Abstract
The class III receptor tyrosine kinase FLT3/FLK2 (FLT3; CD135) represents an important molecule involved in early steps of hematopoiesis. Here we compare cell-surface expression of FLT3 on bone marrow (BM) and cord blood (CB) cells using monoclonal antibodies (MoAbs) specific for the extracellular domain of human FLT3. Flow cytometric analysis of MACS-purified BM and CB cells showed that 63% to 82% of BM CD34+ and 88% to 95% of the CB CD34+ cells coexpress FLT3. Clonogenic assays and morphological characterization of FACS-sorted BM CD34+ cells demonstrate that colony-forming unit–granulocyte-macrophage (CFU-GM) and immature myelo-monocytic precursor cells are enriched in the subpopulation staining most brightly with the FLT3 MoAb whereas the majority of the burst-forming units-erythroid (BTU-E) and small cells with lymphoid morphology are found in the FLT3− population. In contrast, statistically indistinguishable proportions of CFU-granulocyte-erythrocyte-megakaryocyte-macrophage (CFU-GEMM) and more primitive cobblestone area forming cells (CAFC) were detected in both fractions, albeit the FLT3+ fraction consistently showed more CAFC activity than the FLT3− fraction. Although in both, BM and CB the majority of CD34+CD117+ (KIT+), CD34+CD90+ (Thy-1+), and CD34+CD109+ cells coexpress FLT3, three-color phenotypic analyses are consistent with the functional findings and suggest that the most primitive cells defined as CD34+CD38−, CD34+CD71low, CD34+HLA-DR−, CD34+CD117low, CD34+CD90+, and CD34+CD109+ express low levels of cell-surface FLT3 and were therefore not enriched to a statistically significant extent with the bright versus negative sorting scheme. Thus, clear segregation of the most primitive progenitors from BM CD34+ cells was confounded by low apparent levels of FLT3 cell-surface expression on these cells, whereas myeloid progenitors unambiguously segregated with the FLT3 brightest cells and erythroid progenitors with the FLT3 dimmest. Additional phenotypic analyses using MoAbs against progenitor/stem cell markers including the mucinlike molecule MGC-24v (CD164), the receptor tyrosine kinases TIE, FMS (CD115), and KIT (CD117) further illustrate the differences in surface antigen expression profiles of BM and CB CD34+ cells. Notably, CD115 is rarely detected on CB CD34+ cells, whereas 20% to 25% of the BM CD34+FLT3+ cells are CD115+. Furthermore, 80% to 95% of the CB CD34+CD117+ but only 60% to 75% of the BM CD34+CD117+ cells coexpress FLT3. Only a negligible amount of CD34+CD19+ are detected in CB, while in BM 20% to 30% of CD34+CD19+ presumed pro/pre-B cells coexpress FLT3. In contrast, the majority of the CD34+CD164+ and CD34+TIE+ subsets in both CB and BM coexpress FLT3. Analysis of unseparated cells showed that FLT3 expression is not restricted to CD34+ subsets. About 65% to 70% of lymphocyte-gated BM CD34−FLT3+ cells are positive for the monocytic marker CD115 whereas 25% to 30% of these cells consist of CD10 expressing B-cell precursors. Finally, CD34− monocytes in BM, CB, and PB express FLT3 whereas granulocytes are FLT3−. Our data show that detectable FLT3 appears first at low levels on the surface of primitive multilineage progenitor cells and disappears during defined stages of B-cell development, but is upregulated and maintained during monocytic maturation. © 1997 by The American Society of Hematology.
THE ANTIGENIC profile of hematopoietic stem cells and/or their progeny has been extensively studied using monoclonal antibodies (MoAbs) against surface antigens selectively expressed on primitive cells. Antibodies against the heavily glycosylated transmembrane protein, CD34, are of particular interest because its expression is restricted to the stem/progenitor cell compartment.1-4 Thus, bone marrow (BM) cells that express CD34 contain multipotential cells with long-term engraftment capacity.5
In vitro functional activities within the CD34+ population are heterogeneous containing the spectrum from relatively mature colony-forming units (CFUs) to the most primitive cells detectable in vitro, cobblestone area forming cells (CAFC)/long-term culture initiating cells (LTC-IC). In the mouse, the latter activity has been highly correlated with cells contributing to both short-term and durable multilineage engraftment.6-8 To further define phenotypically the CD34+ population enriched for primitive stem cells, additional antibodies have been raised. Antibodies to several receptor tyrosine kinases (RTK) have proven to be suitable to discriminate functionally and phenotypically distinct progenitor/stem cell subsets.9-14 The class III RTK15 CD115/FMS/M-CSF receptor (CD115),9 CD117/KIT/stem cell factor receptor (CD117),10,11 and FLT3/FLK2 (FLT3),12-14 recently clustered as CD135,16 are known to play important roles in the regulation of hematopoiesis. Several studies have shown that both CD117 and FLT3 are expressed on the surface of CD34+ subsets.10-13 Additionally, CD117, but not FLT3 expression, is found on CD34−, immature erythroid cells,17,18 whereas normal and leukemic B-lymphoblastic precursor cells preferentially express FLT3.12-14 In contrast, CD115 expression is apparently restricted to cells showing monocyte-macrophage development potential.9
Recently, expression of the genes encoding the related RTK TIE/TIE-1 (TIE) and TEK/TIE-2 (TEK)19,20 has been described not only in endothelial cells but also in a subset of primitive hematopoietic cells. Moreover, TIE cell-surface expression has been preferentially detected on CD34+ cells with a primitive phenotype.21 22
CD90 (Thy-1) and CD109 belong to a group of glycophosphatidylinositol (GPI)-linked molecules and appear to be selectively expressed on the most immature subpopulation of CD34+ cells.23,24 Craig et al23 have shown that the CD34+CD90+ fraction is highly enriched in LTC-IC. Additionally, Baum et al25 have shown that these cells capable of multilineage repopulation of human fetal bones engrafted into severe combined immunodeficient (SCID) mice. Similarly, LTC-IC enrichment has also been reported in the CD34+CD109+ subset.24 26
Several reports have demonstrated that MoAbs with specificities for the recently clustered variant form of “multi-glycosylated core protein of 24 kD” (MGC-24v; CD164),27 a mucinlike molecule originally identified in a gastric carcinoma cell line,28 not only recognize solid tumors from various origins27 but also erythroid precursor cells and the majority of CD34+ BM cells.27,29-32 Further studies have shown that the highest levels of CD164 expression on CD34+ cells is found in the CD34+CD38− BM subset, suggesting that this molecule plays a crucial role in primitive progenitor cells.30 32
Murine flt3 mRNA expression has been detected in hematopoietic and nervous systems, the gonads, and the placenta.33 In addition, FLT3 cell-surface expression has been described on cycling murine progenitor cells from fetal liver and BM cells.34 Human FLT3 receptor expression has been detected on leukemic blasts,12,35 leukemic cell lines,12,35,36 and on BM cells from normal donors.12,37 Because of the low FLT3 receptor density on normal human hematopoietic cells,37 little information exists about the precise phenotype of the FLT3+ populations. In this study we analyze the coexpression of candidate stem/progenitor as well as lineage-restricted antigens on BM and cord blood (CB) CD34+FLT3± and CD34−FLT3+ cells, and describe the functional and morphological differences of FLT3+ and FLT3− BM CD34+ cells.
MATERIALS AND METHODS
Cells
BM samples from healthy donors and CB samples from normal full-term neonates were obtained after informed consent according to the guidelines of our local ethics committee. Buffy coat peripheral blood (PB) cells were obtained from the Blood Transfusion Department. Mononuclear cells were separated on a Ficoll-Hypaque density gradient (1.077 g/mL) (Biochrome KG, Berlin, Germany).
Selection of CD34+ Cells by MACS
CD34+ cells from BM and CB were purified on magnetic activated cell sorting (MACS) columns using microbead-conjugated antibodies. Separation was performed according to the manufacturer's recommendations (MiniMACS; Miltenyi Biotec, Bergisch-Gladbach, Germany).
Immunization and Hybridoma Production
MoAb 4G8 (anti-FLT3) was raised by immunization of 4- to 8-week-old female Balb/c mice with Ba/F3 cells transfected with the complete coding sequence of the human FLT3/FLK2 cDNA.38 The mice were injected five times intraperitoneally with 107 cells in 2-week intervals, and 10 days after the last injection an intrasplenic boost of 2 × 105 cells was applied. The spleens were removed 4 days later for fusion with the SP2/0 myeloma cell line. The resulting hybridomas were grown in RPMI 1640 (GIBCO-BRL, Eggenstein, Germany) containing 10% fetal calf serum and hypoxanthine-aminopterine-thymidine (HAT; Sigma Chemicals, München, Germany). Culture supernatants were screened on the cells used for immunization, and hybridoma cells secreting antibodies selectively recognizing the transfectant cell line but not parental Ba/F3 cells were cloned twice by limiting dilution. The clone was cultured on a large scale in serum-free medium supplemented with 1% Nutridoma (Boehringer Mannheim, Mannheim, Germany), and antibody was purified from supernatant using T-Gel affinity columns (Bender & Hobein, Munich, Germany) as described by the manufacturer. The IgG1 isotype of the resulting MoAb 4G8 was determined by ELISA (Boehringer Mannheim). MoAb 4G8 was recently assigned to the CD135 (FLT3) cluster during the VIth International Workshop and Conference on Human Differentiation Antigens (HLDA) in Kobe, Japan.16
MGC-24v (CD164)-specific MoAb N6B6 (IgG2a) was raised by immunization of mice with the pre-B cell line NALM-1 which was obtained by the German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany). The specificity was determined by the selective recognition of FDC-P1 cells transfected with the coding sequence of the human MGC-24v gene.30,31 These cells were used to establish the CD164 cluster during the VIth International HLDA conference.27 The generation of MoAb N6B6 is described in more detail by Watt et al.32
Biotinylation of Anti-FLT3 MoAb SF1.340
Purified FLT3-specific antibody SF1.34012 was dialyzed against 0.1 mmol/L sodium borate buffer, pH 9.3, and concentrated to about 1 mg/mL by ultrafiltration (Ultrafree PF-filter, 10.000 NMWL; Millipore, Eschborn, Germany). Antibody concentration was estimated by Coomassie protein assay reagent (Bender & Hobein, Munich, Germany). A freshly prepared solution of 1 mg/mL ε-amino caproic acid N-hydroxy succinimide biotin (Biotin-X-NHS; Calbiochem, Heidelberg, Germany) was added to the antibody at a molar ratio of 30:1 and incubated for 2.5 hours at room temperature. The reaction was stopped by thorough dialysis against Hanks' balanced salt solution (HBSS). The optimal concentration of the biotinylated antibody for flow cytometric analysis was evaluated by incubating FLT3 transfected Ba/F3 cells with serial dilutions of the conjugate and staining with streptavidin-phycoerythrin (SA-PE).
Fluorescein Isothiocyanate (FITC)-Conjugation of FLT3-Specific MoAb 4G8
Purified antibody was dialyzed against 0.1 mol/L sodium carbonate, 0.1 mol/L sodium chloride, pH 9.2, and concentrated to 1 mg/mL. An FITC-celite solution (Sigma, Munich, Germany) of 100 μg/mL (10 μL) dimethylsulfoxide (DMSO) was prepared and slowly added to the antibody at a molar ratio of 40:1. After 30 minutes of incubation the reaction was stopped by pelleting the FITC-celite and conjugated antibody was separated by gelfiltration using Sephadex G-25 (Pharmacia, Freiburg, Germany). Activity, specificity, and fluorescence intensity of 4G8-FITC was estimated by comparative staining of FLT3 transfected and nontransfected Ba/F3 control cells with serial dilutions of the conjugate.
Immunoprecipitation and Western Blot Analysis
Proteins were extracted from Ba/F3 cells transfected with the complete coding sequence of the human FLT3 cDNA.38 Samples were solubilized by 1.5% NP40-TSE-buffer (50 mmol/L TrisHCl, 150 mmol/L NaCl, 1 mmol/L EDTA, pH 8) containing proteinase inhibitors (2 mmol/L phenylmethyl sulfonyl fluoride [PMSF], 20 μg/mL leupeptin, 20 μg/mL aprotinin). Fifty microliters of coated goat-antimouse IgG-Sepharose (Sigma Chemicals) was preincubated for 9 hours on ice with 400 μL hybridoma culture supernatant of MoAb SF1.340, MoAb 4G8, or IgG1 control MoAb, respectively. Antibody-Sepharose complexes were washed twice with TSE and equal amounts of cleared cell lysate were added. After incubation on ice for 3 hours immunocomplexes were washed twice each with 1.5% NP40-TSE, 0.15% NP40-TSE and TSE. Bound proteins were eluted with 2× reducing Laemmli sample buffer, boiled for 3 minutes, and separated by 7.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Electrophoresed proteins were transferred to nitrocellulose, and the membranes were blocked with 3% nonfat milk-TBS-T (10 mmol/L TrisHCl pH 7.5, 100 mmol/L NaCl, 0.1% Tween). A rabbit polyclonal IgG raised against a peptide corresponding to the carboxy terminus of human FLT3 (STK1; Santa Cruz Biotech, IC, Ismaning, Germany) was used as primary antibody (1 μg/mL in blocking buffer), and after thorough washing with TBS-T the filters were incubated with horseradish peroxidase-conjugated secondary antibody (Amersham, Braunschweig, Germany) and detected by ECL (Amersham).
Immunofluorescence Staining and Flow Cytometric Analysis
Two-color staining.Unseparated or MACS-purified CD34+ BM, CB, or PB cells were labeled with HPCA-2-FITC (CD34; Becton Dickinson, Heidelberg, Germany) and SF1.340-biotin (FLT3) for 30 minutes on ice. After washing, cells were stained with streptavidin-PE (SA-PE) for 15 minutes and washed twice before FACS analysis or sorting.
Three-color staining.Ficoll-separated BM and CB cells were incubated in the first step with anti–CD34-PerCP (HPCA-2-PerCP; Becton Dickinson), the FLT3-specific MoAb SF1.340-biotin, and FITC-conjugated antibodies against CD10, CD14, CD15, CD19, CD71, HLA-DR (all from Becton Dickinson), CD33 (Immunotech, Krefeld, Germany), CD117 (Hölzel Diag, Cologne, Germany), or the nonconjugated CD115-specific rat MoAb AB-2 (Calbiochem, Bad Soden, Germany), respectively. In the second step cells were incubated with SA-PE and PE-conjugated rabbit-antirat IgG (Ade, Munich, Germany) to stain SF1.340-biotin and AB-2, respectively. For data acquisition 150,000 to 250,000 events were analyzed and stored on a FACSCalibur flow cytometer (Becton Dickinson) using the Cellquest software (Becton Dickinson).
MACS-purified CD34+ BM or CB cells were incubated in the first step for 30 minutes at 4°C with anti–CD34-PerCP (IgG1), 4G8-FITC (anti-FLT3; IgG1), and the indicated PE-conjugates, or the CD109-specific IgM antibody 59D6, the CD164-specific IgG2a antibody N6B6, or the rat-antihuman CD115-specific antibody AB-2, respectively. The PE-conjugates used in these experiments were anti–CD38-PE, anti–HLA-DR-PE, anti–CD19-PE (Becton Dickinson), 104D2-PE (anti-CD117; conjugated by Dr K. Davis, Becton Dickinson Immunocytometry Systems, San Jose, CA), anti–CD71-PE (Boehringer Ingelheim, Ingelheim, Germany), anti–CD90-PE (PharMingen, Hamburg, Germany), and a pool of PE-conjugated anti-TIE MoAbs 3C4, 7E8, 8G3, and 10F11.21 The CD115-specific rat antibody AB-2 (Calbiochem, Bad Soden, Germany) was stained with PE-conjugated rabbit-antirat IgG and the CD109-specific mouse IgM antibody with PE-conjugated rat-antimouse IgM. To enhance sensitivity the IgM antibody 59D6 was stained in a third step with PE-conjugated rabbit-antirat antibody. In all cases appropriate background staining was done with the corresponding control antibodies and conjugates (mouse IgG1, IgG2a, IgM control antibodies and goat-antimouse IgG2a-PE were from Dunn, Asbach, Germany; rat IgG2a control MoAb from Biermann, Bad Nauheim, Germany; rabbit-antimouse IgM-PE, rabbit-antirat IgG-PE, and rat-antirabbit IgG-PE from Ade, München, Germany). For data acquisition a FACSCalibur flow cytometer (Becton Dickinson) was used. Ten thousand to 30,000 events were stored and analyzed using the Cellquest software.
Cell Sorting
Cell sorting was performed on a FACSVantage (Becton Dickinson) equipped with an air-cooled argon laser. FITC and PE fluorochromes were excited at 488 nm, and emission of FITC was detected through a 530-nm band pass filter, PE through a 570-nm band pass filter. Instrument alignment, and compensation of FITC versus PE signals was accomplished using a mixture of Calibrite beads (Becton Dickinson). For morphological analysis and colony assays, cells stained with anti-CD34 and anti-FLT3 were sorted into tubes containing 200 μL of phosphate-buffered saline (PBS)/40% fetal bovine serum (FBS). Sort windows were set as shown in Figs 5 and 6, respectively. For morphological analysis cells were cytocentrifuged onto glass slides and stained with May-Grünwald-Giemsa.
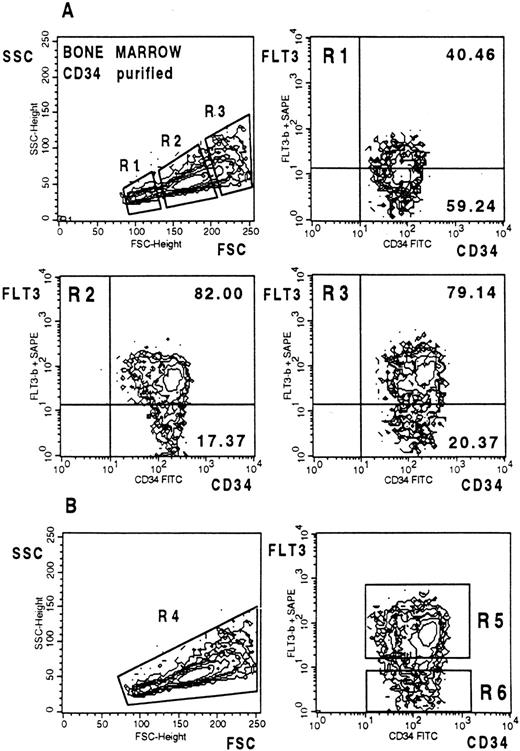
(A) FLT3 expression on MACS-selected CD34+ BM cells. Three gates with different light scatter characteristics (R1-R3) were chosen to analyze FLT3 expression on differently sized cells. Cells were stained and analyzed as described in Fig 2. (B) After dual scatter gate setting on purified CD34+ cells (bottom left) the two indicated sort windows were used to select CD34+FLT3+ and CD34+FLT3− cells for CFC and CAFC assays (bottom right).
(A) FLT3 expression on MACS-selected CD34+ BM cells. Three gates with different light scatter characteristics (R1-R3) were chosen to analyze FLT3 expression on differently sized cells. Cells were stained and analyzed as described in Fig 2. (B) After dual scatter gate setting on purified CD34+ cells (bottom left) the two indicated sort windows were used to select CD34+FLT3+ and CD34+FLT3− cells for CFC and CAFC assays (bottom right).
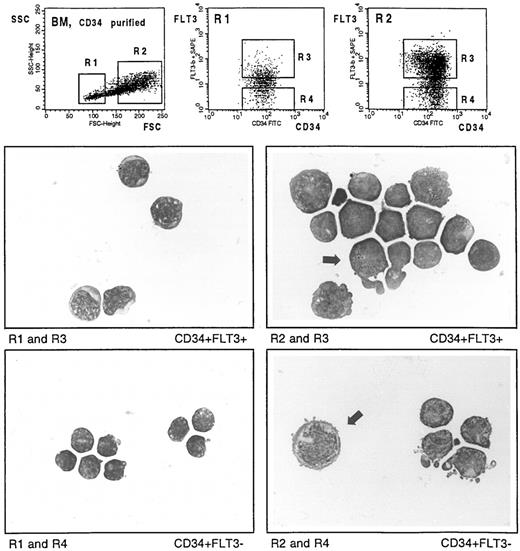
May-Grünwald–stained cytocentrifuge preparations of CD34-selected BM cells labeled with CD34-FITC and the biotinylated FLT3 specific MoAb SF1.340. Cells were fractionated according to the indicated sort windows. Arrows show a FLT3+ cell with promyelocyte-specific azurophile granulation (middle right), and a large FLT3− cell with proerythroblast-like morphology (bottom right), respectively.
May-Grünwald–stained cytocentrifuge preparations of CD34-selected BM cells labeled with CD34-FITC and the biotinylated FLT3 specific MoAb SF1.340. Cells were fractionated according to the indicated sort windows. Arrows show a FLT3+ cell with promyelocyte-specific azurophile granulation (middle right), and a large FLT3− cell with proerythroblast-like morphology (bottom right), respectively.
To determine the frequency of CAFC (for details, see below), defined cell numbers were sorted into 96-well microtiter plates using the automatic cell deposition unit (ACDU). The accuracy of cell deposition was determined by microscopic evaluation of fluorescent beads and cells used for test purposes.
Colony Assays
To determine the number of erythroid (BFU-E), myeloid (CFU-GM), and multipotent (CFU-GEMM) progenitors 2,000 sorted cells were plated in 35-mm tissue culture dishes (Greiner, Nürtingen, Germany) containing 1 mL of methylcellulose-based semisolid culture medium (MethoCult SFBIT H4436; Cell Systems, Remagen, Germany). Each test was performed in duplicate. After 14 days of incubation at 37°C, 5% CO2, the CFU-GM, BFU-E, and CFU-GEMM colonies were enumerated using an inverted microscope. Results are expressed as mean values ± SD from three experiments. Statistical significance was determined using the Student's t-test.
CAFC Assays
CAFC assays were performed as described by Breems et al42 using the murine FBMD-1 stromal cell line. Briefly, confluent stromal layers cultured in 96-well plates in long-term culture medium (MyeloCult H5100; Cell Systems, Remagen, Germany) with freshly added hydrocortisone sodium hemisuccinate (final concentration 10−6 mol/L; Sigma, Munich, Germany) were overlaid with sorted BM CD34+FLT3+, CD34+FLT3−, and unfractionated CD34+ cells, respectively. Serial dilutions of ten replicates ranging from 10 to 320 cells per well were used each. After 5 weeks of coculture at 33°C, 10% CO2 with weekly medium changes, the CAFC frequencies were estimated by limiting dilution analysis. All wells with at least one phase-dark cobblestone area localized under the stromal layer were scored. CAFC frequencies were determined by Poisson statistics and Probit analysis was performed for comparison of groups.43 The recovery was determined as follows: [(CAFC frequency of CD34+ subset × % of sorted CD34+ subset in gate): (CAFC frequency of total CD34+ cells × % of total sorted CD34+ cells)] × 100.
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) Analysis of flt-3 Expression on Sorted CB CD14+ and CD3+ Cells
CB cells stained with anti–CD14-FITC and anti–CD3-PE (Becton Dickinson) were sorted into CD14+ and CD3+ fractions with a FACSVantage cell sorter (Becton Dickinson). RT-PCR analysis was performed essentially as described previously.44 Briefly, 500 CB CD14+ or CD3+ cells were sorted into 100 μL of lysis buffer (4 mol/L guanidine isothiocyanate, 25 mmol/L sodium citrate, pH 5.0, 0.5% sodium lauroyl sarconisate [wt/vol], 100 mmol/L β-mercaptoethanol) containing 20 μg Escherichia coli rRNA (Boehringer, Mannheim, Germany) as carrier, and layered on top of a 100-μL 5.7 mol/L CsCl cushion in RNAse-free 0.3 mL polyallomer tubes. After centrifugation for 6 hours at 45,000 rpm in a TST 60.4 swinging bucket rotor (Du Pont, Bad Homburg, Germany) the total RNA pellet was processed first for DNAse I digestion45 and then for cDNA analysis. Total RNA was dissolved in 10 μL RT buffer (50 mmol/L Tris/HCl, pH 8.3, 75 mmol/L KCl, 3 mmol/L MgCl2) containing 100 U Moloney murine leukemia virus (MMLV) reverse transcriptase (Superscript BRL/LTI; Eggenstein, Germany), 0.2 μg oligo(dT), 15 U RNasin, 10 mmol/L dithiothreitol (DTT), and 0.5 mmol/L of each dNTP and then reverse transcribed for 1 hour at 42°C. For RT-PCR analysis, cDNA from each sample was divided into two aliquots. One aliquot was amplified by an intron-spanning primer pair for the β2-microglobulin gene45 and the other for amplification of the flt-3 cDNA. flt-3 primers recognized positions 2598-2617 and 3003-2983 of the flt-3 cDNA. Each 50-μL PCR reaction contained 2 U AmpliTaq DNA polymerase (Perkin Elmer Cetus, Überlingen, Germany), 200 μmol/L of each dNTP, and 0.5 μmol/L MgCl2, 0.001% gelatin. Reactions were amplified in a DNA thermal cycler (PCR 9600 system; Perkin Elmer Cetus) for 40 cycles using predetermined optimal cycling parameters for each primer pair. The RT-PCR products were electrophoresed through a 2% SEAKEM (FMC BioProducts, BIOzym, Oldendorf, Germany) agarose gel and visualized using ethidium bromide staining.
RESULTS
MoAb 4G8 and SF1.340 Recognize FLT3
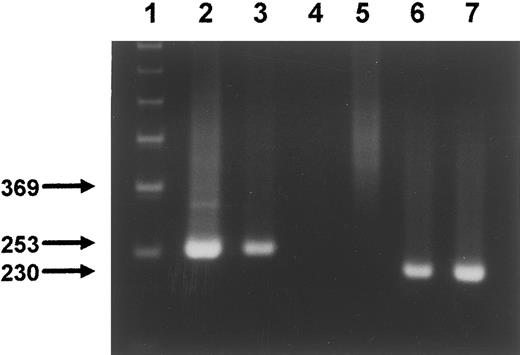
The specificity of MoAb SF1.340 and MoAb 4G8 against the extracellular domain of the FLT3 receptor tyrosine kinase was determined by their specific reactivity with Ba/F3 cells transfected with the complete coding sequence of the human FLT3 cDNA (4G1).38 Both MoAbs reacted strongly with the transfected cells but were negative on the parental Ba/F3 cells (not shown). In addition, immunoprecipitation of 4G1 lysates with MoAbs SF1.340 and 4G8 followed by Western blot analysis with a polyclonal anti-FLT3/STK-1 antiserum showed that both MoAbs are able to immunoprecipitate FLT3 from transfected Ba/F3 cells (Fig 1, lanes 2 and 3). Two bands of 155 kD (mature N-glycosylated form expressed on the cell surface) and 130 kD (immature high mannose glycosylated form) appeared which correspond to published molecular weights of FLT3.12 No bands appeared after blotting the sample obtained with the IgG1 control antibody (lane 1) whereas two faint bands appeared after immunoblotting of the whole lysate (lane 4). The specificity of both MoAbs was also confirmed during the VIth International Workshop and Conference on Human Leukocyte Differentiation Antigens in Kobe, Japan (November 1996) and assigned to the CD135 cluster.16
Immunoprecipitation of FLT3 and Western blotting. Immunoprecipitation of FLT3 from transfected Ba/F3 cells either with MoAb SF1.340 (lane 2), MoAb 4G8 (lane 3) or a control MoAb against human IgG1 (lane 1). Immunoprecipitated lysate or whole cell lysate (lane 4) were electrophoresed on SDS-PAGE 7.5%, blotted with a rabbit polyclonal IgG raised against a peptide corresponding to the carboxy terminus of human FLT3, and visualized by ECL.
Immunoprecipitation of FLT3 and Western blotting. Immunoprecipitation of FLT3 from transfected Ba/F3 cells either with MoAb SF1.340 (lane 2), MoAb 4G8 (lane 3) or a control MoAb against human IgG1 (lane 1). Immunoprecipitated lysate or whole cell lysate (lane 4) were electrophoresed on SDS-PAGE 7.5%, blotted with a rabbit polyclonal IgG raised against a peptide corresponding to the carboxy terminus of human FLT3, and visualized by ECL.
Differential Expression of FLT3 on BM, CB, and PB Cells
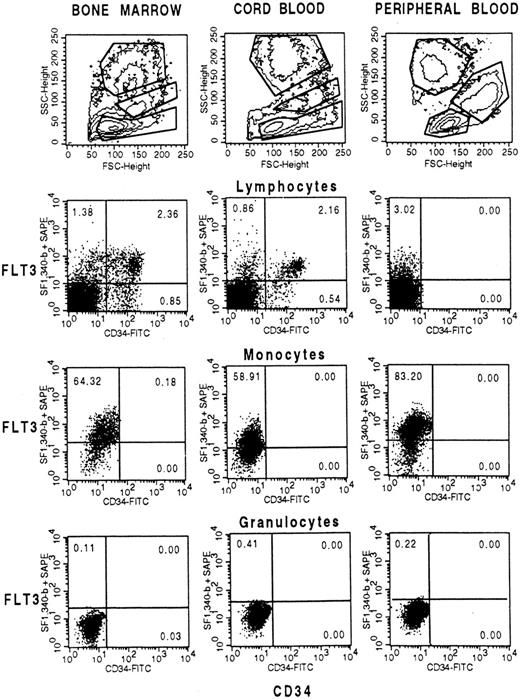
In a first experiment Ficoll-separated BM, CB, and PB cells were double-stained with anti-CD34 and anti-FLT3 antibodies and analyzed by flow cytometry. Cells were either gated on low, intermediate, or high side scatter populations which correspond to lymphocytes, monocytes, or granulocytes, respectively (Fig 2, top row). Because Ficoll-separated PB cells are depleted of granulocytes, a 0.5:1 ratio of erythrocyte-lysed granulocytes were added before staining. Figure 2 (second row) shows that 63% of the BM FLT3+ lymphocytes (range, 45% to 71%; mean, 51%; n = 6) and 71% of the CB FLT3+ cells (range, 41% to 78%; mean, 55%) are positive for CD34. Vice versa, 63% to 82% of the BM CD34+ cells (mean, 74.2%) and 88% to 95% (mean, 92.3%) of the CB CD34+ cells coexpress FLT3. A small percentage of CD34−FLT3+ cells (2% to 3%) was also detected on lymphocyte-gated PB cells. Because FLT3 expression was detected on monocytes from all cell sources (Fig 2, third row) the small FLT3+ population on PB lymphocytes is most likely due to contaminating monocytes not excluded in the dual scatter gate defined in Fig 2 (top right). This is also substantiated by the fact that these cells were CD14+ and FLT3 expression was not found on cells with very low side scatter intensities (data not shown). In contrast to monocytes, granulocytes from all sources were negative for FLT3.
Coexpression of FLT3 and CD34 in BM, CB and PB gated on cells with low side scatter (lymphocytes and progenitor cells), intermediate side scatter (monocytes), or high side scatter (granulocytes). Cells were labeled with anti-CD34-FITC and biotinylated FLT3-specific MoAb SF1.340 and stained with streptavidin-phycoerythrin (SA-PE). A mixture of IgG1-FITC and IgG1-biotin stained with SA-PE was used as control. Fifty thousand cells were analyzed on a FACSCalibur flow cytometer.
Coexpression of FLT3 and CD34 in BM, CB and PB gated on cells with low side scatter (lymphocytes and progenitor cells), intermediate side scatter (monocytes), or high side scatter (granulocytes). Cells were labeled with anti-CD34-FITC and biotinylated FLT3-specific MoAb SF1.340 and stained with streptavidin-phycoerythrin (SA-PE). A mixture of IgG1-FITC and IgG1-biotin stained with SA-PE was used as control. Fifty thousand cells were analyzed on a FACSCalibur flow cytometer.
Flt3 mRNA is Expressed on CB CD14+ Monocytes but not on CD3+ T Cells
Monocytes are known to express high levels of Fc receptors and suboptimal prevention of unspecific antibody binding easily results in an apparent unspecific detection of low levels of surface antigen expression. To address this question flt3 mRNA expression on purified monocytes was analyzed by RT-PCR. CB cells double-stained with anti-CD14 and anti-CD3 were separated by FACS and 500 cells of the resulting populations were used for RT-PCR analysis. Figure 3 (lane 2) shows a strong flt3 signal in the pre-B cell line NALM-1 which is known to express cell surface FLT3.12 A weaker, albeit significant, signal is seen in CD14+ monocytes (lane 3). In contrast, CD3+ T cells (lane 4) and erythroid K562 cells (lane 5) were negative for FLT3. These data are consistent with the detection of FLT3 cell-surface expression on monocytes and suggest that antibody binding to these cells was specific.
RT-PCR of sorted CD14+ and CD3+ CB cells. Five hundred sorted cells from each subset were processed for RT-PCR as described in Materials and Methods. The cDNA from each subset was aliquoted and amplified by PCR in separate reactions using primers for FLT3 (lanes 3 and 4) and β2-microglobulin (lanes 6 and 7), respectively. Lane 1, molecular-weight marker. Lane 2, FLT3 RT-PCR product from NALM-1 cells (positive control). Lane 3, cDNA corresponding to CD14+ monocytes. Lane 4, CD3+ T cells. Lane 5, FLT3 RT-PCR of K562 cells (negative control). Lanes 6 and 7, RT-PCR of CD14+ and CD3+ cells, respectively, using primers recognizing two different exons of the β2-microglobulin gene.
RT-PCR of sorted CD14+ and CD3+ CB cells. Five hundred sorted cells from each subset were processed for RT-PCR as described in Materials and Methods. The cDNA from each subset was aliquoted and amplified by PCR in separate reactions using primers for FLT3 (lanes 3 and 4) and β2-microglobulin (lanes 6 and 7), respectively. Lane 1, molecular-weight marker. Lane 2, FLT3 RT-PCR product from NALM-1 cells (positive control). Lane 3, cDNA corresponding to CD14+ monocytes. Lane 4, CD3+ T cells. Lane 5, FLT3 RT-PCR of K562 cells (negative control). Lanes 6 and 7, RT-PCR of CD14+ and CD3+ cells, respectively, using primers recognizing two different exons of the β2-microglobulin gene.
FLT3 Expression on CD34− BM and CB Cells
Figure 2 shows that 1.4% of the lymphocyte-gated BM cells and 0.9% of the CB cells were CD34−FLT3+. To determine the precise phenotype of these populations, Ficoll-separated cells stained with anti-CD34, anti-FLT3, and antibodies against known cell-surface antigens were analyzed by FACS and sequentially gated on the low side scatter population and on FLT3+ cells (Fig 4, top left). Figure 4 shows that about 30% of the BM CD34−FLT3+ cells express CD10. A similar portion was also positive for CD20 (not shown), suggesting that about one third of BM CD34−FLT3+ cells consist of B-cell precursors. The majority of the CD34−FLT3+ cells (about 65%), however, expressed the monocyte-restricted antigen FMS (CD115), and about 50% of the non-B cells were also positive for CD33. In contrast, the granulocyte-specific antigen CD15 and the late-appearing monocyte antigen CD14 were found only in minor fractions (5% to 7% and 12% to 13.5%, respectively), suggesting that about two thirds of the CD34−FLT3+ cells in the lymphocyte gate consist mainly of not fully differentiated monocytic cells. Our data further show that almost none of these cells express KIT (CD117), suggesting that CD117 is concomitantly lost with CD34 during monocytic differentiation (Fig 4, bottom right). In contrast, the majority of the CD34−FLT3+ cells are CD71low and HLA-DR+.
Dot-blot analysis of unseparated BM cells triple-stained with CD34-PerCP, biotinylated FLT3 specific MoAb SF1.340 (plus SA-PE), and the indicated FITC-conjugates. All plots represent cells sequentially gated on low side scatter and the FLT3+ population (R1).
Dot-blot analysis of unseparated BM cells triple-stained with CD34-PerCP, biotinylated FLT3 specific MoAb SF1.340 (plus SA-PE), and the indicated FITC-conjugates. All plots represent cells sequentially gated on low side scatter and the FLT3+ population (R1).
About 29% of lymphocyte gated CB CD34−FLT3+ cells consisted of CD10+ and 20% of CD20+ B-cell precursors, whereas only about 17% expressed CD33. An even smaller population expressed CD13, CD14, and CD115 (>10%). More than 50% of the CD34−FLT3+ cells could not be assigned to any lineage because, except for the nonlineage markers HLA-DR and CD38, they were negative for the T-cell lineage markers CD7 and CD3, for the erythroid marker glycophorin A, and the natural killer (NK) cell marker CD56 (not shown). Therefore, the precise phenotype of this population remains to be identified.
FLT3 Expression on CD34-Selected BM Progenitor Cells
In the next experiment, FLT3 expression on MACS-selected BM CD34+ cells was analyzed. The purity of the CD34+ cells was routinely 95% to 99.9% (mean, 97.5%; two purification cycles on separate MiniMACS columns were performed) and the ratio of MACS-selected versus unselected CD34+FLT3+ and CD34+FLT3− cells remained relatively unchanged. Two-color FACS analysis of anti-CD34 and anti-FLT3 stained column-purified CD34+ cells shows that the selected cells varied considerably in size (Fig 5A, top left). To estimate differential expression of FLT3 on CD34+ progenitor cells that differ in size, gates were set on small, intermediate, and large cells (Fig 5A, top left). About 40% of the small-sized CD34+ cells, which mainly consist of CD19+ pro/pre B cells (unpublished observation), expressed FLT3 at a comparably low level (Fig 5A, top right). In contrast, about 82% of the medium-sized cells (Fig 5A, middle left) and about 79% of the large-sized cells (Fig 5A, middle right) expressed FLT3 at high average levels. Thus, small BM CD34+ cells express lower FLT3 levels than the majority of the medium-sized and large cells.
Clonogenic Capacity of BM CD34+FLT3+ and CD34+FLT3− Progenitor Cells
The colony-forming capacity of column-purified, sorted CD34+FLT3+ and CD34+FLT3− BM cells was assayed in methylcellulose medium containing defined growth factors. After dual-scatter gating (Fig 5B, left) sort windows were set as indicated in Fig 5B (right). Results from three independent experiments performed in duplicate are summarized in Table 1. The CD34+FLT3+ subsets were highly enriched for CFU-GM but contained only a small population of BFU-E. Conversely, the BFU-E were highly enriched in the CD34+FLT3− subpopulation. In 2 of 3 experiments, the CFU-GEMM frequency was similar in both fractions, whereas in one experiment the multipotent progenitors were predominantly found in the FLT3+ fraction (Table 1). Thus, in contrast to KIT, FLT3 is only rarely found on erythroid progenitor cells.
Colony Numbers of Sorted BM CD34+FLT3+ and CD34+FLT3− Cells
| Fraction . | Colonies . | |||
|---|---|---|---|---|
| . | BFU-E . | CFU-GM . | CFU-GEMM . | CFC Recovery (%) . |
| CD34+ | 57.2 ± 8.9 | 60.3 ± 16.1 | 1.6 ± 1.6 | 100 |
| CD34+FLT3− | 109.3 ± 13.5 | 22.6 ± 3.2 | 1.3 ± 0.8 | 21-28 |
| CD34+FLT3+ | 15.0 ± 3.2 | 94.7 ± 9.5 | 4.1 ± 3.8 | 53-63 |
| Fraction . | Colonies . | |||
|---|---|---|---|---|
| . | BFU-E . | CFU-GM . | CFU-GEMM . | CFC Recovery (%) . |
| CD34+ | 57.2 ± 8.9 | 60.3 ± 16.1 | 1.6 ± 1.6 | 100 |
| CD34+FLT3− | 109.3 ± 13.5 | 22.6 ± 3.2 | 1.3 ± 0.8 | 21-28 |
| CD34+FLT3+ | 15.0 ± 3.2 | 94.7 ± 9.5 | 4.1 ± 3.8 | 53-63 |
Colony-forming capacity of MACS-selected CD34+ BM cells stained with anti–CD34-FITC and anti–FLT3-PE and FACS-sorted according to the sort windows shown in Fig 5B (right). Fractionated cells were plated in semisolid methylcellulose cultures as described in Materials and Methods. Cultures were scored for colony growth after 14 days of incubation at 37°C and 5% CO2. Results represent the mean colony numbers ± SD based on 2 × 103 plated cells from three independent experiments with duplicate determinations.
CAFC Frequency in BM CD34+FLT3+ and CD34+FLT3− Progenitor Cell Subsets
The frequency of more primitive progenitor cells was estimated using the murine stromal cell line FBMD-142 as a feeder layer. BM CD34+ cells were preselected as described for the colony assays, and defined cell numbers were FACS-sorted with the ACDU onto confluent FBMD-1 cells in 96-well plates. The CAFC frequency of sorted CD34+FLT3+, CD34+FLT3−, and CD34+ cells was determined. After 5 weeks of coculture, all wells with at least one cobblestone area were enumerated and the CAFC frequency determined by limiting dilution analysis as described.43 Table 2 shows that the highest mean CAFC frequency was found in the CD34+FLT3+ population. The estimated values derived from three independent experiments ranged from 1/40 to 1/110 CD34+FLT3+ cells compared with CD34+ control cells of 1/50 to 1/200. In contrast, the CAFC frequency in the CD34+FLT3− fractions ranged between 1/80 and 1/250. These data show that immature progenitor cells were present in both, the CD34+FLT3+ and CD34+FLT3− fractions. Furthermore, no significant difference in CAFC frequencies could be assigned to any of the fractions since by probit analysis the 90% confidence intervals from μ and σ were overlapping for each experimental group.43 However, in all experiments a higher frequency was determined in the CD34+FLT3+ subpopulation.
CAFC Frequencies of Sorted BM CD34+FLT3+ and CD34+FLT3− Cells
| Fraction . | Experiment 1 . | Experiment 2 . | Experiment 3 . | |||
|---|---|---|---|---|---|---|
| . | Frequency . | Recovery (%) . | Frequency . | Recovery (%) . | Frequency . | Recovery (%) . |
| CD34+ | 1/50 | 100 | 1/200 | 100 | 1/80 | 100 |
| CD34+FLT3− | 1/80 | 15.6 | 1/250 | 17.6 | 1/110 | 19.6 |
| CD34+FLT3+ | 1/40 | 81 | 1/110 | 113 | 1/105 | 45.6 |
| Fraction . | Experiment 1 . | Experiment 2 . | Experiment 3 . | |||
|---|---|---|---|---|---|---|
| . | Frequency . | Recovery (%) . | Frequency . | Recovery (%) . | Frequency . | Recovery (%) . |
| CD34+ | 1/50 | 100 | 1/200 | 100 | 1/80 | 100 |
| CD34+FLT3− | 1/80 | 15.6 | 1/250 | 17.6 | 1/110 | 19.6 |
| CD34+FLT3+ | 1/40 | 81 | 1/110 | 113 | 1/105 | 45.6 |
CAFC frequency of MACS-selected CD34+ BM cells stained with anti–CD34-FITC and anti–FLT3-PE and FACS-sorted according to the sort windows shown in Fig 5B (right). Cells were ACDU sorted into 96-well plates coated with stromal FBMD-1 cells42 as described in Materials and Methods. CAFC frequencies were determined by Poisson statistics and Probit analysis was performed for comparison of groups.43
Morphological Characterization of BM CD34+FLT3+ and CD34+FLT3− Subsets
MACS-selected CD34+BM cells were stained with anti-FLT3 and anti-CD34, and FLT3+ and FLT3− populations size-fractionated by cell sorting (Fig 6, top left). Cytospin preparations of sorted cells were stained with May-Grünwald solution and microscopically examined. Morphological evaluation of 100 cells from the small-sized populations (gate R1, top left) resulted in 95% lymphocytes and 5% lymphoblastoid cells in the CD34+FLT3− (gate R4, top middle) and in 48% lymphocytes and 51% lymphoblastoid cells in the CD34+FLT3+ fraction (gate R3; top middle). Figure 6 (middle left) illustrates that the majority of CD34+FLT3+ cells are somewhat larger in size with a broader cytoplasmic seam than the CD34+FLT3− population (bottom left). Counting of cells with high forward scatter (gate R2, top left) resulted in 6% lymphocytes/lymphoblastoid cells and 94% blasts in the CD34+FLT3− (gate R4, top right) and in 11% promyelocytes and 88% blasts in the CD34+FLT3+ population. Figure 6 (middle right) shows some cells which are characterized by a promyelocyte-specific azurophile granulation (example indicated by arrow). The other cells in this gate represented agranulated myeloblasts or monoblasts. In contrast, the majority of the large-sized CD34+FLT3− cells appeared to be agranulated and contained nuclei with significant variations in shape (Fig 6, bottom right). Some of these cells were strikingly larger in size and showed a proerythroblast-like morphology (indicated by arrow).
Phenotypic Characterization of BM and CB CD34+FLT3± Cells
Coexpression of defined stem/progenitor cell antigens on BM and CB CD34+FLT3+ and CD34+FLT3− cells was analyzed by three-color analysis. Figures 7 and 8 illustrate contour plots of MACS-purified CD34+ cells (A1) which were gated on the CD34+ population (B1) after triple-staining with anti–CD34-PerCP, anti–FLT3-FITC, and each of the indicated PE-conjugated antibodies. About 65% of the BM CD34+ and about 85% of the CB CD34+ cells were FLT3+.
Contour-plot analysis of triple-stained CD34-selected BM cells. Cells were labeled with CD34-PerCP, FLT3 specific MoAb 4G8-FITC, and the indicated PE-conjugates. All plots represent cells gated on the CD34+ population (not shown).
Contour-plot analysis of triple-stained CD34-selected BM cells. Cells were labeled with CD34-PerCP, FLT3 specific MoAb 4G8-FITC, and the indicated PE-conjugates. All plots represent cells gated on the CD34+ population (not shown).
Contour-plot analysis of triple-stained CD34-purified CB cells. Cells were labeled with CD34-PerCP, FLT3-specific MoAb 4G8-FITC, and the indicated PE conjugates. All plots represent cells gated on the CD34+ population.
Contour-plot analysis of triple-stained CD34-purified CB cells. Cells were labeled with CD34-PerCP, FLT3-specific MoAb 4G8-FITC, and the indicated PE conjugates. All plots represent cells gated on the CD34+ population.
The cell-surface antigen CD38 represents an important marker that allows discrimination between immature and more mature CD34+ hematopoietic progenitors.46 Figures 7 and 8 (A2) show that the highest levels of FLT3 expression were found in the CD34+CD38high population, whereas the CD34+CD38low and CD34+CD38− fractions expressed FLT3 at low levels. A similar distribution pattern was found in the HLA-DR subsets. Most of the CD34+HLA-DR− cells in BM (1%) and CB (3%) coexpressed FLT3 at low levels (Figs 7 and 8, A3). Because primitive BM progenitors are negative for HLA-DR,47 it is likely that these progenitors express FLT3 at low levels.
About 25% of the BM CD34+CD19+ B cells expressed FLT3 at relatively low levels (Fig 7, A4). These cells presumably represent primitive B-cell precursors. In CB, only a negligible amount of CD34+CD19+ B cells was detected that could not be further resolved into FLT3± fractions (Fig 8, A4).
Figures 7 and 8 (B2) show that BM and CB CD34+ cells expressing CD71 at high levels (20% to 27% and 10% to 15%, respectively) were mainly FLT3−, whereas most of CD34+ cells expressing low levels of CD71 coexpressed FLT3. Because erythroid progenitors express CD71 at higher levels than myeloid progenitors,48 this observation is consistent with our findings that BFU-E are mainly FLT3− and CFU-GM are mainly FLT3+.
Recently, expression of the TIE receptor on primitive CD34+CD38− BM and CB cells was reported.21 Figure 7, B3, shows that on BM CD34+ cells lower levels of TIE expression were detected (about 55%) than on CB CD34+ cells (about 88%; Fig 8). Notably, about 60% of the BM CD34+FLT3+ and 90% of CB CD34+FLT3+ cells coexpressed TIE.
The FMS receptor (CD115) was rarely detected on CB CD34+ cells (Fig 8, B4), whereas about 20% of the BM CD34+ cells were CD115+ (Fig 7, B4). Cells expressing CD115 at high levels were also highly positive for FLT3 (Fig 7, B4). Differential dual-scatter gating showed that the percentage of FLT3+CD115+ cells was highest in the population with high forward scatter intensities (not shown).
KIT (CD117) is highly expressed on purified BM and CB CD34+ cells (about 90% and 97%, respectively) and the majority of the CD117+ cells coexpressed FLT3 (Figs 7 and 8, C1). Both BM and CB CD34+ populations contained a subset of FLT3−CD117+ cells (about 25% and 13%, respectively), which likely represent erythroid progenitors because most of the FLT3− cells concomitantly expressed high levels of CD71. Notably, CD34+ cells with the highest level of FLT3 expression showed also the highest level of CD117 expression.
The GPI-anchored molecule Thy-1 (CD90) is expressed on about 20% to 40% of CD34+ BM cells and the double-positive fraction contains primitive stem cells.23 Figures 7 and 8 (C2) show that about 35% of the BM CD34+ cells and 58% of the CB CD34+ cells expressed CD90, which is predominantly expressed on FLT3+ cells. Interestingly, the highest levels of CD90 expression were found in the CD34+ fraction with intermediate levels of FLT3 expression (Figs 7 and 8, C2).
CD109 represents another GPI-anchored membrane glycoprotein expressed at low levels on very primitive progenitor cells.24 26 Figures 7 and 8 (C3) illustrate heterogeneous CD109 expression on CD34+ BM (about 40%) and CB cells (about 75%). The majority of CD34+CD109+ cells expressed FLT3, whereas a minor population of BM and CB CD34+CD109+ cells were FLT3− (about 20% and 10%, respectively).
The mucinlike molecule CD164, originally identified in a gastric carcinoma cell line, is also expressed on hematopoietic progenitor cells.29,32 Indeed, most of the CD34+ cells in BM and CB were positive for CD164 (Figs 7 and 8, C4). Virtually all FLT3+ cells coexpressed CD164. Remarkably, high levels of CD164 expression correlate with low levels of FLT3 expression. Because CD34+CD38− cells express high levels of CD164,32 it is likely that primitive progenitors are FLT3low.
DISCUSSION
Characterization and purification of the most primitive hematopoietic progenitor cells is one of the major goals in experimental hematology. However, targeting of stem cells for transplantation or gene therapy is hampered by the very low frequency of such cells in BM, CB, or mobilized PB. Thus, additional markers are necessary to precisely define the phenotype of stem cells. Using MoAbs against the recently identified “stem cell” receptor tyrosine kinase FLT312 we FACS-separated purified BM CD34+ cells into FLT3+ and FLT3− fractions and analyzed the functional properties of the sorted subsets. Additionally, we characterized the phenotype of CD34+FLT3+, CD34+FLT3−, and CD34−FLT3+ cells in BM and CB using antibodies against defined cell-surface antigens.
FACS-gating on low side scatter mononuclear BM and CB cells showed that the majority of the CD34+ cells also expressed FLT3. However, expression of this RTK is not restricted to the CD34+ population. Both in BM and CB about half of the FLT3+ cells with low side scatter intensity did not express CD34. Phenotypic analysis of CD34−FLT3+ BM cells showed that beside a minor CD10+ B-cell precursor subset , these cells mainly consisted of immature CD115+CD14− monocytic cells. In addition, FLT3 expression was detected in BM, CB, and PB monocytes, whereas granulocytes were negative for FLT3. Specific FLT3 expression on PB monocytes could also be corroborated by a decreased binding of MoAb SF1.340 and 4G8 after receptor downregulation with FLT3 ligand (not shown) and by the specific detection of FLT3 mRNA in CD14+ but not CD3+ CB cells using RT-PCR analysis. Thus, our results show that FLT3 expression is maintained during monocyte development but is abrogated during granulopoiesis after the CFU-GM stage. Our observations that FLT3 expression is not restricted to CD34+ cells are apparently in conflict with the data of Small et al49 who found flt3/stk-1 mRNA expression exclusively in the CD34+ fraction and suggested that the described low flt3/stk-1–specific signal detected in the CD34− fraction was the result of incomplete depletion of CD34+ cells. This discrepancy might be explained by the possibility that the CD34− population was indeed depleted of CD34+ cells in their experimental approach and, therefore, the mentioned flt3/stk-1 signal in the CD34− fraction was probably specific.
To determine the lineage commitment and proliferative potential of CD34+FLT3+ and CD34+FLT3− progenitor cells we FACS-separated CD34+ cells into FLT3+ and FLT3− fractions and estimated the colony-forming and cobblestone area–forming capacities of the sorted populations. We demonstrated that FLT3 is preferentially expressed on progenitors committed to the myelo-monocytic lineage (CFU-GM) and only to a negligible extent on erythroid progenitors (BFU-E). This observation is in agreement with (1) the strong activity of FLT3 ligand (FL) on myeloid and B-cell precursor cells but not on erythroid progenitors,50-52 (2) the fact that FLT3 is expressed at high levels on CD34+CD115+ BM cells which consist of monocytic progenitor cells,9 and (3) the fact that the BFU-E, which reside in the CD34+CD71high fraction, are FLT3−, whereas the CFU-GM, which reside in the CD34+CD71low fraction, are FLT3+.
Mixed colonies (CFU-GEMM) and CAFC could not be significantly enriched in either of the sorted FLT3+ or FLT3− populations although the CFU-GEMM numbers and CAFC frequencies were tendentially higher in the FLT3+ populations. This could be explained by very low levels of FLT3 expression on part of these progenitors and hence in an insufficient separation of the FLT3low fraction using state of the art techniques. Zeigler et al13 showed that primitive murine LTC-IC are found in both FLT3+ and FLT3− fractions which, however, could not be separated into distinct populations by a clear cutoff line. The separated fractions differed in their cycling state and they proposed that the more immature, noncycling cells resided in the FLT3− population. This is apparently in conflict with the fact that FL acts on primitive progenitor cells50-53 and is able to amplify LTC-IC numbers to around 30-fold.54
Several studies showed that primitive progenitor cells are enriched in the CD34+CD38− and CD34+HLA-DR− fractions.46,47,55 In addition, hematopoietic stem cells with long-term engrafting abilities were almost exclusively found in the CD117low population.56,57 We could show by three-color analysis of BM and CB CD34+ cells that the CD38low/−, HLA-DR−, and CD117low cells each expressed low levels of FLT3. Also, expression of the stem cell antigen CD9023 was mainly restricted to the FLT3low population. Low levels of FLT3 were further detected on CD34+CD164high cells, which is consistent with the fact that CD164 expression is highest on primitive CD34+CD38− cells.32 Considerable fractions of BM and particularly CB CD34+FLT3+ cells expressed TIE, which is also coexpressed on primitive CD34+CD38− cells.21,22 Because the level of TIE expression in this population does not differ from the level of other CD34+TIE+ subsets it is unknown whether primitive cells are TIElowFLT3low or TIEhighFLT3low. However, one can speculate that primitive cells are TIEhighFLT3low because significant TIE expression is detected in hemangioblasts and persists until developmentally not precisely defined steps of early adult hematopoiesis. A significant correlation appears between expression levels of the stem cell antigen CD10958 and FLT3, demonstrating that primitive FLT3low cells presumably express CD109 at low levels. Like TIE, CD109 is expressed at higher levels on CB than on BM CD34+ cells. Taken together, these findings suggest that the most primitive progenitors express low levels of FLT3. To more precisely determine FLT3 expression and its role on primitive progenitors, an increased staining intensity of fluorochromes as well as sensitivity of fluorescence detection is desirable. In addition, progenitor cells assayed by LTC-IC and CAFC may not represent the most primitive stem cells. Thus, in vivo systems such as immunodeficient mice are required to investigate primitive stem cells with self-renewal capacity and to assess the long-term repopulating ability of the sorted cells.59
Mutant mice lacking a functional FLT3 receptor are deficient in the growth of very primitive B-lymphoid progenitors, which reflects the important role of FLT3 in early B-cell development.60 In line with this we found low levels of FLT3 expression on a subpopulation of BM CD34+CD19+ cells. It may be speculated that CD34+CD19+FLT3+ cells represent the most primitive B-cell precursors in BM and may correspond to the early B-cell subset affected in FLT3 knock-out mice. Long-term transplantation experiments further showed that targeted FLT3 disruption impaired the developmental capacity of primitive progenitor cells of all hematopoietic lineages with the greatest impact on lymphopoiesis.60 In addition, FLT3 was found to be highly expressed on most “common” acute lymphoblastic leukemia (C-ALL) C-ALL and B-ALL as well as on most B-cell lines.35 36 This suggests that FLT3 is involved in the proliferation/differentiation of early B-lymphoid progenitors and is downregulated during normal B-cell development. In contrast, myelo-monocytic commitment seems to be accompanied by an upregulation of FLT3 expression, which terminates at not precisely defined developmental stages of granulocyte differentiation but is maintained during monocytic development. This is also substantiated by the fact that a subset of BM FLT3+ cells coexpressed FMS, which is exclusively found on progenitors committed to precursors of the monocytic lineage and on mature monocytes. The lack of FMS expression in CB suggests that monocytic precursor cells in CB are either more primitive than in BM or are very rare cells.
In conclusion, we have shown that FLT3 is expressed on myeloid progenitors and early B cells as well as on primitive CAFC. To precisely answer the question to which extent FLT3 is expressed on the most primitive assayable stem cells, sorted CD34+FLT3high, CD34+FLT3low, or CD34+FLT3− cells might be transplanted into irradiated immunodeficient mice and scored for the capacity to initiate and sustain human long-term hematopoiesis.
Supported by the Deutsche Forschungsgemeinschaft, SFB 1646, project A1, and the Deutsche Krebshilfe, project 10362 (H.-J.B.), by a Research Fellowship of the Max-Planck Society and Fortüne project F.1282107.1 of the University of Tübingen (I.R.), Grants No. E/B 41G/T0347/T5920 (B.L.Z.), EC contract no. FI4P-CT 95-0029 (B.L.Z.), and by a Subgroup of the European Concerted Action on Human Hematopoietic Stem Cells (O.R., D.B., W.K., K.A., and H.-J.B).
Address reprint requests to Hans-Jörg Bühring, PhD, Medizinische Universitätsklinik, Abteilung II, FACS-Labor, Otfried-Müller-Str.10, 72076 Tübingen, Germany.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal