CD8 antibodies. The percentage of CD4+ and CD8+ peripheral lymphocytes and the proportion of Fashi cells among these subsets was determined. Two groups of patients (anti-AChR antibody titer <1 nmol/L, ≥1 nmol/L) are distinguished and compared to controls by using the Mann-Whitney test. No significant modification in the proportion of Fashi was observed in MG patient peripheral T-cell subsets.
DISCUSSION
Dysregulation of Fas expression in MG patients with anti-AChR antibody.The main finding in this study is that Fas antigen expression is dysregulated in thymocytes from patients with myasthenia gravis, a human autoimmune disease, who had positive anti-AChR antibody titer. We showed that thymocytes strongly expressing Fas were present in the thymus of control subjects but at a lower proportion than in MG patients. This is in agreement with reports from Debatin et al40 and Yonehara et al,45 who described, in the normal thymus, a thymocyte subpopulation strongly expressing Fas and representing less than 4% of total thymocytes. This population had the same characteristics in control subjects and MG patients: Fashi thymocytes are enriched in activated cells and are mainly in the CD4 lineage (CD4+ and CD4+CD8+), and express intermediate levels of CD3. This phenotype characterizes cells in a transitional state during intrathymic maturation. No Fas expression modification was observed in the thymocytes from MG patients with a negative or borderline anti-AChR antibody titer. MG disease in which anti-AChR antibody is absent is unlikely to be related to thymus function. This is compatible with the absence of thymic hyperplasia and thymoma in seronegative forms of the disease, in which thymus is very often normal or involuted.2
Possible involvement of Fashi thymocytes in the anti-AChR autoimmune response.Previous work has suggested that the autoimmune response against AChR could take place in the thymus of MG patients, given that autoreactive T cells are present10,11; in addition, thymic B lymphocytes isolated from hyperplastic thymuses can produce anti-AChR antibodies.13 Interestingly, we found that the increase in the proportion of Fashi thymocytes correlated strongly with serum anti-AChR antibody titers in MG patients. In addition, among CD4+CD8− cells, Fashi thymocytes preferentially expressed the Vβ5.1 TCR segment but not the Vβ6.7 segment. Previous studies of MG thymocytes have indicated that Vβ5.1-expressing cells are potentially autoreactive (Cohen-Kaminsky et al, manuscript submitted, January 1997) and that their proportion is enhanced among mature single-positive thymocytes and their CD3++CD4+CD8+ late precursors.39,41 Previous work on Fashi thymocytes in humans has suggested that this thymocyte subpopulation could be autoreactive.40 45 In addition, we observed that (1) Fashi cells from MG patients were responsible for the spontaneous proliferation in autologous conditions, and (2) the proliferative response of MG patient thymocytes to two different AChR peptides was abolished when Fashi cells were depleted. Altogether our findings suggest, therefore, that Fashi thymocytes comprise autoreactive T cells that induce the autoimmune response against the AChR in the MG thymus.
To identify any peripheral events associated with Fashi thymocyte accumulation, we analyzed Fas expression in peripheral lymphocytes from the same MG patients collected at the time of thymectomy. We did not observe any significant modification in CD4+Fashi or CD8+Fashi peripheral cell proportion. Therefore, Fas expression modification in MG patients was observed in the thymic but not in the peripheral compartment. Fas ligand was previously shown to play a prominent role in the elimination of autoreactive cells in the periphery26-28; thus, the increase of Fashi thymocytes proportion in MG patient thymuses could be corrected in the periphery.
Why do Fashi thymocytes accumulate in MG thymuses?The main difference we found between control and MG thymuses was the proportion of Fashi thymocytes, which was about four times (up to 10 times) higher in MG patients with positive AChR antibody titers than in controls. Masunaga et al46 found that Fas mRNA was slightly decreased in the thymus of six MG patients, but used a nonquantitative reverse transcription-polymerase chain reaction on total thymic mRNA rather than isolated thymocytes. Fas is also expressed on human thymic epithelial cells (unpublished results, January 1996), and this could explain the apparent discrepancy with our results. Further experiments comparing Fas protein and mRNA expression should involve isolated thymocytes and thymic epithelial cells.
Several hypotheses can be raised to explain the accumulation of Fashi thymocytes in MG patients. Firstly, it could be due to activated PBL reentering the thymus.47 This hypothesis is unlikely for several reasons: (1) in MG patients, the increase in Fashi cell proportion was observed in thymocytes but not in PBL; (2) the increase of Fashi cell proportion was observed not only in CD4+Fashi but also in the CD4+CD8+Fashi population that is mainly a thymic subset; and (3) Fashi PBL, but not Fashi thymocytes, are resistant to Fas-induced cell death in the absence of activation.43
Secondly, Fashi thymocytes from control subjects and MG patients are enriched in activated cells, because they strongly express CD25 and HLA-DR. Activation of peripheral lymphocytes induces an increase in Fas expression.48 The sensitivity or resistance of activated cells to Fas-mediated apoptosis depends on the activation state.43 In addition, stimulation of human thymocytes through the TCR complex induces strong Fas expression45 (unpublished results, June 1996). Our team has previously found several signs of activation in the thymus of MG patients,10,13 especially increased cytokine expression.49 Taken together, these data suggest that the accumulation of Fashi thymocytes in MG patients is related to an increase in activation signals.
Thirdly, the accumulation of activated Fashi thymocytes could be caused by a failure to eliminate these cells in the thymus. However, most Fashi thymocytes in MG patients are sensitive to an agonistic anti-Fas antibody, showing that the Fas receptor is functional and can transduce an apoptotic signal. We could wonder whether another Fas/Fas ligand system dysfunction could be involved in the maintenance of Fashi thymocytes. Defective secretion of the Fas ligand or increased soluble Fas receptor levels could induce an accumulation of cells that normally die of apoptosis mediated by the Fas system. Cells able to produce Fas ligand are poorly characterized in the human thymus, although recent data suggest that thymic epithelial cell lines are good producers50 (unpublished results, July 1996). Soluble Fas receptor is able to inhibit Fas-mediated apoptosis, probably in interacting with available Fas ligand; its level is increased in patients with systemic lupus erythematosus according to Cheng et al,19 although other groups disagree.51,52 In addition, an increase in the level of some cytokines in MG thymuses could induce a less efficient Fas/Fas ligand-mediated cell death; indeed, it was shown that recombinant IL-12 was able to inhibit Fas-mediated apoptosis in human peripheral CD4+ lymphocytes.53 In conclusion, the accumulation of Fashi cells in MG patients could be caused by an imbalance between activation and deletion of thymic cells.
Taken together, our results indicate that a subpopulation of thymocytes strongly expressing Fas antigen may comprise AChR-reactive cells. These thymocytes, enriched in activated cells, accumulate in the thymus of MG patients with positive anti-AChR antibody titers and are not totally eliminated.
ACKNOWLEDGMENT
We thank Profs J.Y. Neveu and C. Planché, and Dr R. Nottin for providing normal thymuses. We are grateful to Prof A. Fisher and Dr J.M. Bertho for critical reading of the manuscript.
Supported by grants from AFM (Association Française contre les Myopathies), CNRS (Centre National de la Recherche Scientifique), and CNAMTS (Caisse Nationale d'Assurance Maladie des Travailleurs Salariés). N.M. received a postdoctoral grant from AFM.
Address reprint requests to Nathalie Moulian, PhD, Laboratoire d'Immunologie Cellulaire et Moléculaire, CNRS URA 1159, Hôpital Marie-Lannelongue, 133, avenue de la Résistance, 92350 Le Plessis Robinson, France.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal