Abstract
Recent clinical studies in China showed that As2O3 is an effective and relatively safe drug in the treatment of acute promyelocytic leukemia (APL). We found previously that As2O3 can trigger apoptosis of APL cell line NB4 cells, which is associated with downregulation of bcl-2 gene expression and modulation of PML-RARα chimeric protein. To further understand the mechanisms of this alternative therapy for APL, we investigated in this report the effects of a wide range of concentrations of As2O3 on cultured primary APL cells, all-trans retinoic acid (ATRA)-susceptible (NB4 cells) and ATRA-resistant (MR2 subclone) APL cell lines. The results indicated that As2O3 had dose-dependent dual effects on APL cells: inducing preferentially apoptosis at relatively high concentrations (0.5 to 2 μmol/L) and inducing partial differentiation at low concentrations (0.1 to 0.5 μmol/L). The rapid modulation and degradation of PML-RARα proteins, which was induced by As2O3 at 0.1 to 2 μmol/L, could contribute to these two effects. Bone marrow and peripheral blood examination showed that myelocyte-like cells, probably as a result of partial in vivo differentiation, and degenerative cells increased after 2 to 3 weeks of continuous in vivo As2O3 treatment when leukemic promyelocytes decreased. In conclusion, combination of induction of apoptosis and partial differention could be the main cellular mechanisms of As2O3 in the treatment of APL, and PML-RARα could play an important role in determining the specific effects of As2O3 on APL cells.
ACUTE promyelocytic leukemia (APL or AML-M3 and M3-variant) represents a unique model for cancer research in terms of the biological and clinical features. It has been shown during the last decade that PML-RARα chimeric protein, as a result of the specific chromosome translocation t(15; 17), plays a central role in APL pathogenesis.1-3 Clinically, APL is sensitive to antileukemic chemotherapy mainly consisting of an anthracycline and cytosine arabinoside, and to the differentiation therapy with all-trans retinoid acid (ATRA).4-9 Several multicenter clinical trials combining ATRA and chemotherapy have further improved the survival of APL patients.10,11 More recently, there were reports in China describing beneficial effects of an ancient drug, arsenic, in APL clinical trial. Arsenic compounds including arsenic trioxide (As2O3) and arsenic disulfide have allowed not only a high complete remission (CR) rate, but also relatively long-term survival in a significant proportion of patients.12-14
Interestingly, there seems to be no obvious cross-resistance between ATRA and As2O3 . This is of clinical relevance because with the currently used ATRA-chemotherapy protocol, there are still 50% of the patients who will relapse. Once the relapse occurs, the majority of the patients (60% to 70% in a recent trial in China) became resistant to ATRA and/or chemotherapeutic drugs.5,8 15 Use of new therapeutic means could bring benefits to relapsed APL patients. Indeed, among 15 relapsed APL patients treated with As2O3 in our Institute, 14 reached CR (see accompanying paper).
To illustrate mechanisms of As2O3 in the treatment of APL, we previously conducted an experimental study on NB4 cells (a human APL cell line with t[15; 17] and PML-RARα chimera) as an in vitro model, and found that As2O3 induced the cell apoptosis, which was associated with the downregulation of bcl-2 gene expression at both mRNA and protein levels and the modulation of PML/PML-RARα proteins.16 However, some clinical findings, such as the development of hyperleukocytosis during the second to third week of arsenic treatment in one third of patients (see accompanying paper), and the appearance of cells mimicking partially differentiated granulocytes in bone marrow (BM) (discussed later), suggest that the mechanisms of action of As2O3 could be more complicated than initially thought.
In the present work, we have addressed the in vitro and in vivo cellular and molecular mechanisms of As2O3 in the treatmet of APL, by using primary APL cell culture, the ATRA-susceptible and resistant APL cell lines (NB4 and the NB4-derived MR2 subclone), and patient samples obtained during arsenic treatment. In addition, a wider range of concentrations of As2O3 (0.05 to 2 μmol/L) and a longer period of observation, if necessary, have been used in the in vitro study in our present work than in the previous studies.16 The results showed that As2O3 may exert dual effects, ie, triggering cell apoptosis and inducing partial cell differentiation in a dose-dependent manner, probably as a result of the degradation of PML-RARα proteins.
MATERIALS AND METHODS
Compounds.A 0.1% As2O3 solution for intravenous administration was provided by the Pharmacy of Chinese Traditional Medicine in the First Hospital affiliated to Harbin Medical University (Harbin, People's Republic of China). Stock solutions were made at the concentration of 1 mmol/L with phosphate-buffered saline and diluted to working concentrations before use. One millimolar ATRA (Sigma, St Louis, MO) stock solution was made in ethanol.
Cell culture and cell viability assay.Primary APL cells were obtained from BM with informed consent from two cases of de novo APL and four cases at relapse. The diagnosis was established according to methods described in the accompanying paper. Fresh cells were separated by centrifugation on Ficoll's solution. ATRA-sensitive NB4 cells and an ATRA-resistant subclone MR2 derived from NB4 cells were also used. All cells were cultured in a humidified atmosphere of 95% air/5% CO2 at 37°C, according to our previously described methods.16 To minimize spontaneous nucleosomal DNA fragmentation and apoptosis, cells were maintained at densities <5 × 105 cells/mL by adjusting cell concentrations daily with adding fresh culture medium and corresponding concentrations of compounds while necessary.17
Cell morphology, cytochemistry, cell adhesion, and phagocytosis assay, and examination of histogramic-related nuclear DNA content.Cells were harvested after As2O3 treatment. Morphology was determined with Wright's stain of cells centrifuged onto slides by cytospin (Shandon, Runcorn, UK; 500 rpm, 4 minutes). Nitroblue tetrazolium (NBT) reduction was performed as previously described.18 Cytochemical reaction for myeloperoxidase, specific esterase (Naphthol AS-D chloracetate esterase), nonspecific esterase (α-Naphthol acetate esterase) and Periodic acid-Schiff (PAS) staining was performed using conventional methods. Cell adhesion test was performed using standard glass adhesion assay. Briefly, 1 mL of cell suspension (50 × 104/mL) was incubated at 37°C for 2 hours in glass tubes on a horizontal position. The tubes were then gently put at the vertical position and the suspension removed to other tubes. The cells in the suspension and those adhering to the tube wall were then counted and the cell adhesion rate (%) calculated. The phagocytosis activity was tested using standard black ink assay. Histogramic-related nuclear DNA contents were tested by flow cytometry using our previously described methods.16 Cells with DNA content below G1 phase were regarded as apoptotic cells. The percentage of apoptotic cells was analyzed by Lysis II software (Becton Dickinson, San Jose, CA).
Western blot analysis.Protein extracts (20 μL) prepared from 2 × 107 cells were loaded on a 10% sodium dodecyl sulfate-polyacrylamide gel, electrophoresed, and transferred to a nitrocellulose membrane, that was subsequently stained with 0.2% Ponceau red to assure equal protein loading and transfer. After neutralization of the membrane in 10% defatted milk powder, the membrane was incubated for 90 minutes with antihuman RARα polyclonal antibody (Lot G266; Santa Cruz Biotechnology, Santa Cruz, CA) in 10% defatted milk powder. The immunocomplex was visualized by enhanced chemiluminescence kit (RPN2108; Amersham Life Science, Buckinghamshire, UK, HP79NA).
Immuno-fluorescence analysis.Cells were centrifuged onto slides as described above and rapidly air-dried. Immunofluorescence staining of the N-terminal region of PML was performed according to a previously described method18 except that a new anti-PML N-terminal antiserum of better quality was used, prepared by Dr Naoe (Nagoya University School of Medicine Branch Hospital, Nagoya, Japan).
Immuno-phenotyping.Expression of cell surface differentiation antigens CD11b and CD33 were performed with previously described methods to measure differentiation induction.18 Becton Dickinson Simultest Control r1 /r2α (IgG1 /fluorescein isothyiocyanate/IgG2αPE) was used as a negative control.
In situ terminal deoxynucleotidyl transferase (TdT) labeling.The labeling reaction was performed on cytospin slides (for cultured cells) and smear slides (for previously Wright stained BM sample) according to the protocols recommended by Apop Tag in situ Apoptosis Detection Kit (Catalog: S7110-KIT; Oncor Technical Assistance, USA). Slides were viewed and images were recorded using fluorescence microscope with filter sets for viewing fluorescein or PI and Kodak 400 film (Eastman Kodak, Rochester, NY).
Analysis of BM picture during in vivo As2O3 treatment.As2O3 treatment was performed in 15 relapsed APL patients, as described in the accompanying paper. BM pictures were analyzed before and during As2O3 treatment, and after As2O3 -induced CR in all patients. In seven cases, the myelogram was performed once a week during the treatment. BM smears were stained with Wright's staining and at least 500 cells were analyzed for morphological classification. Some smears were also used for immunofluoresence of PML/PML-RARα.
Fluorescence in situ hybridization (FISH) analysis of PML-RARα fusion gene on patient BM cells.Two yeast artificial chromosomes (YACs) from Centre d'Etude du Polymorphisme Humain (CEPH) library, namely 417D9 with a genomic localization just down stream of RARα gene and 185B02 including PML locus, were used as probe to reveal the t(15; 17) FISH was performed using previously described method.19
RESULTS
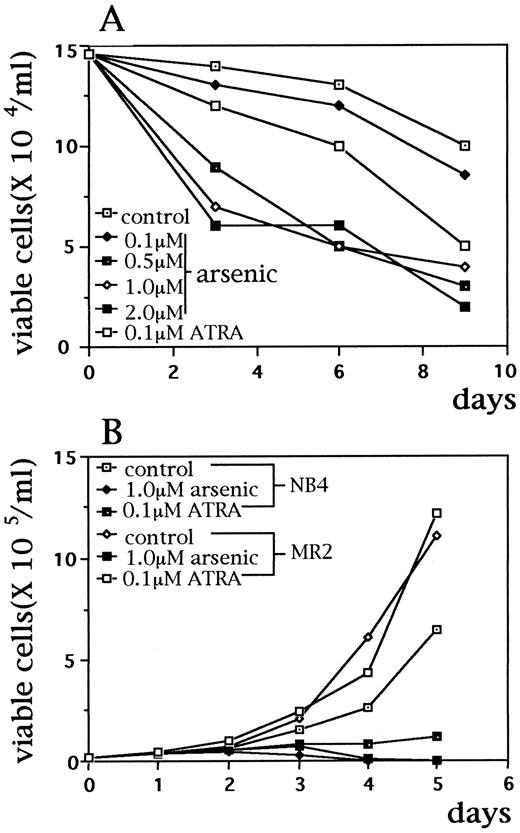
As2O3 induces apoptosis and partial differentiation of APL cells in a dose-dependent manner.During in vitro culture, unlike NB4 cells, primary APL cells showed little proliferation and the cell viability was gradually decreased when no drug was added. Treatment with relatively high concentrations (0.5 to 2 μmol/L) of As2O3 significantly reduced the number of viable cells in samples from six patients. However, use of lower concentration (0.1 μmol/L) of the drug for 9 days did not change the cell viablity profile compared with control group (Fig 1A). Similarly, 0.1 μmol/L As2O3 treatment for 10 days did not alter the growth and survival of NB4 cells (data not shown). Morphologically, after treatment with 0.5 to 2 μmol/L of As2O3 for 24 to 72 hours, primary APL cells presented some changes such as the decreased cytoplasmic granules, condensation of chromatin, decreased nuclear/cytoplasm ratio, and partial disappearance of nucleoli, suggesting a certain degree of differentiation. Nuclear fragmentation characteristic of apoptosis could be seen as well (Fig 2E, F ). It may be interesting to note that APL cells from different patients showed some differences on effect of As2O3 : nuclear fragmentation was easily seen in primary cells from some cases after incubation with As2O3 , whereas cells from others mainly presented morphologic features corresponding to a partial differentiation. It was difficult to evaluate the morphological changes of primary APL cells under lower concentration (0.1 μmol/L) of arsenic, since no obvious changes were observed through 1- to 8-day culture while afterwards the cells became degenerative like in the control samples.
Effects of As2O3 on APL cell growth. (A) Primary BM cells from one relapsed APL patient were incubated with As2O3 (0.1 to 2.0 μmol/L) and ATRA (0.1 μmol/L) for 9 days. Fresh APL cells from five other patients had similar results (not shown); (B) ATRA-susceptible NB4 cells and ATRA-resistant–MR2 cells were incubated with As2O3 (1 μmol/L) and ATRA (0.1 μmol/L) for 5 days. Viable cell numbers were calculated with typan-blue exclusion. Every point represents the mean of triplicated samples.
Effects of As2O3 on APL cell growth. (A) Primary BM cells from one relapsed APL patient were incubated with As2O3 (0.1 to 2.0 μmol/L) and ATRA (0.1 μmol/L) for 9 days. Fresh APL cells from five other patients had similar results (not shown); (B) ATRA-susceptible NB4 cells and ATRA-resistant–MR2 cells were incubated with As2O3 (1 μmol/L) and ATRA (0.1 μmol/L) for 5 days. Viable cell numbers were calculated with typan-blue exclusion. Every point represents the mean of triplicated samples.
As2O3 induces morphological changes in cultured primary APL cells and NB4 cells. (A through D): NB4 cells were treated without (A) and with 1 μmol/L (B) As2O3 for 24 hours, and 0.5 (C) and 0.1 μmol/L (D) As2O3 for 10 days; (E and F): BM cells from one relapsed APL patient were cultured and treated in vitro without (E) and with (F ) 2 μmol/L As2O3 for 24 hours. Similar results were also observed in three other APL cases. Cells were centrifuged onto slides by cytospin (Shandon, 500 rpm, 4 minutes) and stained with Wright's staining.
As2O3 induces morphological changes in cultured primary APL cells and NB4 cells. (A through D): NB4 cells were treated without (A) and with 1 μmol/L (B) As2O3 for 24 hours, and 0.5 (C) and 0.1 μmol/L (D) As2O3 for 10 days; (E and F): BM cells from one relapsed APL patient were cultured and treated in vitro without (E) and with (F ) 2 μmol/L As2O3 for 24 hours. Similar results were also observed in three other APL cases. Cells were centrifuged onto slides by cytospin (Shandon, 500 rpm, 4 minutes) and stained with Wright's staining.
To test if As2O3 could really induce apoptosis of primary APL cells, the examination of histogramic related nuclear DNA contents on flow cytometry and the in situ TdT labeling reaction were performed. As shown in Fig 3A, a portion of apoptotic cells (sub-G1) was present in the control culture, which was possibly due to the absence of hematopoietic growth factors. As2O3 treatment at 0.1 μmol/L showed no effect on this portion. However, treatment at concentrations of 0.5 to 2 μmol/L caused significantly increased numbers of the apoptotic cells in dose- and time-dependent way. TdT labeling also showed DNA fragmentation, confirming the presence of apoptosis (data not shown).
The percentage of apoptotic APL cells after As2O3 treatment. (A) Primary APL cells were incubated with different concentrations of As2O3 for 3 and 6 days; (B) NB4 cells and MR2 cells were incubated with As2O3 (1.0 μmol/L) and ATRA (0.1 μmol/L) for 1, 3, and 5 days. The percentage of apoptotic cells or sub-G1 cells was analyzed by flow cytometer.
The percentage of apoptotic APL cells after As2O3 treatment. (A) Primary APL cells were incubated with different concentrations of As2O3 for 3 and 6 days; (B) NB4 cells and MR2 cells were incubated with As2O3 (1.0 μmol/L) and ATRA (0.1 μmol/L) for 1, 3, and 5 days. The percentage of apoptotic cells or sub-G1 cells was analyzed by flow cytometer.
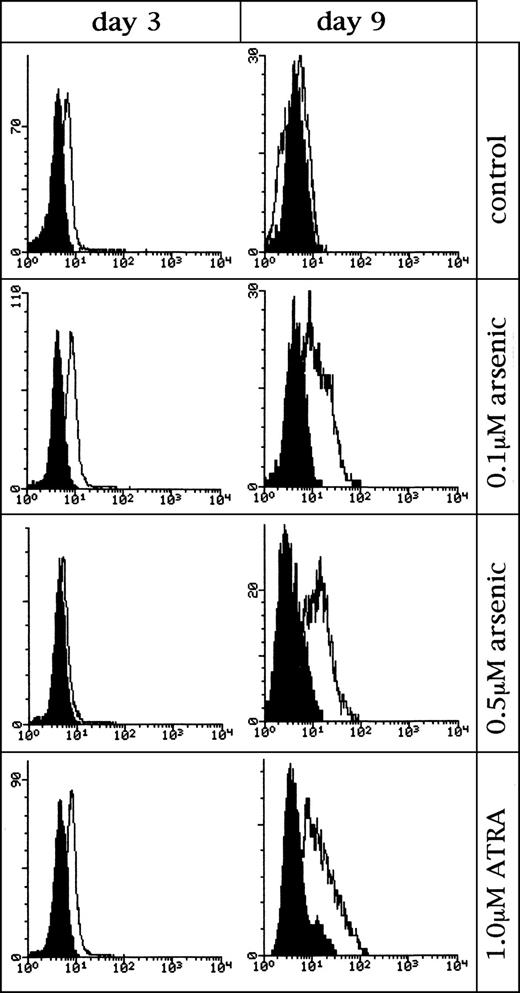
The possibility of cell differentiation induced by As2O3 was addressed using analysis of differentiation antigens in primary APL cell (Fig 4, and not shown). Interestingly, 0.1 to 0.5 μmol/L As2O3 treatment could modulate the expression of CD11b and CD33. CD11b level was increased gradually while CD33 was decreased. Although these changes were in line with those seen in granulocyte differentiation triggered by ATRA, the extent of modulation was lower than that induced by ATRA. At higher concentration (1 to 2 μmol/L), As2O3 did not alter CD11b expression whereas there was a significant reduction of CD33 level, which might be related to increased cell death. However, unlike the effect of ATRA, no elevation of the NBT reduction was seen after treatment (not shown).
Histogram on effects of low-concentration of As2O3 on the expression of differentiation antigen CD11b on fresh leukemic cells from one APL patient. Control: untreated cells. Shadowed peaks represent Becton Dickinson Simultest Control and the open peaks represent CD11b. Similar results were obtained in cells from other two APL patients and NB4 cell line.
Histogram on effects of low-concentration of As2O3 on the expression of differentiation antigen CD11b on fresh leukemic cells from one APL patient. Control: untreated cells. Shadowed peaks represent Becton Dickinson Simultest Control and the open peaks represent CD11b. Similar results were obtained in cells from other two APL patients and NB4 cell line.
To get further insight into the differentiation-inducing effect of As2O3 , NB4 cells were used as model. Experimental conditions were designed so that cells could be exposed to arsenic at a wider range of concentrations and, when possible, with longer duration than in the previous report.20 As shown in Fig 2A, untreated NB4 cells had very immature morphology, such as high nuclear/cytoplasmic ratio, clear nucleoli, and particularly very basophilic cytoplasm containing no granules, suggesting that they could be situated in a less differentiated stage than the majority of fresh APL cells in patient BM. Strikingly, the treatment of As2O3 at 0.1 to 0.25 μmol/L for more than 10 days could produce significant morphological changes, such as condensed chromatin, smaller nuclei, decreased nuclei/cytoplasm ratio, appearance of granules (including neutrophilic granules) in cytoplasm (Fig 2D). The majority of cells look like myelocytes, and a few may correspond to metamyelocytes, although the nucleoli are still visible in their nuclei. Interestingly, when NB4 cells were treated with As2O3 at 0.5 μmol/L for 10 days, both apoptotic and partly differentiated cells were visible (Fig 2C). Immunophenotypic analysis showed that low concentrations of As2O3 could indeed modulate the expression of differentiation antigens, especially that of CD11b in NB4 cells (not shown). Cytochemical studies also showed an increased expression of myeloperoxidase (POX) and chloracetate esterase (NAS-DCE) in 0.1 μmol/L As2O3 -treated NB4 cells (Table 1). Functional tests showed that untreated NB4 cells had relatively strong phagocytotic activity, which was not changed after treatment with 0.1 μmol/L As2O3 (Table 2). However, the ability of cell adhesion was largely increased in treated cells (Table 2). It is worth noting, nevertheless, that as in primary APL cell culture, no terminal morphological differentiation (presence of polynucleated granulocytes) was achieved. Another finding not consistent with a typical differentiation is that NBT reduction was always negative despite morphological changes (data not shown).
Cytochemical Reaction of NB4 Cells Untreated and Treated With 0.1 μmol/L As2O3
| . | . | Score . | |||||
|---|---|---|---|---|---|---|---|
| . | . | (−) . | (±) . | (+) . | (++) . | (+++) . | |
| POX | C | 0 | 25 | 47 | 28 | 0 | (%) |
| T | 0 | 0 | 39 | 58 | 30 | (%) | |
| NAS-DCE | C | 100 | 0 | 0 | 0 | 0 | (%) |
| T | 78 | 10 | 12 | 0 | 0 | (%) | |
| α-NAE | C | 0 | 0 | 91 | 9 | 0 | (%) |
| T | 0 | 0 | 83 | 15 | 2 | (%) | |
| α-NAE + NaF | C | 0 | 100 | 0 | 0 | 0 | (%) |
| T | 0 | 88 | 12 | 0 | 0 | (%) | |
| PAS | C | 0 | 0 | 26 | 42 | 32 | (%) |
| T | 0 | 0 | 13 | 56 | 35 | (%) | |
| . | . | Score . | |||||
|---|---|---|---|---|---|---|---|
| . | . | (−) . | (±) . | (+) . | (++) . | (+++) . | |
| POX | C | 0 | 25 | 47 | 28 | 0 | (%) |
| T | 0 | 0 | 39 | 58 | 30 | (%) | |
| NAS-DCE | C | 100 | 0 | 0 | 0 | 0 | (%) |
| T | 78 | 10 | 12 | 0 | 0 | (%) | |
| α-NAE | C | 0 | 0 | 91 | 9 | 0 | (%) |
| T | 0 | 0 | 83 | 15 | 2 | (%) | |
| α-NAE + NaF | C | 0 | 100 | 0 | 0 | 0 | (%) |
| T | 0 | 88 | 12 | 0 | 0 | (%) | |
| PAS | C | 0 | 0 | 26 | 42 | 32 | (%) |
| T | 0 | 0 | 13 | 56 | 35 | (%) | |
Abbreviations: C, control; T, treatment with As2O3 ; POX, myeloperoxidase; NAS-DCE, Naphthol AS-D chloracetate esterase; α-NAE, α-Naphthol acetate esterase; α-NAE + NaF:α-Naphthol acetate esterase + NaF; PAS, Periodic acid-Schiff stain. (−), negative; (±), weak or few positive; (+), moderately positive; (++), moderately strong positive; (+++), strongly positive.
Functional Tests of NB4 Cells Untreated and Treated With 0.1 μmol/L As2O3
| . | . | Score . | |||||
|---|---|---|---|---|---|---|---|
| . | . | (−) . | (±) . | (+) . | (++) . | (+++) . | |
| Phagocytosis | C | 0 | 30 | 35 | 25 | 10 | (%) |
| T | 0 | 25 | 30 | 30 | 15 | (%) | |
| . | . | Score . | |||||
|---|---|---|---|---|---|---|---|
| . | . | (−) . | (±) . | (+) . | (++) . | (+++) . | |
| Phagocytosis | C | 0 | 30 | 35 | 25 | 10 | (%) |
| T | 0 | 25 | 30 | 30 | 15 | (%) | |
| Adhesion rate | C | 8.7 ± 1.2% |
| T | 49.7 ± 10.5% |
| Adhesion rate | C | 8.7 ± 1.2% |
| T | 49.7 ± 10.5% |
Abbreviations: C, control; T, treatment with As2O3 .
Mean ± SD of 4 independent assays.
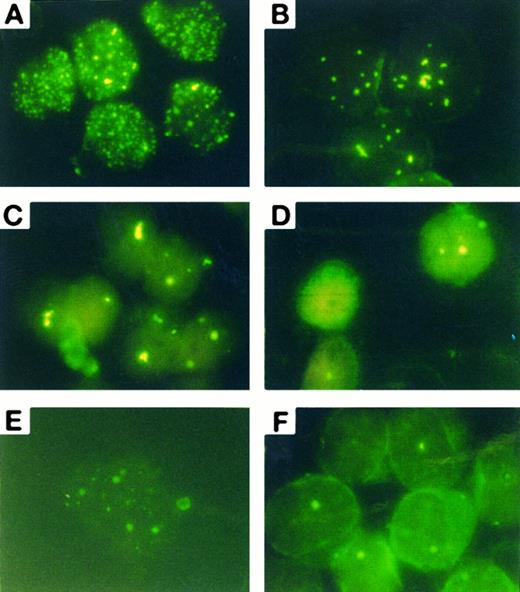
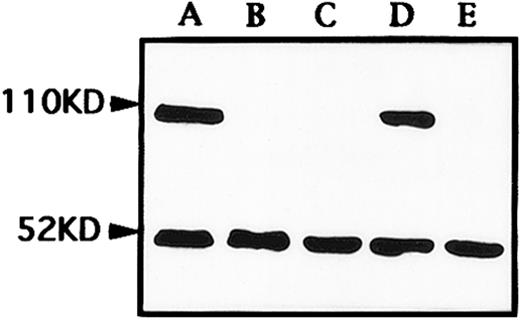
As2O3 causes a rapid degradation of PML/PML-RARα chimeric proteins in APL cells.Previously, we showed that in the NB4 cell line, As2O3 can induce a remarkable modulation of PML/PML-RARα staining pattern.16 When primary APL cells were treated by As2O3 in vitro, similar effects could be seen. Untreated primary cells exhibited a great number of micropunctates in the nucleus and cytoplasm, corresponding to the previously described abnormal staining pattern caused by PML-RARα and/or PML-RARα/PML heterodimers (see Fig 5A).18 After incubation with As2O3 at 0.1 to 2 μmol/L, PML speckles of normal appearance (known as PODs or nuclear bodies 20 through 22), about 8 to 10 in numbers in each cell nucleus, could be restored at about 12 hours (Fig 5B). These speckles further aggregated to form 2 to 3 large granules at 24 hours (Fig 5C) before their disappearance at 48 hours (Fig 5D). Such a process of PML staining pattern changes was quicker with high drug concentration (1 μmol/L) than that observed with low concentration (0.1 μmol/L) (Fig 5E). No obvious accumulation of PML staining in the cytoplasm was noted on primary APL cells. Compared to our previous experiment in NB4 cell line, the modulation in PML-RARα/PML in primary APL cells seems to need longer time, since the similar modulation was observed within 12 to 24 hours in NB4 cells (Zhu J, et al, manuscript submitted). To further explore the effect of As2O3 at protein level, Western blot analysis for PML-RARα and wild-type RARα was performed on an NB4 cell line. The surprising finding is that As2O3 triggered a rapid degradation of PML-RARα, but not the wild-type RARα protein (Fig 6).
Immuno-fluorescence analysis of the subcellular localization of PML-RARα/PML in cultured primary APL cells from a patient. (A) Control; (B) 1 μmol/L As2O3 treatment for 12 hours; (C) 1 μmol/L As2O3 treatment for 24 hours. (D) One micromolar As2O3 treatment for 48 hours; (E) 0.1 μmol/L 1 μmol/L As2O3 treatment for 24 hours; (F ) in vivo BM cells on day 15 of treatment.
Immuno-fluorescence analysis of the subcellular localization of PML-RARα/PML in cultured primary APL cells from a patient. (A) Control; (B) 1 μmol/L As2O3 treatment for 12 hours; (C) 1 μmol/L As2O3 treatment for 24 hours. (D) One micromolar As2O3 treatment for 48 hours; (E) 0.1 μmol/L 1 μmol/L As2O3 treatment for 24 hours; (F ) in vivo BM cells on day 15 of treatment.
Western blot analysis of RARα and PML-RARα expression in NB4 cells untreated (A), treated with 1 μmol/L As2O3 for 24 hours (B) and 48 hours (C), and with 0.1 μmol/L As2O3 for 24 hours (D) and 48 hours (E). Both bands of 52 kD and 110 kD represented wild-type RARα and PML-RARα chimeric proteins, respectively.
Western blot analysis of RARα and PML-RARα expression in NB4 cells untreated (A), treated with 1 μmol/L As2O3 for 24 hours (B) and 48 hours (C), and with 0.1 μmol/L As2O3 for 24 hours (D) and 48 hours (E). Both bands of 52 kD and 110 kD represented wild-type RARα and PML-RARα chimeric proteins, respectively.
Effects of As2O3 on ATRA-resistant MR2 subclone.Since As2O3 showed a therapeutic effect in APL patients resistant to ATRA, it was important to examine the effect of As2O3 on ATRA-resistant APL cells. For this, MR2 subclone is a good in vitro model, whose resistance to ATRA was conformed by morphologic changes and NBT reduction (Fig 1B and data not shown). In fact, the growth and survival of MR2 cells could be inhibited effectively by As2O3 , like NB4 cells (Fig 1B), and the examination of cytomorphology and histogramic related nuclear DNA contents on flow cytometry (Fig 3B) confirmed that As2O3 could also trigger apoptosis in this subclone. MR2 cells displayed the same PML/PML-RARα staining pattern as NB4 cells. With 10−7 mol/L ATRA treatment for 48 hours, these micropunctates did not restore into normal POD or nuclear bodies, unlike that seen in ATRA-treated NB4 cells. However, similar to NB4 and primary cultured APL cells, 1 μmol/L As2O3 treatment significantly decrease PML staining in the nuclei of MR2 cells (data not shown).
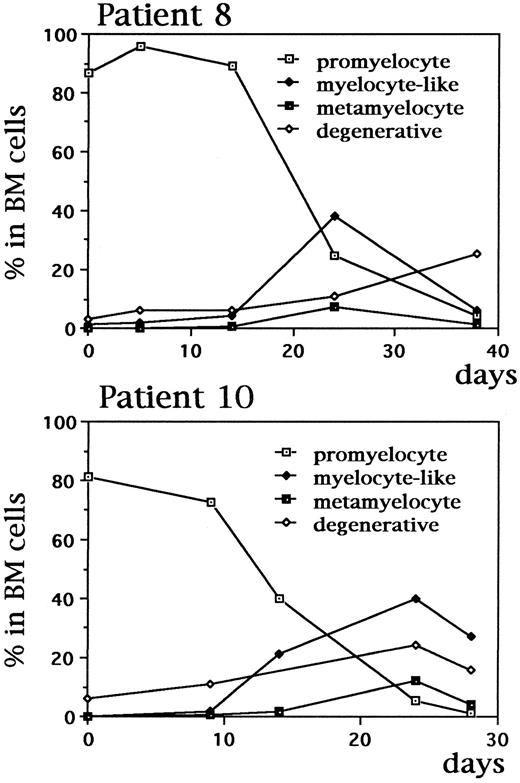
In vivo studies during continuous As2O3 treatment in APL patients.As shown in Fig 7A, leukemic cells from one relapsed APL patient presented a typical morphology of APL. During the treatment with As2O3 , typical nuclear fragmented cells were seen only after scrutiny. However, at the third and fourth week of arsenic therapy, a large percentage of cells presenting smaller volume, decreased azurophile granules but increased neutrophile granules, condensed chromatin, and decreased nuclear-cytoplasmic ratio, could be observed in both BM and peripheral blood when abnormal promyelocytes gradually decreased (Fig 7B, Fig 8). Although with some similarities to myelocytes, these cells showed morphologic features seen in myelodyspoiesis including relatively small size and imbalanced nuclear/cytoplasmic maturation, and thus were called myelocyte-like cells to distinguish them from the normal myelocytes. FISH analysis confirmed that these cells did contain the t(15; 17) translocation using PML and RARα YAC probes (data not shown). In addition, many degenerative, dying cells with condensed or coarse nucleus losing cytoplasm (“nude” nucleus) could also be easily observed (Fig 7C, D, and Fig 8). Some of the cells seem to be at a transitional stage between the myelocyte-like cells and the degenerative one. Of note, TdT labeling revealed a positive staining in a large portion of these cells, suggesting that these cells, after a partial differentiation, were undertaking apoptosis (Fig 9, see page 3350). Analysis of a large number of BM elements allowed to see nuclear fragmentation in a few cells. However, the amount of terminally differentiated elements, namely polynucleated granulocytes did not increase with As2O3 treatment. In addition, decreased PML-RARα proteins in nuclear matrix could also be seen in in vivo BM cells after As2O3 treatment (Fig 5F ).
In vivo BM cell morphologic changes in an APL patient during continuous As2O3 treatment. (A) Promyelocytic leukemic cells before the treatment; (B) myelocyte-like cells on day 21 of treatment; (C and D) “nude nuclear” cells with coarse (C) or condensed (D) nuclei on day 28 of the treatment. Similar changes also appeared in peripheral blood (not shown).
In vivo BM cell morphologic changes in an APL patient during continuous As2O3 treatment. (A) Promyelocytic leukemic cells before the treatment; (B) myelocyte-like cells on day 21 of treatment; (C and D) “nude nuclear” cells with coarse (C) or condensed (D) nuclei on day 28 of the treatment. Similar changes also appeared in peripheral blood (not shown).
Dynamic changes of the percentages of promyelocyte, myelocyte-like cells, metamyelocyte and degenerative cells among BM nucleated cells during As2O3 treatment in two APL patients. Similar results were also seen in other five patients investigated.
Dynamic changes of the percentages of promyelocyte, myelocyte-like cells, metamyelocyte and degenerative cells among BM nucleated cells during As2O3 treatment in two APL patients. Similar results were also seen in other five patients investigated.
In situ TdT labeling of BM smear from an APL patient. (A) Before the treatment; (B) on day 24 of As2O3 treatment. Similar results were seen in another patient.
In situ TdT labeling of BM smear from an APL patient. (A) Before the treatment; (B) on day 24 of As2O3 treatment. Similar results were seen in another patient.
DISCUSSION
The clinical trials of As2O3 in APL showed that this treatment is quite different from the conventional cytotoxic chemotherapy but has some similarities to ATRA treatment, in that it did not cause BM depression and did not exacerbate APL-related bleeding diathesis14 (and accompanying paper). In our preliminary study using the APL-cell line NB4 as a model, we suggested that induction of apoptosis may be one of the mechanisms for As2O3 to treat APL.16 The concentrations of the drug used in that in vitro study were 0.5 to 2 μmol/L, within the known plasma concentrations present in APL patients according to pharmacokinetic data. In the present work, we confirmed this in primary APL cells by using morphological and biochemical parameters (histogramic related nuclear DNA contents on flow cytometry and in situ TdT labeling reaction) after in vitro treatment of As2O3 at the same concentrations. In addition, in an ATRA-resistant subclone (MR2) derived from NB4-cell line, As2O3 was able to trigger cell apoptosis with the similar effectiveness as that in NB4 cells. However, some clinical data suggest that other mechanisms could also be involved in the induction of clinical remission. Importantly, during the third and the fourth weeks of in vivo As2O3 treatment, a large number of myelocyte-like cells carrying t(15; 17) could be seen in BM and in peripheral blood when abnormal promyelocytic cells decreased. In one third of the patients, there was a hyperleucocytosis mainly composed of the myelocyte-like elements (see accompanying paper). These results suggest that As2O3 could trigger the differentiation of leukemic promyelocytes.
Indeed, it was found in this work that low-dose (0.1 to 0.5 μmol/L) As2O3 could induce, to some extent, the differentiation of APL cells. In vitro studies indicated that primary APL cells and NB4 cells could be induced to present morphological features resembling those of myelocytes and metamyelocyte after relatively long-time exposure to low concentration of As2O3 . In concordance with this, low concentration of As2O3 could also modulate the expression of differentiation antigens CD11b and CD33 as well as some enzymes (myeloperoxidase, specific esterase) associated with APL-cell differentiation. In parallel to these changes was the increased ability of cell adhesion.
However, it is important to point out that the differentiation induced with As2O3 is not a typical one. On the one hand, the maturation seemed seldomly beyond the metamyelocyte stage and most cells were blocked at the myelocyte-like stage. On the other hand, these incompletely differentiated cells were functionally deficient since they were unable to cause NBT reduction and the extent of modulation of cell surface differentiation antigen was lower compared to that induced by ATRA. An important question can be raised: what is the outcome of the partially differentiated APL cells?
Interestingly, when the myelogram performed during the As2O3 treatment showed a large amount of myelocyte-like elements, the BM also contained a high percentage of “nude nuclear” cells. It is possible that an in situ cytolysis exists to eliminate the aberrantly differentiated myelocyte-like cells. In fact, in situ TdT labeling showed that these cells were experiencing a process of DNA fragmentation characteristic of the apoptosis.
Taking as a whole the in vitro and in vivo findings in the present work, we suggest that As2O3 has dose-dependent dual effects on APL cells: triggering cell apoptosis at high concentration and inducing partial differentiation at low concentration. It is possible that in vivo arsenic administration causes both apoptosis and a partial differentiation of APL cells. Leukemia cells partially differentiated during the first 2 to 3 weeks of treatment then enter into cell death. A combination of these two effects of As2O3 might confer to its clinical effectiveness in the treatment of APL patients. The next question is: by which pathway(s) does As2O3 produce these effects?
Previous studies have reported that overexpression of PML-RARα can not only block U937 cell differentiation induced by vitamin D3 and transformed growth factor-β (TGF-β), but can also inhibit cell apoptosis triggered by growth factor withdrawal.23 Recently, ATRA has been shown to be able to induced the degradation of PML-RARα, probably involving the proteosome system.24 These findings provide us with an important clue that PML-RARα protein may contribute to the selective effect of As2O3 on APL. As demonstrated in the present and previous work16 (and Zhu J, et al, manuscript submitted), As2O3 significantly decreases the immunofluorescence staining of PML-RARα as well as the wild-type PML proteins in NB4 cells and fresh APL cells. After a transient restoration of PODs, PML staining became aggregated and then declined. This effect is different from that observed with ATRA treatment since ATRA restores the subcellular localization of PML but does not cause its disappearance.18 Western analysis showed that As2O3 could rapidly degradate the chimeric protein. Besides, As2O3 , but not ATRA, could modulate PML-RARα/PML in MR2 subclone, suggesting that As2O3 must function through different mechanisms from ATRA. Therefore, we suggest the following senario: the transient restoration of PODs and the subsequent degradation of PML-RARα protein induced by As2O3 can release the block of differentiation, allowing the leukemic promyelocytes to make a few steps of differentiation, to become morpholocially myelocyte-like elements. Since this differentiation process could be coupled with cell division, it may explain the hyperleucocytosis observed in some patients during the remission induction with As2O3 . However, this differentiation is different from that triggered by ATRA because no activation of wild-type retinoic acid receptors is provided. Therefore, leukemic cells, after a temporary and partial restoration of differentiation, tend to enter into apoptosis. Meanwhile, the withdrawal of the inhibition of apoptosis through degradation of PML-RARα may also lead to the release of the cell suicide program in APL cells and directly induces leukemic promyelocytes to die.
Various human genes and proteins can regulate or contribute to cell apoptotic and differentiation processes.25-37 It has been known that As2O3 can effectively downregulate apoptosis inhibitor bcl-2 in NB4 cells at both mRNA and protein levels.16 This could also contribute to As2O3 -induced fresh APL cell apoptosis. Nevertheless, whether As2O3 regulates bcl-2 expression directly or through other molecules such as PML-RARα protein is still unknown. In addition, it is well known that As2O3 can modulate some enzyme activity by combination with -SH group of amino acid.38 Therefore, it is conceivable that As2O3 -induced apoptosis may be associated with other important molecules, such as interleukin-1β–converting enzyme-like cysteine protein enzymes, which are necessary for cell apoptosis induced by many signals and has been considered as one of the death effector molecules.39 40
In conclusion, As2O3 can be used effectively to treat APL through induction of apoptosis and partial differentiation. The precise cellular and molecular mechanisms of its therapeutic effects remain to be illustrated.
NOTE ADDED IN PROOF
The data by Zhu J, et al listed as submitted is now in press (Zhu J, Koken M, Quignon F, Chelbi-Alix M, Degos L, Wang ZY, Chen Z, de The H: Proc Natl Acad Sci USA 1997).
ACKNOWLEDGMENT
We thank Dr M. Lanotte for providing the NB4 cell line. We also thank Prof L. Degos, Saint-Louis Hospital (Paris, France), and Prof R. Ohno, Hamamatsu University School of Medicine (Hamamatsu, Japan) for constructive discussion.
Partly supported by National Natural Science Foundation of China (NSCF , Beijing), National Ministry of Public Health (Beijing), Shanghai Municipal Foundation for Outstanding Young Researcher, Clyde Wu Foundation of Shanghai Institute of Hematology (Shanghai), Samuel Waxman Cancer Research Foundation (New York), and la Fondation de la France contre Leucemie (Paris).
G.-Q.C. and X.-G.S. should be considered as equal first authors.
Address reprint requests to Zhu Chen, MD, PhD, Shanghai Institute of Hematology, Ruijin Hospital, Shanghai Second Medical University, 197 Ruijin Road II, Shanghai, 200025, China.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal