Abstract
Cross-linking of major histocompatibility complex (MHC) class II antigens by anti-HLA-DR monoclonal antibody (MoAb; H81.9; IgG2a) results in inhibition of hematopoiesis in canine and human models. Inhibition of hematopoiesis is associated with apoptosis in a proportion of marrow cells. Since in murine macrophages class II cross-linking triggers nitric oxide (NO) production, and NO is thought to affect regulation of hematopoiesis, we investigated whether NO was involved in our models. In murine J774 monocytes/macrophages, MoAb H81.9 did induce NO. NO production was blocked by NG-monomethyl-L-arginine (NMMA), an inhibitor of NO synthase (NOS), and by the antioxidant N-acetylcysteine (NAC). In human and canine long-term marrow cultures (LTMCs) and in enriched marrow monocytes, however, no measurable increase in NO production was noted after H81.9 exposure. Nevertheless, NAC protected LTMCs against H81.9 induced inhibition of hematopoiesis. Therefore, we determined the effect of an exogenous NO donator, sin-1 (3-morpholinosydnonimine), on canine and human LTMCs and on apoptosis. Sin-1 at concentrations ≥100 μg/mL inhibited LTMCs and induced apoptosis; at low concentrations (1 μg/mL), however, sin-1 stimulated the generation of colony-forming unit granulocyte-macrophage. Combined treatment with sin-1 at 100 μg/mL and MoAb H81.9 resulted in profound inhibition of hematopoiesis in both canine and human LTMCs, and had an additive effect on apoptosis. At 1 μg/mL sin-1 counteracted the effect of H81.9 on hematopoiesis. The effect of sin-1 on apoptosis and hematopoiesis in LTMC was largely prevented by NAC. These results are consistent with the hypothesis that HLA-DR mediated apoptosis and inhibition of hematopoiesis involve oxidative stress. However, the biphasic response of hematopoiesis to sin-1 suggests a complex regulatory network possibly related to differences in NO sensitivity of distinct subpopulations of cells. Signals in addition to NO appear to be involved in the effect of anti-HLA-DR MoAb on hematopoiesis.
NITRIC OXIDE (NO) is an unstable free radical that plays an important role in immune modulation, neurotransmission, and regulation of vascular tone.1-3 NO is generated from L-arginine by the enzyme nitric oxide synthase (NOS). In vivo NO decomposes into the stable inorganic nitrogen oxides, nitrate and nitrite. NOS exists in both a constitutive form present in endothelial cells, neurons, and platelets4,5 and in an inducible form (iNOS) expressed in macrophages, neutrophils, endothelial cells, and hepatocytes.6-8 Expression of iNOS is stimulated by cytokines such as interleukin-1 (IL-1), tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), and IL-2, resulting in the augmentation of NO production both in vitro and in vivo.6,8-11 However, the effect of NO is dependent on the respective tissue, costimulatory signals and even the sequence of signals.12-14 NO induced by various inflammatory stimuli and cytokines has been reported to suppress cellular proliferation in murine bone marrow (BM) cells15 and to affect growth and differentiation of normal human BM cells.11,16 NO inhibits the growth of fresh acute myelocytic leukemia cells and may induce differentiation into a monocytic phenotype.17 NO has also been implicated as a mediator of apoptosis in murine macrophages.18 Since physiologic NO is derived from oxidized arginines, this fact supports the concept of oxidative metabolism leading to programmed cell death.19
We have described a model of hematopoietic failure induced by monoclonal antibodies (MoAbs) directed at monomorphic HLA-DR determinants.20,21 In this model we showed that while dogs treated with total body irradiation and transplanted with autologous marrow cells without further interventions recovered normal hematopoiesis, dogs given anti-HLA-DR MoAb (H81.9) intravenously on days 0 to 4 posttransplant showed only a transient rise in granulocytes which was followed by marrow aplasia. In vitro, similarly, MoAb H81.9 when added to canine or human long-term marrow cultures (LTMCs), inhibited the generation of colony-forming units granulocyte-macrophage (CFU-GM) from nonadherent and adherent cells and led to the exhaustion of cultures.22 Inhibition of hematopoiesis was associated with the development of apoptosis in a small proportion of adherent and nonadherent marrow cells.22 Since cross-linking of major histocompatibility complex (MHC) class II antigens with MoAbs in murine monocytes induces NO production23 and because NO has been implicated as a mediator of apoptosis, we investigated in the present study whether NO was involved in MoAb H81.9 induced inhibition of hematopoiesis.
MATERIALS AND METHODS
Cells
J774 cells (mouse monocyte/macrophage cell line) were obtained from ATCC (Rockville, MD) and cultured in RPMI 1640 media containing 10% fetal calf serum (FCS) and 10 μg/mL each of penicillin G and streptomycin. J774 served as a standard control in experiments of nitrite production.
BM mononuclear cells (BMMNCs).Canine BM aspirates were obtained from the humerus of anesthetized normal dogs as described.24 Human BM samples were obtained from normal volunteer donors who had given informed consent according to the procedures approved by the Institutional Review Board of the Fred Hutchinson Cancer Research Center (FHCRC). Cells were fractionated over Ficoll-Hypaque gradients and processed as described.22 25
Monocytes.Canine or human monocytes were isolated from BMMNCs by way of adherence to glass or plastic as described.26 Briefly, BMMNCs were resuspended in serum-free Dulbecco's modified Eagle's medium (DMEM) at 2 × 106 cells/mL. Ten milliliters of cells was added to 75-cm2 tissue culture flasks (Costar, Cambridge, MA) and incubated for 1 hour at 37°C in a humidified 5% CO2 incubator. Medium and nonadherent cells were removed. Adherent cells were washed twice with serum-free DMEM and collected into RPMI medium by gently scraping with a plastic cell scraper.
MoAbs and Reagents
MoAb H81.98.21 (H81.9; IgG2a), generated against mouse MHC class II (I-E) antigens, is cross-reactive with human HLA-DR and canine MHC class II framework determinants.27 28 Isotype control MoAb G3G6, reactive with human platelet associated protein gpIIb/IIIa, was a gift from Chris Badger (Seattle, WA).
Lipopolysaccharide (LPS; Escherichia coli serotype 0128:B12) and N-acetylcysteine (NAC) were purchased from Sigma Chemical Co (St Louis, MO). Sin-1 (3-morpholinosydnonimine hydrochloride) was purchased from Research Biochemicals International (Natick, MA). NG-monomethyl-L-arginine (NMMA) was obtained from Calbiochem (La Jolla, CA). Serum-free culture medium, Nutridoma HU, was obtained from Boehringer Mannheim (Indianapolis, IN). Recombinant canine and human c-kit ligand (stem cell factor; SCF ) were generously provided by Ian McNiece, Amgen, Inc. (Thousand Oaks, CA).
LTMCs
Canine LTMCs were established as described.29 Briefly, marrow cells were washed, resuspended in Iscove's modified Dulbecco's medium (GIBCO, Grand Island, NY) supplemented with 12.5% each of heat inactivated horse serum and FCS, 0.4 mg/mL of L-glutamine, 1 mmol/L sodium pyruvate, 10−6 mol/L hydrocortisone, 10−4 mol/L 2-mercaptoethanol, 100 U/mL penicillin, and 100 μg/mL of streptomycin (Dexter medium).29 30 2 × 107 cells were cultured in 25 cm2 tissue culture flasks (Costar) and maintained at 37°C in a humidified atmosphere of 5% CO2 in air. After 1 week, nonadherent cells were removed, and half of the spent medium plus an equal volume of fresh medium and fresh autologous marrow buffy coat cells (2 × 107) were recharged onto the adherent layers. Cultures were maintained for ≥4 weeks after recharging.
Human two-stage LTMCs were performed as previously described.30 31 Briefly, stromal layers were established in T25 tissue culture flasks (Corning, Corning, NY) by adding 10 mL of Dexter medium containing 2 × 106 BMMNC/mL and cultured at 37°C in 5% CO2/air atmosphere. Flasks were demidepleted of cells at weekly intervals, and at 2 to 3 weeks the adherent cells were irradiated with 1,600 cGy and used as stromal layers. These layers were then “recharged” with fresh BMMNCs. Starting 1 week after recharging, nonadherent cells were procured every week, pelleted, and counted, and aliquots were assayed for CFU-GM (see below). The remaining cells were returned to the long-term culture flasks with spent and fresh medium mixed 1:1. Sin-1 (100 μg/mL) with or without H81.9 (10 μg/mL) was added every week beginning at the time of recharging; in cultures using low concentrations of sin-1 (1 μg/mL) sin-1 was added either weekly or daily to the culture flask. Preliminary experiments using concentrations of sin-1 of 1 to 100 μg/mL had shown that the maximum achievable concentration of NO, measured in the form of nitrite, was reached reproducibly within one hour of sin-1 addition to cultures. Thus, “effective” concentrations of NO could be expected to be available without substantial delay after sin-1 addition. NAC in the respective experiments was added only at the time of recharging. Since serum, in particular horse serum, can serve as a source of nitrite and thereby affect the results, some experiments were also carried out in serum-free (Nutridoma) medium.
CFU-GM Assays
Nonadherent cells from canine LTMCs were assayed for CFU-GM as described elsewhere.29 Briefly, MNCs, 7.5 × 104, were cultured for 14 days at 37°C in a humidified atmosphere of 5% CO2 in air in 35 × 10-mm Petri dishes (Corning, NY) containing 2 mL of agar medium. The agar medium consisted of an equal volume mixture of 0.6% (wt/vol) Bacto agar (Difco Lab, Detroit, MI) and double-strength DMEM (GIBCO) containing 40% (vol/vol) heat-inactivated human AB plasma. Three replicate cultures per test were assayed.
CFU-GM assays from human LTMCs were performed as described for canine LTMCs except that 40% FCS instead of human AB plasma was used. A colony was defined as a group of 30 or more cells.
NO Measurement
J774 cells, canine, or human BMMNCs or monocytes were seeded in 96-well microtiter plates (Costar) at densities of 5 × 105/well and incubated with LPS, H81.9, NAC, NMMA alone, or in combination. At the timepoints indicated, cell-free supernatants were collected, centrifuged, and stored at −70°C until analysis. At room temperature NO quickly degrades into stable metabolites. We followed standard procedures and measured NO production by assessment of nitrite (NO−2) levels. NO, quantified by the accumulation of nitrite in the culture medium, was determined by microplate assay method.6 Supernatants were mixed with Griess reagent containing 40 μL sulfanilamide (25 mg/mL) in 15% HCl and 20 μL naphthylethylene diamine HCl (1 mg/mL) in a final volume of 260 μL, and incubated at room temperature for 10 minutes. Absorbance at 540 nm was detected in a microplate reader (Molecular Devices, Menlo Park, CA). Standard curves of various concentrations of sodium nitrite (0 to 20 nmol) were generated in parallel as control. Unless otherwise stated, data are expressed as nmol nitrite produced per 5 × 105 cells.
Flow Cytometric Analysis for Apoptosis
Apoptosis was determined by means of DNA histograms to identify hypodiploid nuclei (sub-G1 peak) using flow cytometric analysis.32,33 Briefly, 1.5 × 106 cells/sample were washed, pelleted, and resuspended with phosphate-buffered saline (PBS) containing 2% FCS and 2% human AB serum. Cells were fixed by adding dropwise 5 mL of cold 75% ethanol while gently vortexing. Cells were then incubated in the dark at 4°C for 30 minutes, pelleted, and resuspended in 1 ml of PBS containing 0.25% Triton-X 100 (Sigma). After further incubation in the dark at room temperature for 5 minutes, cells were pelleted, washed, and resuspended in 400 μL propidium iodide (PI) solution (2 μg/mL) containing RNase (10 μg/mL) and 0.1% sodium citrate. Samples were kept in the dark for 30 minutes at room temperature. Fluorescence analysis of individual nuclei was performed using a FACScan flow cytometer equipped with argon laser excitation at 488 nm and 250 mW light output (Becton Dickinson, San Jose, CA). A minimum of 10,000 events per sample were counted, stored, and analyzed by Multicycle software developed by Dr P. Rabinovitch (University of Washington, Seattle, WA). The PI fluorescence of individual nuclei was registered using the FL2 detector, and the percentage of cells containing hypodiploid DNA (sub-G1 peak) was used as a measure for cells undergoing apoptosis.22 Necrotic cells were excluded on the basis of forward and side scatter profiles.
Statistics
Results of NO production and colony formation in control and manipulated cultures were compared using the one-sample t-test.
RESULTS
Generation of NO by Murine J774 Cells, and Canine and Human Monocytes Activated by MoAb H81.9
Unstimulated J774 cells produced barely detectable amounts of NO. However, exposure to either MoAb H81.9 or LPS triggered J774 cells to generate NO in a dose- and time-dependent manner (Fig 1). The NOS inhibitor, NMMA, blocked NO generation induced by MoAb H81.9, suggesting that NO production by J774 cells treated with MoAb H81.9 was because of induction of NOS (Fig 1).
MoAb H81.9-induced nitric oxide (nitrite) generation in murine J774 cells is prevented by the NOS inhibitor NMMA. The vertical axis shows nmol of nitrite, the horizontal axis time after initiation of cultures. Results are expressed as the mean ± SEM of triplicate cultures of three to four experiments. Where indicated the NOS inhibitor NMMA at a concentration of 0.5 mmol/L was added concurrently with MoAb H81.9. (P < .05 at 48 and 72 hours for cells treated with H81.9 v untreated controls or cells treated with H81.9 + NMMA.)
MoAb H81.9-induced nitric oxide (nitrite) generation in murine J774 cells is prevented by the NOS inhibitor NMMA. The vertical axis shows nmol of nitrite, the horizontal axis time after initiation of cultures. Results are expressed as the mean ± SEM of triplicate cultures of three to four experiments. Where indicated the NOS inhibitor NMMA at a concentration of 0.5 mmol/L was added concurrently with MoAb H81.9. (P < .05 at 48 and 72 hours for cells treated with H81.9 v untreated controls or cells treated with H81.9 + NMMA.)
In contrast to J774 cells, unfractionated canine or human BMMNCs treated with MoAb H81.9 did not produce significant amounts of nitrite compared with untreated controls (data not shown). Because monocytes/macrophages are a major source of NO in the murine system,23 we next examined nitrite generation by monocytes which had been isolated from canine or human BMMNCs, enriched to 58% to 74% by plastic adherence and activated by MoAb H81.9 or LPS. No significant nitrite generation in either canine or human monocytes was observed. Measurements over 72 hours showed 2.5 to 3.0 nmol of nitrite/5 × 105 in both control and H81.9 stimulated canine and human cultures. Modification of culture conditions such as supplementation with L-arginine (as additional substrate) or assaying in Nutridoma (serum-free) medium yielded identical results. Also, results were not different when monocytes were obtained by means of elutriation.
Effect of Sin-1 on Colony Formation in LTMCs
The above experiments failed to provide evidence for NO production in activated canine or human marrow cells and monocytes. Nevertheless, studies by others have suggested that NO does affect hematopoiesis.11,15,16 34 Therefore, we tested the effect of an exogenous NO donator, sin-1, in our model systems, ie, in human and canine LTMCs. As shown in Fig 2A, at sin-1 concentrations of 100 μg/mL the generation of CFU-GM was reduced to 27% to 56% in canine LTMCs (P < .05 at 1, 2, 3, and 4 weeks). At 1 μg/mL of sin-1, however, the generation of CFU-GM was increased by 50% to 150% (P < .05 at 2, 3, and 4 weeks), while at an intermediate concentration of 10 μg/mL numbers of CFU-GM did not differ significantly from controls. Similar results were observed in human LTMCs (Fig 2B): High concentrations of sin-1 (100 μg/mL) inhibited colony formation by 22% to 55% (P < .05 at 1, 3, and 4 weeks) while low concentrations (1 μg/mL) stimulated colony formation by 10% to 175% (P < .01 at 2, 3, and 4 weeks) (Fig 2B). These observations indicated, therefore, that exogenous NO had a biphasic effect on in vitro hematopoiesis.
Effect of sin-1 on the generation of CFU-GM from canine (A) and human (B) LTMCs. Sin-1 was added every week beginning at the time of recharging. High concentrations of sin-1 (100 μg/mL) inhibited colony formation, whereas low concentrations (1 μg/mL) increased the numbers of CFU-GM (see text). The columns represent means ± SEM of three experiments (triplicate cultures each).
Effect of sin-1 on the generation of CFU-GM from canine (A) and human (B) LTMCs. Sin-1 was added every week beginning at the time of recharging. High concentrations of sin-1 (100 μg/mL) inhibited colony formation, whereas low concentrations (1 μg/mL) increased the numbers of CFU-GM (see text). The columns represent means ± SEM of three experiments (triplicate cultures each).
Sin-1–Induced Inhibition of Hematopoiesis Is Associated with Apoptosis
In several model systems inhibition of hematopoiesis involves programmed cell death.22,35,36 Since NO mediates some pathways of apoptosis,19 we next examined sin-1–treated cultures for evidence of apoptosis. As shown for human cells in Fig 3A, DNA histograms from control BMMNCs (n = 4) cultured for 5 days showed 1.9 ± 0.6% cells in the sub-G1 peak representing apoptotic nuclei. In contrast, 9.6 ± 2.5% of cells were apoptotic in 5-day cultures at 16 to 24 hours after adding sin-1 at 100 μg/mL (n = 4) (Fig 3C). The apoptotic peak with sin-1 at 1 μg/mL (Fig 3B) was only slightly increased compared to control. A similar pattern was observed with canine cells (not shown).
DNA histograms of human marrow mononuclear cells. Human marrow cells from untreated cultures (A, control) and cultures treated with sin-1, 1 μg/mL (B); sin-1, 100 μg/mL (C); H81.9, 10 μg/mL (D); sin-1, 1 μg/mL + H81.9 (E); and sin-1, 100 μg/mL + H81.9 (F ) were obtained after a 16-hour incubation, stained with PI and analyzed using flow cytometry. Relative numbers of nuclei are shown on the vertical axis and DNA content on the horizontal axis. Apoptotic nuclei are represented by the sub-G1 peak marked by the percentage. Apoptotic features were confirmed by electron microscopy.22 Results of one of four similar experiments are shown.
DNA histograms of human marrow mononuclear cells. Human marrow cells from untreated cultures (A, control) and cultures treated with sin-1, 1 μg/mL (B); sin-1, 100 μg/mL (C); H81.9, 10 μg/mL (D); sin-1, 1 μg/mL + H81.9 (E); and sin-1, 100 μg/mL + H81.9 (F ) were obtained after a 16-hour incubation, stained with PI and analyzed using flow cytometry. Relative numbers of nuclei are shown on the vertical axis and DNA content on the horizontal axis. Apoptotic nuclei are represented by the sub-G1 peak marked by the percentage. Apoptotic features were confirmed by electron microscopy.22 Results of one of four similar experiments are shown.
Additive Effects of Sin-1 and MoAb H81.9 on Hematopoiesis and Apoptosis
Treatment of canine or human LTMCs with MoAb H81.9 induces apoptosis in a proportion of cells.22 36 We hypothesized that if the pathways by which H81.9 and sin-1 inhibited hematopoiesis and induced apoptosis differed, a combination of both agents was likely to lead to a more profound suppression of CFU-GM and to an increase in apoptotic cell death. In both canine (Table 1A) and human LTMC (Table 1B) treatment with a combination of sin-1 (100 μg/mL) and MoAb H81.9 (10 μg/mL) intensified the suppression of CFU-GM beyond the extent observed with either agent alone. It was of note that sin-1 at low concentrations (1 μg/mL) when combined with MoAb H81.9 slightly increased colony numbers above those observed with H81.9 alone. Thus, a biphasic response to sin-1 occurred even in the presence of MoAb H81.9, providing further evidence that the H81.9/HLA-DR mediated effect was at least in part mediated by a mechanism independent of NO.
Effect of sin-1 Plus Anti–HLA-DR MoAb H81.9 on CFU-GM Production in Canine and Human LTMCS
| LTMC (wk of CFU-GM Assay) . | Culture Conditions . | |||
|---|---|---|---|---|
| . | Control . | H81.9* . | H81.9* + Sin-1 (1 μg/mL) . | H81.9* + Sin-1 (100 μg/mL) . |
| (A)Canine | ||||
| 1 | 100 | 24 ± 8 | ND | 12 ± 6 |
| 2 | 100 | 13 ± 7 | ND | 11 ± 7 |
| 3 | 100 | 45 ± 5 | ND | 21 ± 9 |
| 4 | 100 | 30 ± 6 | ND | 17 ± 7 |
| (B)Human | ||||
| 1 | 100 | 32 ± 4 | 51 ± 5 | 18 ± 3 |
| 2 | 100 | 10 ± 7 | 21 ± 6 | 1 ± 1 |
| 3 | 100 | 2 ± 4 | 20 ± 3 | 1 ± 1 |
| 4 | 100 | 0 ± 1 | 18 ± 4 | 0 ± 1 |
| LTMC (wk of CFU-GM Assay) . | Culture Conditions . | |||
|---|---|---|---|---|
| . | Control . | H81.9* . | H81.9* + Sin-1 (1 μg/mL) . | H81.9* + Sin-1 (100 μg/mL) . |
| (A)Canine | ||||
| 1 | 100 | 24 ± 8 | ND | 12 ± 6 |
| 2 | 100 | 13 ± 7 | ND | 11 ± 7 |
| 3 | 100 | 45 ± 5 | ND | 21 ± 9 |
| 4 | 100 | 30 ± 6 | ND | 17 ± 7 |
| (B)Human | ||||
| 1 | 100 | 32 ± 4 | 51 ± 5 | 18 ± 3 |
| 2 | 100 | 10 ± 7 | 21 ± 6 | 1 ± 1 |
| 3 | 100 | 2 ± 4 | 20 ± 3 | 1 ± 1 |
| 4 | 100 | 0 ± 1 | 18 ± 4 | 0 ± 1 |
Results summarize data from two experiments each for canine and human cells. At each timepoint CFU-GM assays were set up in triplicate. Results are expressed as percent of control. Colony numbers in canine control cultures were 123 ± 14, 81 ± 12, 79 ± 15, and 62 ± 18, and in human cultures 240 ± 28, 215 ± 30, 180 ± 27, and 160 ± 36 at 1, 2, 3, and 4 weeks, respectively.
Abbreviation: ND, not done.
10 μg/mL in all assays.
The proportion of apoptotic cells among human BMMNCs treated with MoAb H81.9 alone (Fig 3D) was of the same order of magnitude as observed with sin-1 at 100 μg/mL (Fig 3C). However, combined treatment with sin-1 at 100 μg/mL and MoAb H81.9 (Fig 3F ) or sin-1 at 1 μg/mL and MoAb H81.9 (Fig 3E) resulted in an increase in apoptosis above the levels observed in cultures treated with either sin-1 or MoAb H81.9 alone. Interestingly, as shown in Fig 4, the addition of the NOS inhibitor NMMA to cultures reduced the extent of apoptosis triggered by H81.9, suggesting that NO production did contribute to H81.9-induced apoptosis.
Prevention of H81.9 induced apoptosis by NMMA. Human marrow mononuclear cells were analyzed for apoptotic nuclei (DNA histograms) as described for Fig 3. The relative number of nuclei is shown on the vertical axis, the DNA content on the horizontal axis. In the presence of NMMA the apoptotic (sub-G1 ) peak was reduced from 12.5% (A; H81.9 alone) to 1.2% (B; H81.9 + NMMA).
Prevention of H81.9 induced apoptosis by NMMA. Human marrow mononuclear cells were analyzed for apoptotic nuclei (DNA histograms) as described for Fig 3. The relative number of nuclei is shown on the vertical axis, the DNA content on the horizontal axis. In the presence of NMMA the apoptotic (sub-G1 ) peak was reduced from 12.5% (A; H81.9 alone) to 1.2% (B; H81.9 + NMMA).
NAC Protects Against Sin-1–Induced Apoptosis and Inhibition of Hematopoiesis
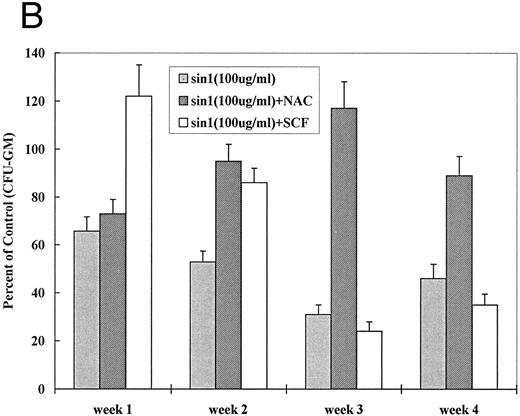
If oxidative stress and NO are involved in apoptosis, a counterregulatory effect would be expected from treatment with antioxidant. NAC is an effective antioxidant capable of direct reduction of reactive oxygen species and of increasing glutathione (GSH) production.37,38 We first determined optimal concentrations of NAC for J774 and BMMNCs. NAC at 10 to 15 mmol/L completely inhibited LPS-induced (data not shown) and MoAb H81.9 initiated NO production in J774 cells (Fig 5A). While the same concentrations of NAC were toxic to both canine and human BMMNCs, NAC at 0.5 to 1 mmol/L exerted no toxic effect, and apoptosis triggered by MoAb H81.9 was prevented as determined by DNA histograms. Furthermore, in canine (Fig 5B) and human (not shown) LTMCs, sin-1–induced inhibition of colony formation was prevented by concurrent treatment of cultures with NAC at 0.5 mmol/L; the c-kit ligand SCF, previously shown to protect LTMCs against the inhibitory effect of MoAb H81.9,21 22 showed only a transient protective effect against sin-1 (Fig 5B).
Effect of NAC and SCF on nitric oxide generation and hematopoietic colony formation. (A) Nitric oxide generation (measured in the form of nitrite) in J774 cells. J774 cells were incubated with H81.9 (10 or 100 μg/mL) with or without the addition of NAC (15 mmol/L) for 1 to 3 days. In J774 cells NO production triggered by H81.9 was significant at 48 and 72 hours (P < .01); results in the presence of NAC were not different from controls. (B) Effect of sin-1, alone or in combination with NAC or SCF on colony formation from canine LTMCs. Sin-1 (100 μg/mL), NAC (0.5 mmol/L), and SCF (100 ng/mL) were added to LTMCs weekly beginning at the time of recharging. CFU-GM in sin-1–treated cultures were lower than in controls at all timepoints (see Fig 3). NAC significantly protected colony formation at 2, 3, and 4 weeks (P < .01). SCF had a protective effect in weeks 1 and 2 (P < .01) but not at later points. Shown are the means ± SEM of three experiments expressed as percent of controls.
Effect of NAC and SCF on nitric oxide generation and hematopoietic colony formation. (A) Nitric oxide generation (measured in the form of nitrite) in J774 cells. J774 cells were incubated with H81.9 (10 or 100 μg/mL) with or without the addition of NAC (15 mmol/L) for 1 to 3 days. In J774 cells NO production triggered by H81.9 was significant at 48 and 72 hours (P < .01); results in the presence of NAC were not different from controls. (B) Effect of sin-1, alone or in combination with NAC or SCF on colony formation from canine LTMCs. Sin-1 (100 μg/mL), NAC (0.5 mmol/L), and SCF (100 ng/mL) were added to LTMCs weekly beginning at the time of recharging. CFU-GM in sin-1–treated cultures were lower than in controls at all timepoints (see Fig 3). NAC significantly protected colony formation at 2, 3, and 4 weeks (P < .01). SCF had a protective effect in weeks 1 and 2 (P < .01) but not at later points. Shown are the means ± SEM of three experiments expressed as percent of controls.
DISCUSSION
NO is an important physiological regulator5 in many systems, including hematopoiesis.11,34 NO may either have a protective or an agonistic effect, dependent on the model system studied.12,13 Multiple interactions between cytokines such as IFN-γ, TNF-α, or IL-1 and NO have been described8-10 and a direct involvement in apoptosis has been postulated.12,13,18,19,39 However, major species differences have been reported particularly in regard to iNOS dependent NO generation in mononuclear cells: While murine 6,9,14,23 and bovine40 macrophages contain easily inducible NOS, canine41 and caprine40 macrophages show little or no induction of NOS, and results with human cells are somewhat controversial.10,11,42 43
We have been studying the effect of MHC class II cross-linking on in vivo and in vitro hematopoiesis in canine20-22 and human25,36,41,44 models. The observation that inhibition of hematopoiesis in these models was associated with apoptosis, and the finding that cross-linking of MHC class II on murine macrophages induced the production of NO, a radical known to be involved in apoptosis,23 led us to investigate whether NO served as a mediator of MHC class II-mediated inhibition of hematopoiesis. In agreement with studies by others,23 anti-MHC class II (HLA-DR) MoAb H81.9 triggered dose- and time-dependent NO production in the murine macrophage cell line J774. Addition of the NOS inhibitor NMMA blocked NO production, an indication that MHC class II-initiated signals involved NOS. However, MoAb H81.9 at concentrations even higher than those effective with murine cells failed to induce measurable amounts of NO in human and canine marrow cells or enriched marrow monocytes. While these findings were not supportive of a role for NO in MHC class II-mediated downregulation of hematopoiesis, ancillary experiments had suggested that the antioxidant NAC when added to canine or human LTMCs treated with MoAb H81.9 reduced the extent of inhibition of hematopoiesis. It is possible that NAC neutralized radicals other than NO,19 or directly affected transcription factors, eg, NFKB45; however, NOS is expressed in human marrow cells, particularly after stimulation with cytokines such as IFN-γ or TNF-α.11 We have shown that treatment of human and canine marrow with MoAb H81.9 upregulates TNF-α,36 44 a result which suggests such a pathway to be operative in the present model.
To further explore the possibility of an effect of NO on hematopoiesis and apoptosis in canine and human marrow cells we used sin-1, a synthetic NO donator as a probe. Experiments with sin-1 showed, indeed, a striking effect on apoptosis and interactions with H81.9 mediated signals. Of note, the response to sin-1 was biphasic, with an increase in hematopoietic colony formation at low and inhibition at high concentrations. An explanation is not immediately apparent. However, as discussed above the impact of NO may vary between different cell populations; both protective and negative effects have been reported.12 13 Conceivably, at low concentrations a negative effect of NO was restricted to subpopulations of marrow cells that function as negative regulators of hematopoiesis (directly or via secreted mediators), and elimination of these regulators (eg, monocytes; TNF-α) would, therefore, enhance hematopoiesis. Such a concept is consistent with the observation that even at concentrations of sin-1 that enhanced hematopoiesis there was a slight increase in the proportion of apoptotic cells in the marrow (Fig 3B). Why the stimulating effect of low concentration sin-1 was somewhat more sustained in canine than in human cultures is not clear. At high concentrations a broader spectrum of cells, including hematopoietic precursors would be affected, resulting in an increase in apoptosis and a decline in hematopoiesis.
The effect of combinations of MoAb H81.9 and sin-1 was also dependent on the concentration of sin-1. At low concentration sin-1 preserved some colony formation in the presence of H81.9, whereas at high concentration sin-1 and H81.9 effects were additive. These data fit the proposed scheme: if at low concentration sin-1 eliminates cells with a negative regulatory function, they would not be able to contribute to the inhibitory effect of MoAb H81.9. This would be particularly effective if such cells were MHC class II+, eg, macrophages, and, therefore, would be triggered to generate negative regulators such as TNF-α on exposure to anti-MHC class II MoAb.44 46 At high concentrations sin-1 and H81.9 effects were additive, for both inhibition of hematopoiesis and for apoptosis. H81.9-induced apoptosis in human cells was blocked by the NOS inhibitor NMMA, suggesting that H81.9 did indeed trigger NO generation.
Similar to previous observations showing that the c-kit ligand SCF prevented H81.9-induced apoptosis and inhibition of hematopoiesis,21,22,25 the addition of exogenous SCF to cultures had a protective effect on hematopoiesis. However, this effect was present only during the early phase of cultures; subsequently the numbers of CFU-GM measured in LTMCs treated with sin-1 and SCF did not differ from those obtained from cultures treated with sin-1 only. This might be related to the derivation of earlier developing colonies from c-kit+ cells (which could be rescued by SCF ) and later maturing colonies from c-kit− cells, which were not affected by SCF.31 Alternatively, more primitive precursors required for colony formation later in culture might be more susceptible to sin-1. In contrast to SCF, the protective effect of NAC persisted through the entire culture period, apparently protecting cells at various stages of differentiation.
In summary, these studies are consistent with a role of NO in the regulation of canine and human hematopoiesis, including models of HLA-DR–mediated inhibition of hematopoiesis. A physiologic source for NO is not readily apparent at this time; our results indicate complex interactions. While HLA-DR–initiated signals involved nitric oxide synthase, HLA-DR–mediated signals did not appear to be restricted to this pathway. Marrow responses to exogenous NO were biphasic and suggest differential sensitivity of different target populations. Subpopulations of interest, in addition to macrophages and hematopoietic precursors, are endothelial cells, which are a constitutive source of NO and are thought to play an important role in the regulation of hematopoiesis.
ACKNOWLEDGMENT
We thank all volunteer donors who have contributed marrow samples to these studies; Michelle Fisher, Jill Johnson, and Doug Jones of the shared animal facilities for obtaining the canine marrow samples; and Harriet Childs and Bonnie Larson for typing the manuscript. We appreciate comments on the statistics by Ted Gooley, PhD. We thank Peter Kiener, PhD, for providing elutriated monocytes.
Supported by Grants No. HL36444, CA18029, CA18221, and AI25606 from the National Institutes of Health. J.W.L. was supported by a fellowship from Catholic University Medical College, Seoul, Korea.
Address reprint requests to H. Joachim Deeg, MD, Fred Hutchinson Cancer Research Center, 1124 Columbia St, M318, Seattle, WA 98104.








This feature is available to Subscribers Only
Sign In or Create an Account Close Modal