Abstract
We report here that interleukin-4 (IL-4) induces homotypic aggregation of cultured human mast cells, grown from cord blood mononuclear cells in the presence of stem cell factor and IL-6. This aggregation was specifically induced by IL-4, because other cytokines including IL-1α, IL-1β, IL-2, IL-3, IL-5, IL-9, IL-10, interferon-γ, IL-12, granulocyte-macrophage colony-stimulating factor, NGF-β, and tumor necrosis factor-α failed to show such effect. Flow cytometric analysis of the cultured mast cells showed that IL-4 increases the expression of lymphocyte function-associated antigen-1 (LFA-1) and intercellular adhesion molecule-1 (ICAM-1), but not of very late antigen (VLA) family adhesion molecules or vascular cell adhesion molecule-1 (VCAM-1). Neutralizing monoclonal antibodies specific for LFA-1α, LFA-1β, or ICAM-1 inhibited the IL-4–induced homotypic aggregation of the mast cells, indicating that the aggregation was mediated mainly by LFA-1/ICAM-1 interaction. In addition, IL-4–treated but not untreated mast cells bound to immobilized ICAM-1. This binding was also inhibited by anti-LFA-1 or anti-ICAM-1. These results show that IL-4 promotes expression of ICAM-1 and LFA-1 molecules on mast cells, and suggest that IL-4 may contribute to the migration of mast cells into the inflamed tissue and to the cellular interaction with other inflammatory cells by upregulating adhesion molecules.
ADHESION MOLECULES on mast cells are believed to be involved in the tissue distribution of mast cells. In contrast to other bone marrow (BM)-derived cells, mast cells exclusively exist within tissues, suggesting that the expression of adhesion molecules needed for the interaction with tissue stromal cells and/or extracellular matrix is strictly regulated in mast cells. Furthermore, increase in mast cell numbers at sites of inflammation has been observed.1 Although this increase could be caused by the proliferation and maturation of mast cells, adhesion molecules expressed on mast cells may also be involved in the mast cell migration into inflamed tissues. In addition, mast cells are known to secrete several chemokines and cytokines that support the migration of inflammatory cells including lymphocytes, macrophages, neutrophils and eosinophils. Adhesion molecules expressed on mast cells may contribute to the interaction of mast cells with these inflammatory cells that accumulate into the site of inflammation and cause the late-phase allergic reaction. Furthermore, several adhesion molecules are known to induce activation signals that upregulate degranulation and cytokine production by mast cells.2 3 Thus, adhesion molecules on mast cells may play important roles in the tissue distribution, interaction with inflammatory cells, and the activation of mast cells.
The expression and regulation of adhesion molecules on mast cells has been investigated in the murine system using BM-derived mast cells (BMMC). BMMC express large amounts of very late antigen-5 (VLA-5), and very low amounts of VLA-4, VLA-6, and intercellular adhesion molecule-1 (ICAM-1). Kinashi and Springer4 observed that stem cell factor (SCF ) induced activation of VLA-5 and increased the binding of BMMC to fibronectin. Stimulation of FcεRI on BMMC upregulated the binding to laminin and fibronectin.5,6 In analogy to murine BMMC, rat peritoneal mast cells (RPMC) also express VLA-4, VLA-5, and vitronectin receptors. Activation with phorbol myristate acetate (PMA) increases the adhesion of RPMC to fibronectin and vitronectin, and degranulation of RPMC was augmented after binding to fibronectin.7
In the human system, several adhesion molecules have been found to be expressed on tissue mast cells. In previous reports, VLA-4, VLA-5, and ICAM-1 were expressed on mast cells in lung, uterus, heart, and skin.8-11 However, the regulation of adhesion molecules on human mast cells is poorly understood because of the difficulties in purification and culture of human mast cells. Instead, HMC-1, which is a human leukemic mast cell line, has been used to study the regulation of adhesion molecules on mast cells, and the upregulation the ICAM-1 expression by IL-4 was reported.12
Recently we established a culture system of human mast cells derived from cord blood mononuclear cells in the presence of SCF and interleukin-6 (IL-6).13 More than 99% of the cultured cells express c-kit antigen and contain tryptase and histamine, indicating that these cultured cells posses the typical features of mast cells. This culture system provides us with an excellent model for the study of the regulation of adhesion molecules on human mast cells by cytokine stimulation.
Here we report that IL-4 promotes the expression of lymphocyte function-associated antigen-1 (LFA-1) and ICAM-1 on the cultured human mast cells and induces homotypic aggregation of mast cells. Monoclonal antibodies (MoAbs) specific for LFA-1 or ICAM-1 completely blocked the IL-4–induced homotypic aggregation of mast cells as well as the adhesion of mast cells to immobilized ICAM-1. These results demonstrate the role of IL-4 to upregulate adhesion molecules on mast cells, and further suggest that IL-4 may play an important role in the migration and cellular interaction of mast cells in the allergic reaction.
MATERIALS AND METHODS
Cultured human mast cells.Cultured human mast cells were obtained as previously described.13 Briefly, cord blood mononuclear cells (CB MNCs) were grown in tissue culture flasks (Becton Dickinson & Co, San Jose, CA) in α-MEM (GIBCO-BRL, Life Technologies Inc, Gaithersburg, MD) supplemented with 20% fetal calf serum (FCS), in the presence of SCF (100 ng/mL; AMGEN, Thousand Oaks, CA) and IL-6 (80 ng/mL; Ajinomoto Co, Ltd, Tokyo, Japan) for more than 10 weeks.
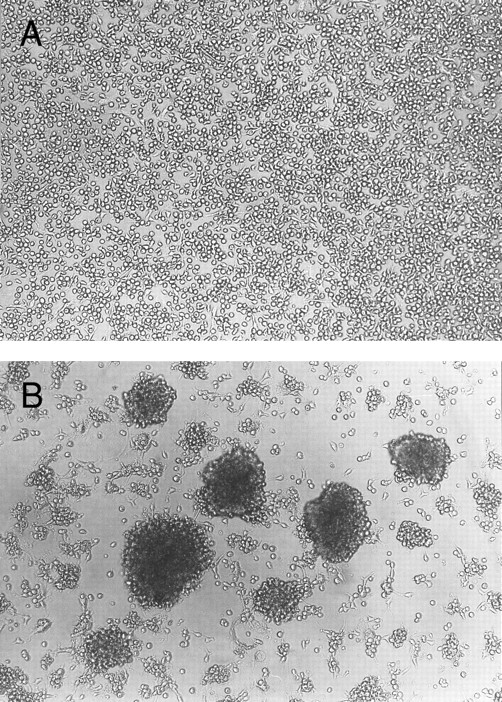
Homotypic aggregation of cultured human mast cells induced by IL-4. Cultured human mast cells were grown from CB MNCs in the presence of SCF (100 ng/mL) and IL-6 (80 ng/mL) for 10 weeks. Cells were then incubated for 10 days without (A) or with (B) IL-4 (10 ng/mL) in the presence of SCF and IL-6. Half of the media was changed on the fifth day of the culture. This experiment has been repeated five times with similar results.
Homotypic aggregation of cultured human mast cells induced by IL-4. Cultured human mast cells were grown from CB MNCs in the presence of SCF (100 ng/mL) and IL-6 (80 ng/mL) for 10 weeks. Cells were then incubated for 10 days without (A) or with (B) IL-4 (10 ng/mL) in the presence of SCF and IL-6. Half of the media was changed on the fifth day of the culture. This experiment has been repeated five times with similar results.
MoAbs and flow cytometry.Human mast cells (2 × 105) cultured with IL-4 (10 ng/mL) (a gift from DNAX, Palo Alto, CA) in the presence of SCF (100 ng/mL) and IL-6 (80 ng/mL) for the indicated periods were incubated with mouse MoAbs specific for human ICAM-1 (RR1/1)14; LFA-1α (TS1/22)14; LFA-1β (TS1/18)14; Mac-1 (OKM1; American Type Culture Collection [ATCC], Rockville, MD); p150.95 (FK24; Nichirei Co, Ltd, Tokyo, Japan); VLA-1 (TS2/7; T Cell Diagnostics, Inc, Cambridge, MA); VLA-2 (P1E8; GIBCO-BRL); VLA-3 (J143)15; VLA-4 (SG/73)15; VLA-5 (KH/33)15; VLA-6 (GoH3; Immunotech, Marseille, France); β1 (SG/19)15; VCAM-1 (1G11; Biodesign International, Kennebunk, ME); or c-kit (Nichirei Co, Ltd, Tokyo, Japan). After exposure to MoAbs, the cells were stained with fluorescein isothiocyanate (FITC)-conjugated goat antimouse IgG (Silenus Laboratories, Ltd, Victoria, Australia) and analyzed by flow cytometry (CytoACE; JASCO, Tokyo, Japan). Mouse IgG1 or IgG2b (Coulter Immunology, St Hialeah, FL) were used as subclass matched control antibodies.
Blocking of homotypic aggregation by MoAbs.Cultured human mast cells were incubated in flat-bottomed 96-well tissue culture plates (Costar Corp, Cambridge, MA) in 200 μL/well of medium containing 10% FCS, SCF (100 ng/mL), IL-6 (100 ng/mL), IL-4 (10 ng/mL), and various blocking MoAbs (20 μg/mL). After 5 days of culture, photographs were taken using an inverted microscope.
Adhesion assays.The adhesion assays with immobilized ICAM-1 were performed as previously described.16 17 Briefly, cultured human mast cells treated with or without IL-4 (10 ng/mL) in the presence of SCF (100 ng/mL), IL-6 (100 ng/mL) for 3 days were labeled with 2′, 7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein (BCECF ). The cells were transferred into polystyrene microtiter plate (Libro/Titertek; ICN Biomedicals, Inc, Aurora, OH) coated with immunoaffinity purified ICAM-1 (2 μg/well) or bovine serum albumin (BSA) (1.0%), and then incubated at 37°C for 30 minutes in 10% FCS-supplemented α-MEM with each of the following blocking antibodies: TS1/18 (20 μg/mL), TS1/22 (20 μg/mL), KH/33 (20 μg/mL), RR1/1 (20 μg/mL), or medium alone. After incubation, nonadherent cells were removed with six 21-gauge needle aspirations. Bound cells were quantitated in the 96-well plate using a Pandex fluorescence concentration analyzer (IDEXX Laboratories, Inc, Westbrook, ME). The level of adhesion was calculated by dividing bound fluorescence by input fluorescence.
Statistical analysis.Statistical analysis was performed by a paired two-way Student's t-test. Data are expressed as mean ± SD. A P value of <.05 was considered statistically significant.
RESULTS
Homotypic aggregation of cultured human mast cells induced by IL-4.Human mast cells derived form cord blood mononuclear cells and cultured in the presence of SCF and IL-6 were exposed to various cytokines in vitro. The addition of IL-4 consistently induced homotypic aggregation of cultured human mast cells as shown in Fig 1. Without IL-4, few aggregates were observed. Other cytokines tested, including IL-1α, IL-1β, IL-2, IL-3, IL-5, IL-9, IL-10, interferon-γ, IL-12, granulocyte-macrophage colony-stimulating factor, nerve growth factor-β (NGF-β), and tumor necrosis factor-α, failed to induce mast cell aggregation (tested twice, data not shown). Aggregation was observed as early as 8 hours after the addition of IL-4, reached maximum intensity in 3 to 5 days, and then decreased. The aggregation was stably observed if IL-4 was continuously added. The effect of IL-4 on the induction of mast cell homotypic aggregation was reversible, since when IL-4 was withdrawn from the culture, homotypic aggregation was gradually decreased. Within 20 days of IL-4 withdrawal, little homotypic aggregation could be observed (data not shown).
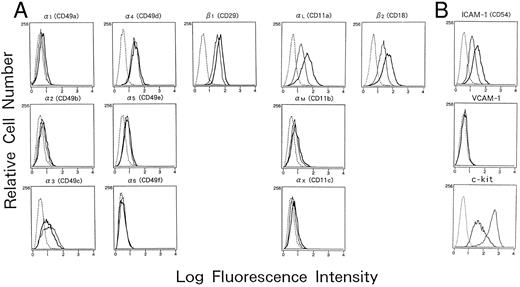
Adhesion molecules expressed on human mast cells cultured with or without IL-4. Human cultured mast cells (2 × 105) established from CB MNCs were cultured with or without IL-4 (10 ng/mL) for 5 days in the presence of SCF (100 ng/mL) and IL-6 (80 ng/mL), and the expression of various adhesion molecules was analyzed by flow cytometry. Cells were incubated with different murine MoAbs specific for (A) integrins; and (B) adhesion molecules belonging to Ig superfamilies; (━), mast cells cultured with IL-4; —— , cultured without IL-4), or subclass matched control antibodies (- - - -), and were subsequently stained with FITC-conjugated goat antimouse IgG. The results are one representative out of three.
Adhesion molecules expressed on human mast cells cultured with or without IL-4. Human cultured mast cells (2 × 105) established from CB MNCs were cultured with or without IL-4 (10 ng/mL) for 5 days in the presence of SCF (100 ng/mL) and IL-6 (80 ng/mL), and the expression of various adhesion molecules was analyzed by flow cytometry. Cells were incubated with different murine MoAbs specific for (A) integrins; and (B) adhesion molecules belonging to Ig superfamilies; (━), mast cells cultured with IL-4; —— , cultured without IL-4), or subclass matched control antibodies (- - - -), and were subsequently stained with FITC-conjugated goat antimouse IgG. The results are one representative out of three.
These results suggest that IL-4 induces adhesion molecules on the surface of cultured mast cells, resulting in homotypic aggregation.
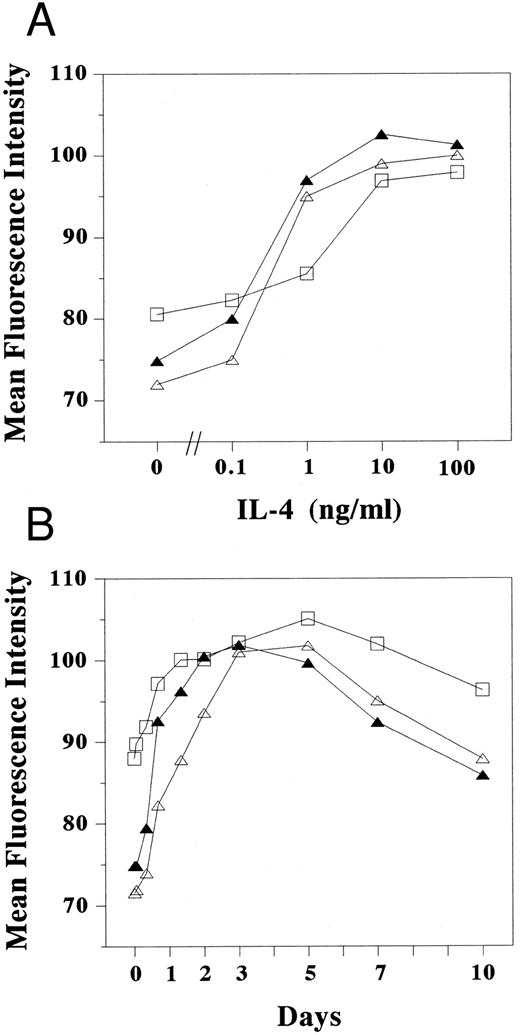
Adhesion molecules expressed on cultured human mast cells treated with IL-4.To identify the IL-4–induced adhesion molecules causing mast cell aggregation, we examined the expression of various surface adhesion molecules on mast cells cultured with or without IL-4 on flow cytometry (Fig 2A and B). In the absence of IL-4, cultured human mast cells constitutively expressed several adhesion molecules including ICAM-1 (CD54), LFA-1α (αL or CD11a), LFA-1β (β2 or CD18), VLA-3 (α3 or CD49c), and VLA-4 (α4 or CD49d), whereas they failed to express detectable amounts of VLA-1 (α1 or CD49a), VLA-2 (α2 or CD49b), VLA-6 (α6 or CD49f ), and VCAM-1 (CD106). Weak expressions of VLA-5 (α5 or CD49e), Mac-1 (αM or CD11b), and p150.95 (αX or CD11c) were observed in less than 5% of the cultured mast cells. When IL-4 was added to the mast cell cultures, the expression of LFA-1α, LFA-1β, and ICAM-1 markedly increased (mean fluorescence intensity: 71.5 to 96.6, 72.0 to 99.6, and 70.4 to 90.0, respectively) (Fig 2A and B). In contrast, the expression of VLA-3 and c-kit decreased by the addition of IL-4. IL-4 had little effects, if any, on the expression of other adhesion molecules tested including VLA-1, VLA-2, VLA-3, VLA-4, VLA-5, VLA-6, Mac-1, p150.95, and VCAM-1. The increase of LFA-1α, LFA-1β, and ICAM-1 could be observed as low as 0.1 ng/mL IL-4. The lowest concentration of IL-4 that induced the maximum expression was 10 ng/mL (Fig 3A), which is same as the concentration optimal for the induction of homotypic aggregation and for the growth of human cultured mast cells.18
Dose response and kinetics of LFA-1 and ICAM-1 expression induced by IL-4. Cultured human mast cells (2 × 105) stimulated (A) with various concentration of IL-4 for 3 days and (B) with IL-4 (10 ng/mL) for the periods indicated in the presence of SCF and IL-6 were incubated with mouse MoAbs specific for human ICAM-1 (RR1/1) (□), LFA-1α (TS1/22) (▵), and LFA-1β (TS1/18) (▴), respectively, and were subsequently stained with FITC-conjugated goat antimouse IgG. Cells were then analyzed by flow cytometery. The mean fluorescence intensity was measured in log scale and calculated by dividing the log scale axis equally into 255 channels. Each result is one representative out of three.
Dose response and kinetics of LFA-1 and ICAM-1 expression induced by IL-4. Cultured human mast cells (2 × 105) stimulated (A) with various concentration of IL-4 for 3 days and (B) with IL-4 (10 ng/mL) for the periods indicated in the presence of SCF and IL-6 were incubated with mouse MoAbs specific for human ICAM-1 (RR1/1) (□), LFA-1α (TS1/22) (▵), and LFA-1β (TS1/18) (▴), respectively, and were subsequently stained with FITC-conjugated goat antimouse IgG. Cells were then analyzed by flow cytometery. The mean fluorescence intensity was measured in log scale and calculated by dividing the log scale axis equally into 255 channels. Each result is one representative out of three.
Kinetic studies showed that the increase of LFA-1α, LFA-1β, and ICAM-1 expression could be detected as early as 8 hours after the addition of IL-4, and that expression reached maximum level 3 to 5 days after the addition of IL-4 (Fig 3B), similar to the kinetics of homotypic aggregation induced by IL-4.
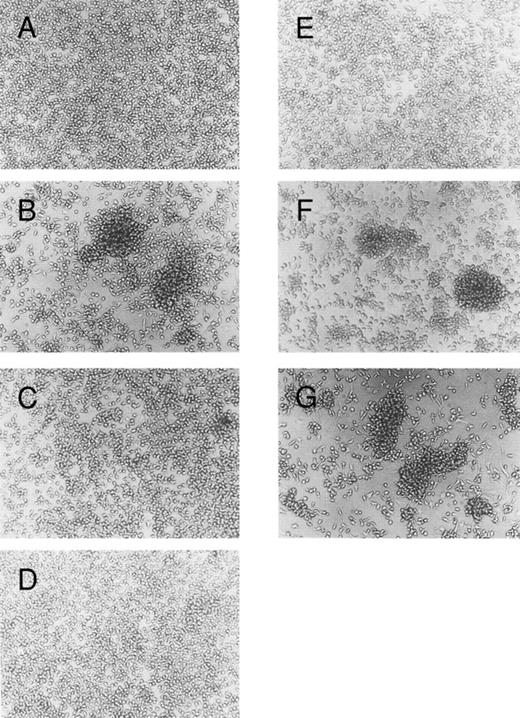
Blocking of IL-4–induced mast cell aggregation by anti–LFA-1 and anti–ICAM-1 MoAbs.We next examined the effect of several MoAbs specific for adhesion molecules on IL-4–induced homotypic aggregation of cultured mast cells. As shown in Fig 4, mast cell aggregation was completely blocked by the addition of MoAb specific for ICAM-1. MoAbs specific for LFA-1α or LFA-1β also effectively inhibited the homotypic aggregation. These results are consistent with the result of the flow cytometric analysis that showed a significant increase of the ICAM-1, LFA-1α, or LFA-1β expression by cultured mast cells exposed to IL-4. In contrast, MoAbs specific for VLA-5 or VLA-4 had no inhibitory effect on the IL-4–induced mast cell aggregation, although these molecules are constitutively expressed on the surface of cultured mast cells (Fig 2A). These results indicate that the IL-4–induced-mast-cell aggregation is mediated mainly by the LFA-1/ICAM-1.
Blocking of homotypic aggregation with antibodies specific for LFA-1α, LFA-1β, or ICAM-1. Human mast cells were cultured in the presence of SCF (100 ng/mL) and IL-6 (80 ng/mL); (A) without, (B) with IL-4 (10 ng/mL). Mouse MoAbs (20 μg/mL) specific for (C) LFA-1α (TS1/22), (D) LFA-1β (TS1/18), (E) ICAM-1 (RR1/1), (F ) VLA-5 (KH/33), or (G) VLA-4 (SG/73) were added to the culture in the presence of SCF, IL-6, and IL-4. The cells were incubated for 5 days. This experiment has been repeated three times with similar results.
Blocking of homotypic aggregation with antibodies specific for LFA-1α, LFA-1β, or ICAM-1. Human mast cells were cultured in the presence of SCF (100 ng/mL) and IL-6 (80 ng/mL); (A) without, (B) with IL-4 (10 ng/mL). Mouse MoAbs (20 μg/mL) specific for (C) LFA-1α (TS1/22), (D) LFA-1β (TS1/18), (E) ICAM-1 (RR1/1), (F ) VLA-5 (KH/33), or (G) VLA-4 (SG/73) were added to the culture in the presence of SCF, IL-6, and IL-4. The cells were incubated for 5 days. This experiment has been repeated three times with similar results.
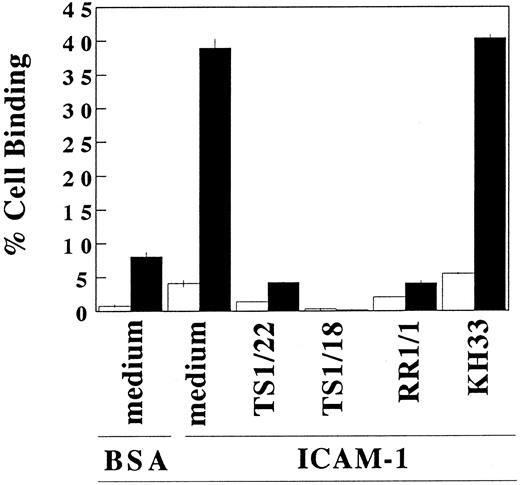
Binding of IL-4–treated mast cells to purified ICAM-1.To further clarify the effect of IL-4 on the LFA-1 molecule on cultured mast cells, we studied the adhesion of IL-4 treated or nontreated mast cells to purified ICAM-1–coated polystyrene microplates (Fig 5). Without IL-4 treatment, only 4.2% ± 0.5% of the cultured human mast cells adhered to immobilized ICAM-1. After IL-4 treatment for 3 days, 39.0% ± 1.3% of the mast cells adhered to ICAM-1–coated wells. This binding was completely blocked by addition of anti–ICAM-1 (RR1/1), anti-LFA-1α (TS1/22), or anti–LFA-1β (TS1/18), but was not inhibited by anti–VLA-5 (KH/33). Incubation of mast cells with IL-4 for 30 minutes, which is a sufficient period to convert inactive form of LFA-1 into active form but not a sufficient period to increase the expression of LFA-1,19-21 failed to augment the adhesion to ICAM-1 (data not shown). These results indicate that the amount of LFA-1 constitutively expressed on mast cells not exposed to IL-4 is insufficient to support adhesion to ICAM-1, and that the effect of IL-4 on the binding to ICAM-1 is due predominantly to an increase in the amount of expressed LFA-1.
IL-4–induced adhesion of cultured human mast cells to immobilized ICAM-1. After the cells were preincubated with (▪) or without (□) IL-4 (10 ng/mL) for 3 days, cultured human mast cells were incubated in ICAM-1 (2 μg/well)-, or BSA (1%)-coated wells. Indicated blocking antibodies (20 μg/mL) were added to each well. Cell binding (%) was measured and calculated as described in Materials and Methods. Each value represents mean ±SD (bar) of triplicate samples. IL-4–treated mast cells showed statistically significant binding ability to ICAM-1 compared with IL-4–nontreated mast cells (P < .001). This IL-4–induced binding to ICAM-1 was inhibited by TS1/22 (anti–LFA-1α MoAb) (P < .005), TS1/18 (anti–LFA-1β MoAb) (P < .001), and RR1/1 (anti–ICAM-1 MoAb) (P < .001). Similar results were obtained in three independent experiments.
IL-4–induced adhesion of cultured human mast cells to immobilized ICAM-1. After the cells were preincubated with (▪) or without (□) IL-4 (10 ng/mL) for 3 days, cultured human mast cells were incubated in ICAM-1 (2 μg/well)-, or BSA (1%)-coated wells. Indicated blocking antibodies (20 μg/mL) were added to each well. Cell binding (%) was measured and calculated as described in Materials and Methods. Each value represents mean ±SD (bar) of triplicate samples. IL-4–treated mast cells showed statistically significant binding ability to ICAM-1 compared with IL-4–nontreated mast cells (P < .001). This IL-4–induced binding to ICAM-1 was inhibited by TS1/22 (anti–LFA-1α MoAb) (P < .005), TS1/18 (anti–LFA-1β MoAb) (P < .001), and RR1/1 (anti–ICAM-1 MoAb) (P < .001). Similar results were obtained in three independent experiments.
DISCUSSION
Using a newly developed culture system, we found that human mast cells express LFA-1 and ICAM-1, and that IL-4 increases the expression of these adhesion molecules. The finding that LFA-1α and LFA-1β is expressed by cultured mast cells differs from previous reports that showed a lack of LFA-1α and LFA-1β expression on mast cells purified from human skin, lung, and heart.8,10,11 The reason of the differences between our observation and previous reports is unknown. However, because human uterine mast cells express LFA-1β, and the human leukemic mast cell line, HMC-1, expresses both LFA-1α and LFA-1β,22 it is likely that LFA-1 is inducible in mast cells under certain conditions. In addition, tissue-derived mast cells were not purified from inflamed tissue.8,10,11 Our observation that IL-4 increases LFA-1 expression by cultured human mast cells raises the possibility that mast cells purified from noninflamed tissues may express LFA-1 if stimulated with IL-4. In this context, it is also possible that mast cells at the site of allergic inflammation may express LFA-1, since it is known that T cells from patients with allergic disorders reportedly produce high amounts of IL-4.23 We are now studying these possibilities.
Despite the fact that cultured human mast cells express low but detectable amount of LFA-1 in the absence of IL-4 as was shown by flow cytometry, few homotypic aggregates were observed without IL-4. Consistently, mast cells not exposed to IL-4 failed to bind to purified ICAM-1. Only when cultured with IL-4, which induced increased expression of LFA-1, could mast cells bind to ICAM-1. These results indicate that the amount of LFA-1 expression by mast cells not treated with IL-4 is insufficient for adhesion to ICAM-1. Because treatment of mast cells with IL-4 for 30 minutes, which is sufficient to activate LFA-1 molecules,19-21 failed to increase the binding of mast cells to immobilized ICAM-1, it is likely that IL-4–induced adhesiveness to ICAM-1 is mainly caused by a quantitative change of LFA-1 expression on cultured mast cells.
LFA-1 and ICAM-1 on mast cells may contribute to cell-cell interaction of mast cells, especially during the late phase of an allergic reaction. ICAM-1 expressed by mast cells may facilitate the interaction with lymphocytes, neutrophils, macrophages, and eosinophils expressing LFA-1. Similarly, LFA-1 expressed by mast cells might contribute to migration of mast cells into inflamed tissues, because the expression of ICAM-1 by epithelial cells and dermal fibroblasts is known to be induced by inflammatory cytokines.24,25 LFA-1 on mast cells may also be involved in the interaction with other inflammatory cells expressing ICAM-1, including activated T lymphocytes, B lymphocytes, and macrophages.24,26 Because IL-4 is known to increase LFA-1 and ICAM-1 in a number of inflammatory cell types,12,25 27-30 it is likely that IL-4 plays an important role in the upregulation of cellular interaction between mast cells and inflammatory cells.
We have previously reported various effects of IL-4 on the cultured human mast cells.18 IL-4 promotes proliferation and induces FcεRI expression on the surface of cultured human mast cells. IL-4–treated mast cells release more histamine than nontreated mast cells in response to FcεRI cross-linking.18 In this report we showed that IL-4 induces expression of LFA-1 and ICAM-1 adhesion molecules, resulting in homotypic aggregation of mast cells. These data strongly support the hypothesis that IL-4 is a potent activator of human mast cells. The signal transduction pathway of the IL-4 on the activation of mast cells, which is likely to be regulated in transcriptional level, should be determined. Because IL-4 is produced in increased amounts by T cells of patients with allergic disorders,23 mast cells of allergic individuals may be in a constant hyper-reactive state and contribute to all processes of allergic inflammation.
ACKNOWLEDGMENT
We thank Dr H. Ishida for providing cytokines, I. Tanaka for technical assistance, and Drs S. Sasaki, I. Kamiyama, and the staffs of Matsushima Obstetric and Pediatric Hospital for providing human cord blood. We thank Dr Hans D. Ochs for reviewing the manuscript.
Supported by Uehara Memorial Foundation, Kowa Life Science Foundation, and Ministry of Education, Science and Culture (SN 08670860).
Address reprint requests to Hano Toru, MD, Department of Pediatrics, School of Medicine, Tokyo Medical and Dental University, Yushima 1-5-45, Bunkyo-ku, Tokyo 113, Japan.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal