Abstract
Hemoglobin A2 (HbA2 ), which contains δ-globin as its non–α-globin, represents a minor fraction of the Hb found in normal adults. It has been shown recently that HbA2 is as potent as HbF in inhibiting intracellular deoxy-HbS polymerization, and its expression is therefore relevant to sickle cell disease treatment strategies. To elucidate the mechanisms responsible for the low-level expression of the δ-globin gene in adult erythroid cells, we first compared promoter sequences and found that the δ-globin gene differs from the β-globin gene in the absence of an erythroid Krüppel-like factor (EKLF ) binding site, the alteration of the CCAAT box to CCAAC, and the presence of a GATA-1 binding site. Second, serial deletions of the human δ-globin promoter sequence fused to a luciferase (LUC) reporter gene were transfected into K562 cells. We identified both positive and negative regulatory regions in the 5′ flanking sequence. Furthermore, a plasmid containing a single base pair (bp) mutation in the CCAAC box of the δ promoter, restoring the CCAAT box, caused a 5.6-fold and 2.4-fold (P < .05) increase of LUC activity in transfected K562 cells and MEL cells, respectively, in comparison to the wild-type δ promoter. A set of substitutions that create an EKLF binding site centered at −85 bp increased the expression by 26.8-fold and 6.5-fold (P < .05) in K562 and MEL cells, respectively. These results clearly demonstrate that the restoration of either an EKLF binding site or the CCAAT box can increase δ-globin gene expression, with potential future clinical benefit.
THE HUMAN δ-globin gene encodes the non–α-globin subunits of the minor adult hemoglobin (Hb), HbA2 . HbA2 (α2δ2 ) comprises only 2.5% of total adult Hb, whereas HbA (α2β2 ) accounts for greater than 95% of the Hb found in adult hemolysate.1 HbA2 differs from HbA by only 10 amino acids and has functional properties that are nearly identical to those of HbA. HbA2 has been of interest to those studying Hb diseases, as HbA2 has been known for many years2 to inhibit the polymerization of deoxy-HbS in sickle cell anemia patients, although preliminary results showed that an extremely high level of HbA2 may be associated with red blood cell abnormalities in a transgenic mouse model.3 Indeed, recent results suggest that it is as potent as HbF in inhibiting intracellular deoxy-HbS polymerization.4 Increasing the concentration of HbF has been a major focus of laboratory and clinical studies in therapies for sickle cell anemia and β-thalassemias (for reviews, see Rodgers5 and Schechter, Rodgers).6 However, HbA2 is distributed pancellularly, whereas HbF is restricted to a subpopulation (10% to 30%) of erythrocytes (F cells).7 8 Therefore, pharmacologic increases in HbA2 production may protect a larger fraction of cells from sickling compared with an elevation of HbF. Accordingly, understanding how the δ-globin gene is regulated could have important therapeutic implications.
The human δ-globin gene is closely linked to the major adult β-globin gene (β) on the short arm of chromosome 11. The β-globin gene encodes the non–α-globin subunit of adult major Hb, HbA. The δ- and β-globin genes are believed to be derived from a common ancestral globin gene through gene duplication.1,9 Although the δ- and β-globin genes have strong sequence homology in the coding regions, in which there are only 31 nucleotide differences, the expression of these two genes is strikingly different. The mechanisms accounting for the low level of δ-globin gene expression have not been completely elucidated, and they may include both transcriptional and posttranscriptional regulation. For example, Wood et al10 reported that the half-life of δ-globin mRNA was less than one third that of β-globin mRNA, suggesting that the relative instability of δ-globin mRNA contributes to the low level of δ-globin. Production of stable mRNA in transfected monkey kidney cells is 50-fold less for the δ-globin gene than for the β-globin gene, approximating the ratio of δ and β mRNA observed in human bone marrow cells.11 However, δ gene 5′ flanking region regulatory elements have not yet been defined. Sequence comparison of the first 100 base pairs (bp) of the promoter regions of the human δ- and β-globin genes shows a striking change in the CCAAT box to CCAAC in the δ-globin gene, which was assumed to be the cause of the low-level expression of δ-globin gene,12 although no experimental evidence has been obtained. Additionally, the recently identified erythroid-specific transcription factor, erythroid Krüppel-like factor (EKLF ), has not been investigated in δ-globin gene. Transcriptional regulation of the δ-globin gene is likely the result of interactions of multiple factors, but neither the cis-acting DNA sequences nor the trans-acting factors leading to the low-level expression (hypofunction) of the δ-globin gene have been fully defined.
In the present study, we compared the promoter sequences of the two human adult β-like globin genes. Using a transient transfection assay with different deletion and substitution plasmids linked to a luciferase (LUC) reporter gene, three major regulatory sequences in the 5′ flanking region of the δ-globin gene were identified. We also demonstrate, by site-directed mutagenesis, that the low-level expression of the δ-globin gene is at least partially the result of the absence of the EKLF binding site and an alteration in the “CCAAT box” (binding site for CP1) sequence located 70 bp upstream from the cap site of the δ-globin promoter, in which the normal CCAAT box is replaced by CCAAC.
MATERIALS AND METHODS
Alignment of DNA sequences.Sequences of the human δ-globin and human β-globin genes were compared using the program sim.13 The initial scoring scheme for the pairwise alignments was +1 for a match, −1 for a mismatch (transition or transversion), −6 to open a gap, and −0.2 per position to extend the gap. A larger alignment was also obtained by relaxing the gap-open parameter to −4.
Preparation of plasmids containing deletions and mutations.A series of deletional mutants in the δ promoter were prepared in the promoterless LUC reporter gene vector, pGL2-Basic (Promega, Madison, WI). Point mutations or insertions in the β-globin or δ-globin gene promoters were introduced by two-step polymerase chain reaction (PCR) site-specific mutagenesis.14 The structure of all plasmids was verified by sequencing. Detailed information for each of the deletional plasmids is shown in Fig 2.
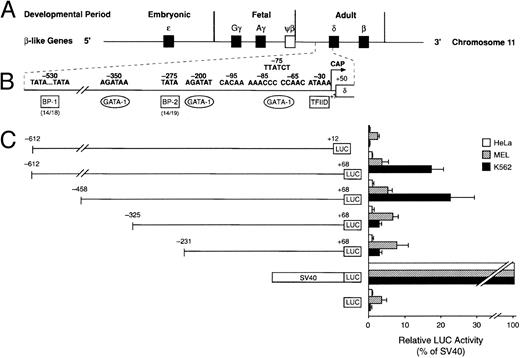
Cell-specific expression of the human δ-globin gene 5′ flanking sequence. (A) β-Like globin gene arrangement on chromosome 11. (B) Promoter region of the human δ-globin gene and possible binding sites of transcription factors. (C) Relative LUC activity of the serial deletion plasmids. The left panel shows human δ-globin promoter-LUC deletion plasmids. The right panel is the relative LUC activity of each plasmid. Data represent the mean ± SD of three individual experiments using three different plasmid DNA preparations.
Cell-specific expression of the human δ-globin gene 5′ flanking sequence. (A) β-Like globin gene arrangement on chromosome 11. (B) Promoter region of the human δ-globin gene and possible binding sites of transcription factors. (C) Relative LUC activity of the serial deletion plasmids. The left panel shows human δ-globin promoter-LUC deletion plasmids. The right panel is the relative LUC activity of each plasmid. Data represent the mean ± SD of three individual experiments using three different plasmid DNA preparations.
Transfections and LUC assay.K562 cells and HeLa cells were maintained in RPMI 1640 medium or modified Eagle's medium, respectively, supplemented with 10% fetal bovine serum plus glutamine, penicillin, and streptomycin. MEL cells were grown in RPMI medium containing 15% fetal bovine serum and antibiotics. Plasmid DNA was introduced into K562 cells by electroporation of a total of 3.2 × 107 cells with 20 μg of each of the LUC plasmids. The cells were then diluted with fresh medium containing 20 μmol/L hemin for induction. MEL cells were similarly transfected with the exception that after electroporation the cells were resuspended in medium containing 15% fetal bovine serum and 2% dimethylsulfoxide (DMSO) for induction. HeLa cells were transfected with DOTAP liposomal transfection reagent (Boehringer Mannheim, Indianapolis, IN). Plasmid DNA (5 μg) was added to the cells plated at 1 × 106 cells/mL 24 hours before lipofection. Cells were harvested 48 hours after transfection and assayed for LUC activity. The amount of cellular extract used in the LUC assay was normalized according to the protein concentration from each sample. Transfection efficiency was monitored by cotransfection of a β-galactosidase control vector. Each transfection was performed at least three times. Student's t test was used to determine the statistical significance of differences in LUC activity between pairs whenever appropriate.
RESULTS
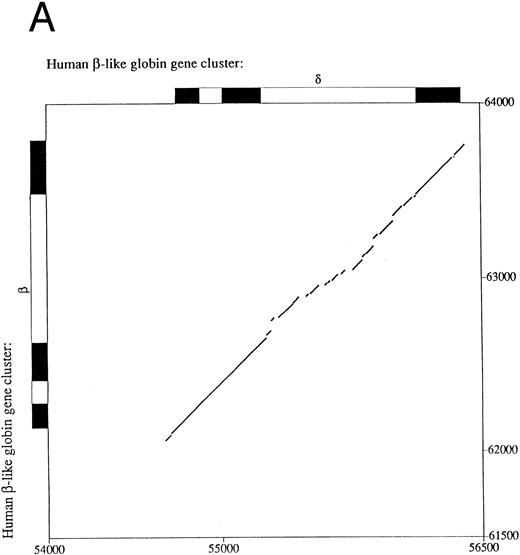
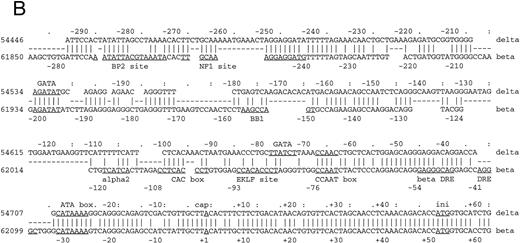
Sequence comparison between primate β- and δ-globin genes.The β-globin gene is expressed in adults as the major non–α-globin gene. To define possible sequence alterations that might account for the low-level expression of the similar human δ-globin gene, we compared the β- and δ-globin gene sequences. The analyses revealed a high degree of sequence similarity in their coding regions, approaching 95% with only 31 nucleotide differences. This very high similarity extends into the 5′ flanking region for only 70 bp upstream from the cap sites (Fig 1A), a pattern that demarcates the region of conversion between the human δ- and β-globin genes and differs slightly from previously published results.15 Decreasing the gap-open penalty to 4 extends the alignment through a less similar region to 546 bp 5′ to the δ-globin gene cap site (part of the sequence alignment is shown in Fig 1B). Presumably, these less similar sequences were not included in the most recent gene conversion between the δ- and β-globin genes. Besides the previously documented difference in the CCAAT box at −70 bp, the conserved EKLF binding sequence motif CCACACCCT (also referred to as the β-globin CACCC box) in the β-globin gene16 does not exist in the δ-globin gene. The absence of the CACCC box is evolutionarily conserved among primate δ-globin genes (data not included). Since the CCAAT box and the CACCC box are required for normal transcription of rabbit β- and mouse βmajor-globin genes17,18 and mutations in the human β-globin CACCC box can lead to a β-thalassemia phenotype,19 the sequence differences in these two boxes may be leading elements contributing to low-level expression of the δ-globin gene.
Comparison of 5′ flanking sequences between β- and δ-globin genes. (A) Alignment of the sequences between human β-and δ-globin genes. The program sim was used to compute local alignments between the 2 sequences, and positions of the alignment were plotted by the program laps. (B) Pairwise alignment of the sequences of human δ-globin and β-globin genes. Sequences were aligned using the program sim with a gap-open penalty of 4. Only the part of the alignment beginning at position −294 and ending at position +62 of the δ-globin gene is shown, but the complete alignment begins at −546 bp of the δ-globin gene and continues through exon 3. On the line between the sequences, a vertical line (‖) marks a match, a hyphen (-) indicates a gap introduced to optimize the alignment, and a space indicates a mismatch. The sequences are numbered as in the GenBank sequence file HUMHBB on the far left, and positions with respect to the cap site are given above the δ-globin gene sequence and below the β-globin gene sequence. The right-most digit in each number aligns with the numbered nucleotide. Binding sites for specific proteins are labeled above the δ-globin and below the β-globin gene sequences, as summarized in Hardison et al.29 ini, start site for translation. Periods (.) and colons (:) above the δ-globin gene sequence mark every fifth and tenth position in the alignment.
Comparison of 5′ flanking sequences between β- and δ-globin genes. (A) Alignment of the sequences between human β-and δ-globin genes. The program sim was used to compute local alignments between the 2 sequences, and positions of the alignment were plotted by the program laps. (B) Pairwise alignment of the sequences of human δ-globin and β-globin genes. Sequences were aligned using the program sim with a gap-open penalty of 4. Only the part of the alignment beginning at position −294 and ending at position +62 of the δ-globin gene is shown, but the complete alignment begins at −546 bp of the δ-globin gene and continues through exon 3. On the line between the sequences, a vertical line (‖) marks a match, a hyphen (-) indicates a gap introduced to optimize the alignment, and a space indicates a mismatch. The sequences are numbered as in the GenBank sequence file HUMHBB on the far left, and positions with respect to the cap site are given above the δ-globin gene sequence and below the β-globin gene sequence. The right-most digit in each number aligns with the numbered nucleotide. Binding sites for specific proteins are labeled above the δ-globin and below the β-globin gene sequences, as summarized in Hardison et al.29 ini, start site for translation. Periods (.) and colons (:) above the δ-globin gene sequence mark every fifth and tenth position in the alignment.
Functional analysis of the δ-globin gene 5′ flanking region.To identify cis-acting regulatory elements in the human δ-globin promoter, a DNA fragment containing the sequence from −612 bp to +68 bp of δ was linked to a LUC reporter gene. δ-LUC activity was expressed at 10% the level of a LUC plasmid containing the SV40 promoter, consistent with previous results that endogenous δ-globin gene is expressed in induced K562 cells.20 We first deleted the −612 plasmid from the 3′ end (+12 to +68 bp). The deletion removes part of the binding site of the RNA polymerase II complex for initiation of transcription (30 bp downstream of the start site21-23 ), the 5′-untranslated region (5′-UTR) beginning from +1 to +50 bp from the cap site, and 18 bp of the sequence inside the coding region. The basal level of expression was completely abolished in K562 cells (Fig 2), indicating that the 5′-UTR plays a positive role in δ-globin gene expression, transcriptionally or posttranscriptionally. Next, a series of 5′ deletions were constructed and tested for transient expression in K562 cells. The resulting fragments began at −458, −325, and −231 bp and extended to +68 bp. After the LUC activities were normalized to the protein concentration in the extracts, the relative transcriptional activity conferred by each of these plasmids was expressed as the percentage of LUC activity produced by the SV40 control promoter. The efficiency of the transfection was monitored by cotransfection with a β-gal gene control vector. Figure 2 shows that deletion from −612 to −458 bp results in a slight increase in LUC activity of approximately 20% compared with controls (P < .05), indicating that weak negative regulatory activity resides in the region between −612 and −458 bp, which is consistent with a role for BP1 in the negative control of δ by interaction in this region (data not shown). DNase I footprinting shows protein binding at a site 350 bp upstream from the δ-globin cap site, where sequence analysis predicts a potential binding site for the transcription factor GATA-1 (manuscript in preparation). Deletion of the region from −458 to −325 bp led to a sevenfold reduction of promoter activity. No obvious alteration of transcription was observed when the sequence between −325 and −231 was deleted. The alignment in Fig 1B shows that the δ-globin gene sequence partially matches that of the β-globin gene putative silencer BP2 binding site. It is possible that the BP2 site in the δ-globin gene promoter also requires the binding of another silencer, such as BP1, to have an effect.
The changes from the deletional plasmid introduced into MEL cells are not as great as in K562 cells. In MEL cells, at least three protein-binding sites upstream of the EKLF site have been implicated in the inducibility of β-globin gene expression — NF1, GATA-1, and BB1 binding sites (Fig 1B). We note that while the GATA-1 binding site is present, the BB1 binding site is not present in the δ-globin gene sequence and the NF1 binding site has an internal deletion. It is likely therefore that the δ-globin gene is not strongly inducible in MEL cells. The effect of the positive element between −458 and −325 bp is not apparent in MEL cells, showing that the two cell types respond differently to the upstream regulatory sequences. Cell-specific regulation of the δ-globin gene was also demonstrated by analysis of these deletional plasmids in a nonerythroid HeLa cell line (Fig 2). Erythroid factors such as EKLF or GATA-1 are not present in the HeLa cell line. Therefore, potential upregulation of the promoter by GATA-1 does not affect δ-globin gene expression in HeLa cells and the expression is low, as expected.
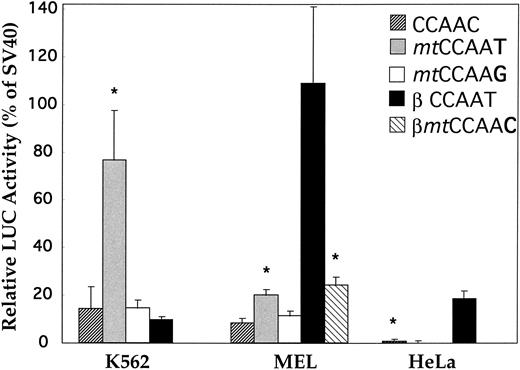
Restoration of the CCAAT box of the δ gene.The CCAAC box in the human δ-globin gene has been speculated to be responsible for the low-level δ expression. However, no experimental evidence has been provided supporting this hypothesis. We mutated the CCAAC box of δ to CCAAT in the δ (−612 to +68) plasmid, thereby restoring the CCAAT motif as found in the β-globin promoter by this single-bp substitution. The specificity of the CCAAT box for activity was confirmed by a second mutational plasmid with the CCAAG sequence replacing the CCAAC box. The wild-type δ sequence (CCAAC) and the mutation plasmids (mtCCAAT and mtCCAAG) were transiently transfected into two erythroleukemia cell lines (human K562 and murine MEL) and a nonerythroid line (human HeLa). Figure 3 shows that LUC activity produced by the plasmid with the promoter containing CCAAT was effectively elevated in both erythroid cell lines (P < .01). Increases of 5.6- and 2.4-fold were recorded in K562 cells and MEL cells, respectively. There was no statistical difference in promoter activity between the wild-type (CCAAC) and the C to G mutation plasmid. This suggested that this particular C to T mutation in the CCAAC box restored the binding capacity of a positive regulatory factor. To compare the promoter strength of β- and δ-globin genes, we made a pβGL plasmid including 5′ flanking β-globin DNA (−640 to +50 bp) fused to the LUC reporter gene. In DMSO-induced MEL cells, the β-globin gene is highly expressed, at a level approximately 13 times greater than that of the δ-globin gene (Fig 3). After converting the CCAAC box to the CCAAT box, δ-globin promoter activity increased such that expression of the β-globin gene is only 5.5-fold greater than that of the δ-globin gene. Activity of the β-globin gene was approximately 30 times greater than that of the δ-globin gene in HeLa cells, approximating the ratio of β- and δ-globin gene expression observed in monkey kidney COS cells.11 This ratio is similar to the ratio of β- and δ-mRNA observed in normal human bone marrow cells. No increase of LUC activity was found when the δ mtCCAAT mutated plasmid was introduced into HeLa cells.
Activation of the human δ-globin gene promoter by restoring the CCAAT box. CCAAC site mutations (mtCCAAT and mt CCAAG) within the δ (−612 to +68)LUC plasmid were made by 2-step PCR site-directed mutagenesis. Transient transfection assays were performed in K562, MEL, and HeLa cell lines. LUC activity is relative to that of the SV40 promoter. The β promoter LUC plasmid used here spans sequences from −640 to +50 bp. Results are the mean ± SD of 3 independent experiments. *Difference (P < .05) from the wild-type (CCAAC) δ plasmid. (Because there is no or extremely low-level expression of β-globin gene in K562 cells,20 transfection of the βmtCCAAC plasmid was not performed).
Activation of the human δ-globin gene promoter by restoring the CCAAT box. CCAAC site mutations (mtCCAAT and mt CCAAG) within the δ (−612 to +68)LUC plasmid were made by 2-step PCR site-directed mutagenesis. Transient transfection assays were performed in K562, MEL, and HeLa cell lines. LUC activity is relative to that of the SV40 promoter. The β promoter LUC plasmid used here spans sequences from −640 to +50 bp. Results are the mean ± SD of 3 independent experiments. *Difference (P < .05) from the wild-type (CCAAC) δ plasmid. (Because there is no or extremely low-level expression of β-globin gene in K562 cells,20 transfection of the βmtCCAAC plasmid was not performed).
To further test the hypothesis that the CCAAT box is essential for regulation of adult β-like globin genes, we introduced a “T to C” transition (βmtCCAAC) into the β-globin promoter at −65 bp. The mutation of the CCAAT box in the β-globin promoter decreased the expression to only one fifth the level of the wild type in MEL cells (Fig 3).
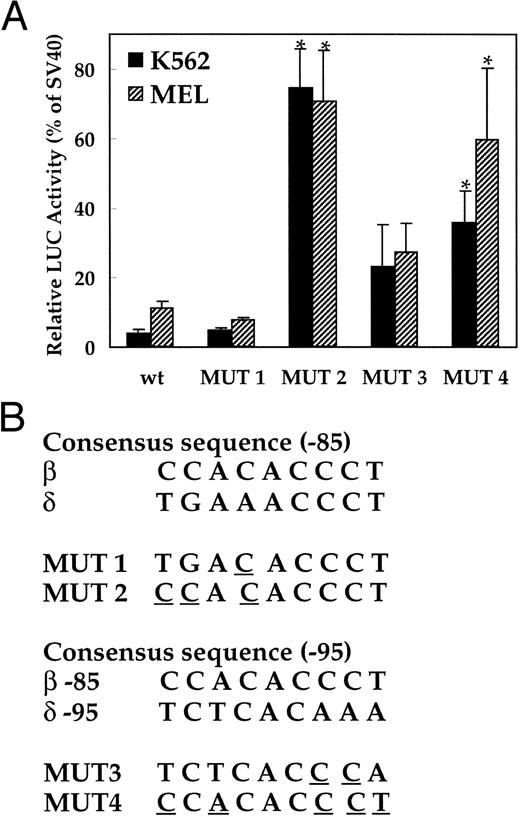
Insertions of the EKLF binding site into the human δ-globin gene.To investigate the role of the absence of transcription factor EKLF in the low-level expression of the δ-globin gene, two mutations were introduced at the −82 AAACCC motif in δ-globin promoter (Fig 4B). The first mutation (MUT 1) contains the core elements of the EKLF binding site (CACCC), and the second mutation (MUT 2) has a consensus nine-bp binding site. LUC activity of the plasmid with the nine-bp EKLF binding sequence was increased 26.8-fold and 6.5-fold in K562 and MEL cells, respectively, versus the wild-type δ sequence (P < .01, Fig 4A). In contrast, replacement of only the core element did not increase LUC activity. The sequence comparison suggested that the −95 position upstream region of δ could be a potential EKLF binding site, since it matches partially with the upstream CAC box of the β-globin gene (Fig 1B). Therefore, two more mutation plasmids (MUT 3 and MUT 4) were created with similar changes to the −85 position and transfected into K562 and MEL cells. LUC activity was significantly increased in plasmids containing EKLF binding sequences (Fig 4A) in both K562 and MEL cells.
Activation of the human δ-globin gene promoter by inserting EKLF binding sequences. LUC activity is relative to that of the SV40 promoter. (A) LUC activity of the mutant plasmids transfected into K562 cells and MEL cells. (B) Consensus sequence of the EKLF binding site and the mutation created in each plasmid. Since the EKLF binding site in the β-globin gene does not match well with the δ-globin gene sequence (Fig 1B), 2 segments of the δ-globin gene with partial matches to a CAC box (centered at about −85 and −95) were mutated as indicated and tested for effects on expression. The wt plasmid contains the −612 to +68 bp portion of the δ-globin promoter. *Difference (P < .05) from the wild-type plasmid.
Activation of the human δ-globin gene promoter by inserting EKLF binding sequences. LUC activity is relative to that of the SV40 promoter. (A) LUC activity of the mutant plasmids transfected into K562 cells and MEL cells. (B) Consensus sequence of the EKLF binding site and the mutation created in each plasmid. Since the EKLF binding site in the β-globin gene does not match well with the δ-globin gene sequence (Fig 1B), 2 segments of the δ-globin gene with partial matches to a CAC box (centered at about −85 and −95) were mutated as indicated and tested for effects on expression. The wt plasmid contains the −612 to +68 bp portion of the δ-globin promoter. *Difference (P < .05) from the wild-type plasmid.
DISCUSSION
The object of this study was to identify cis-acting elements through which transcription factors can regulate the human δ-globin gene and eventually use this information in gene therapy for the hemoglobinopathies. On the basis of sequence comparisons, and functional and mutational assays of different promoter regions of the δ-globin gene, we demonstrated that the absence of the EKLF binding site and the mutated CCAAT box account for the low δ-globin gene expression in erythroid cells. Plasmids used for transfection experiments did not contain locus control region (LCR) sequences. The presence of LCR sequences would increase the level of expression of all the plasmids. It would also artificially provide positive cis-acting sequences that could complement some of the defects in the δ-globin promoter. Thus, it would appear more advisable to study the restoration of a debilitated promoter without the potential complications of the LCR's strong positive regulator nearby on the plasmids.
The restoration of a CCAAT box from the CCAAC box in the δ-globin promoter increased expression in both K562 cells and MEL cells. These gain-of-function results are consistent with the 10-fold decrease in expression of the rabbit β-globin gene and threefold to sevenfold decrease in expression of the mouse βmajor-globin gene by single- or double-base mutations in the canonic CCAAT box.17 These data support the hypothesis that the altered CCAAC box in the δ promoter contributes to the low level of δ-globin expression. Although restoration of the CCAAT box (T → C) activates the δ-globin promoter, the level of elevated δ-globin promoter is only one fifth that of the β-globin promoter in MEL cells. Furthermore, mutation of the CCAAT box in the β-globin promoter still leaves one fifth the activity of the wild-type β-globin promoter, comparable to that of the elevated δ-globin promoter. These data suggest that other factors such as EKLF must be involved in the regulation of δ-globin expression besides CP1. It is of interest that the increase in LUC activity mediated by the mutant CCAAT box is erythroid-specific, suggesting that an erythroid-specific cofactor may play a role in K562 and MEL cell globin gene expression.
Comparison of human β- and δ-globin gene promoter sequences showed striking differences in the nuclear protein-binding sites, including the β-globin CCAAT and CACCC box (EKLF ) site in the proximal region. EKLF provides a crucial transactivation function for β-globin expression, and mutations in its binding site lead to a β-thalassemia phenotype.19 Embryonic mice deficient in EKLF by gene targeting die of anemia during fetal liver erythropoiesis and show hematologic features of β-thalassemia.24 EKLF is also a developmental stage-enriched protein that is involved in human γ- to β-globin gene switching and preferentially activates human β-globin gene expression.25 Thus, the phylogenetic sequence analysis along with experimental analysis of expression results implicate the EKLF binding site and other sequences further 5′ as important for high-level expression of the β-globin gene. Our mutagenesis experiments demonstrated that restoration of an EKLF binding site in the δ-globin promoter boosts expression in both K562 cells and MEL cells. These data are consistent with the positive effect of the erythroid-specific factor EKLF on the expression of adult β-like globin genes, and they indicate that the absence of its binding site contributes to the decreased level of expression of the δ-globin gene. Recently, Donze et al26 showed in an independent investigation that restoration of the EKLF binding site (−95) increased expression of the δ-globin gene. Based on our collective findings, it would be of interest to determine if a double “corrected” mutant (CCAAT and EKLF binding sites) provides an even greater level of expression than in the single correction.
Analysis of 5′ deletion mutants demonstrated that both negative and positive regulatory regions exist in the human δ-globin gene. A weak negative regulatory activity in the region from −612 to −458 bp correlates with a footprint centered at about −535 bp. The positive regulatory region between −458 and −325 bp contains a binding site for the erythroid-specific factor GATA-1. No obvious alteration of transcription was observed when the sequence between −325 and −231 bp was deleted. The GATA-1 binding site in the proximal δ-globin gene promoter has been demonstrated to be important in δ-thalassemia. The T → C substitution in δ0-thalassemia at position −77 bp (upstream to the CCAAC motif ) is located within the sequence 5′-TTATCT-3′ (changed to 5′-TCATCT-3′ ), which is the reverse complement of the binding site for GATA-1 (5′-AGATAA-3′ ). Studies have indicated that the T → C substitution contributes to the impaired expression of the δ-globin gene by affecting the GATA binding site.27 The difference between β- and δ-globin gene sequences is also apparent in IVS II. Baseline expression of human chimeric β-globin genes containing δIVS II in place of βIVS II was dramatically decreased when stably transfected into MEL cells.28 These results suggest that the IVS-II of the δ-globin gene also contributes to the reduction of its expression, as well, by mechanisms that are not yet clear.
In conclusion, we have shown that the altered CCAAT box (CCAAC) and the absence of an EKLF binding site in the promoter are at least partially responsible for the low level of δ-globin gene expression. Moreover, several regulatory elements within the region that extends 612 bp upstream of the promoter are involved in the modulation of erythroid cell–specific expression, albeit low, of the human δ-globin gene. The roles of the CCAAC box and the (absent) CACCC motif in the expression of the δ-globin gene remain to be confirmed directly in primary adult erythroid cells or in a transgenic animal model. These studies should permit quantitative determination of the relative contribution of the mutated CP1 and EKLF binding sites, as well as the IVS II, to the low-level expression of the δ-globin gene in adult erythroid cells.
ACKNOWLEDGMENT
We thank Dr Webb Miller for providing the sequence alignments, Dr Alan N. Schechter for helpful discussions and support, and Dr Steven G. Shapiro for careful reading and discussion of the manuscript.
Supported in part by National Institutes of Health Grants No. DK27635, LM05773, and LM05110 (R.C.H.).
Address reprint requests to Griffin P. Rodgers, MD, Laboratory of Chemical Biology, NIDDK, NIH, Bldg 10, Room 9N318, Bethesda, MD 20892-1822.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal