Abstract
The native form of soluble c-kit ligand (KL) is a noncovalent dimer. We have isolated a soluble, disulfide-linked dimer of murine KL (KL-CD) by expressing KL in Escherichia coli and refolding the denatured protein under conditions that promote the formation of both noncovalent dimers (KL-NC) and KL-CD. KL-CD exhibits a 10- to 15-fold increase in the ability to stimulate the growth of both the human megakaryocytic cell line MO7e and murine bone marrow-derived mast cells relative to KL-NC. Colony-forming assays of murine bone marrow progenitor cells also reflected this increased potency. However, KL-CD and KL-NC are equally able to prime mast cells for enhanced IgE-dependent degranulation in vitro and activate mast cells in vivo. Improving the growth-promoting activity of KL without changing its mast cell activation potential suggests that KL-CD or a related molecule could be administered in the clinic at doses that stimulate hematopoietic recovery while avoiding significant mast cell activation.
KL, THE LIGAND FOR the c-kit proto-oncogene, plays an essential role in hematopoiesis, gametogenesis, and melanogenesis.1 In the hematopoietic system it plays a particularly important role in the development of both erythroid and mast cell lineages. A role in the development of other cell lineages has been demonstrated through its growth and differentiation activity in vitro in combination with other hematopoietic cytokines. Of particular interest is the ability of KL to stimulate early stem cells and multipotential progenitors. It is because of this and other biologic activities that KL is also known as stem cell factor, mast cell growth factor, or steel factor, in recognition of its genetic locus in the mouse.2-7
KL shares many characteristics with macrophage colony-stimulating factor (M-CSF )8,9 and the Flt-3/Flk-2 receptor ligand (FL).10 Like M-CSF and FL, there are multiple forms of KL that are generated from alternative transcripts that encode transmembrane proteins. The most abundant form (KL-1) has a proteolytic cleavage site within the extracellular domain at amino acids 164-165 and is readily cleaved to a soluble protein of 30 to 35 kD.11-13 The second form of KL (KL-2) is derived from a message in which the exon encoding the proteolytic cleavage site has been spliced out, resulting in a protein that remains primarily associated with the cell surface. Although all of the ligands of this family, which also includes platelet-derived growth factor (PDGF ), exist as dimers, soluble KL (KL-1) as well as the Flt-3/Flk-2 ligand differ in that they lack an intermolecular disulfide bond, resulting in dimers associated through noncovalent interactions.2,10 14
Mechanisms by which KL activates its cell surface receptor c-kit are thought to be similar to those involved with other PDGF receptor family members.15 Like the PDGF-R, c-kit has five extracellular Ig-like domains, a transmembrane domain, and a highly conserved tyrosine kinase domain that is interrupted in this family of receptors by a nonhomologous stretch of amino acids.16,17 It has been proposed that cellular activation by the PDGF family of receptors is due to the binding of individual subunits of the dimeric ligand to separate receptor molecules that either leads to the formation of and/or stabilization of receptor dimers. The available biochemical and genetic evidence suggests that c-kit activation also results from a similar ligand-induced dimerization or oligomerization.18-20
The therapeutic potential of recombinant KL was suggested by the phenotype of W and Sl animals and by its efficacy in several preclinical animal models. Administration of KL to rodents at doses of 100 to 200 μg/kg/d led to significant increases in platelets, reticulocytes, and white blood cells and to a dramatic increase in the number of circulating progenitor cells.21-23 KL was also found to protect against a lethal dose of irradiation in mice.24 Studies in primates demonstrated an important dose-response effect of KL.25 KL had little effect on the hematopoietic system at doses of 10 to 25 μg/kg/d, but significant effects were seen at 50, 100, and 200 μg/kg/d. Furthermore, the effects of KL have been shown to be exerted on cells with long-term repopulating activity.26
Despite the encouraging animal studies, the therapeutic potential of native KL may be limited by its acting as a mast cell priming factor or secretagogue.27-29 In vitro, KL primes human lung and skin mast cells by enhancing their release of histamine and leukotrienes when triggered through the high-affinity IgE receptor.27,28 In mice, KL triggers mast cell degranulation in vivo without any apparent requirement for a second stimulus such as IgE and antigen.30 When KL is injected intradermally into rodents, local mast cells are activated and mediators are released that lead to vascular leakage and local swelling. In phase 1 clinical trials of KL administered to patients undergoing chemotherapy, a significant number of patients experienced serious side effects that were apparently related to the systemic activation of mast cells immediately after the KL therapy.31,32 Although patients who received lower doses of KL (<25 μg/kg/d) exhibited minimal side effects, this dose has little effect on stimulating hematopoietic recovery, although significant mobilization of progenitor cells to the peripheral blood was demonstrated.33 34
In this report, we describe a novel recombinant form of KL that contains at least one intermolecular disulfide bond. This modified KL displays increased growth factor activity but no increase in mast cell activation and may therefore be a more suitable candidate for clinical application.
MATERIALS AND METHODS
Expression and purification of KL-NC and KL-CD. A truncated mouse KL cDNA encoding amino acids 1-164 plus an N-terminal methionine was expressed in Escherichia coli using a phage λ PL promoter system.3 A typical protocol for the expression and purification of KL-CD is as follows. A 15-L culture of E coli strain DH5-α (GIBCO-BRL, Gaithersburg, MD) containing this vector was grown to mid log phase at 28°C and then shifted to 42°C to induce expression. The cells (80 g wet weight) were harvested, incubated for 10 minutes at room temperature in 400 mL wash buffer (50 mmol/L Tris-HCl, pH 7.4, 200 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L phenylmethyl sulfonyl fluoride [PMSF ]) plus 1 mg/mL lysozyme, and disrupted by sonication. Inclusion bodies containing insoluble KL were isolated by centrifugation at 10,000g for 20 minutes at 4°C and then washed three times by centrifugation and resuspension in 150 mL wash buffer. Inclusion bodies were solubilized by stirring for 1 hour at 4°C in 300 mL of deionized 6 mol/L urea, and insoluble material was removed by centrifugation for 20 minutes at 10,000g. KL was refolded by dialyzing the supernatant using 6,000 to 8,000 molecular weight cutoff Spectra/Por membrane (Spectrum Houston, TX) against 6 L of 20 mmol/L Tris, pH 8.5, at 4°C for 48 to 72 hours with 3 changes of dialysis buffer. Insoluble material was removed by centrifugation at 10,000g for 20 minutes, and the soluble protein was applied at a flow rate of 6 mL/min to a Waters (Milford, MA) Delta-Pak C18 25 × 100 mm column previously equilibrated with 25% n-propanol/0.1 mol/L ammonium acetate, pH 6.0. The column was washed with equilibration buffer, and proteins were eluted with the following gradient: 25% to 36% n-propanol from 0 to 15 minutes, 36% n-propanol from 15 to 25 minutes, 36% to 39% n-propanol from 25 to 45 minutes, and 39% n-propanol from 45 to 60 minutes. After this step, KL-NC is greater than 95% pure as assessed by high-performance liquid chromatography (HPLC)-gel filtration chromatography monitored at A215 . Fractions from the C18 column containing active KL-CD and some contaminating inactive KL-CD were pooled, diluted threefold with equilibration buffer (50 mmol/L Tris-HCl, pH 7.0), and loaded onto 15 mL of Q-Sepharose Fast Flow (Pharmacia, Piscataway, NJ) resin. Proteins were eluted with a step-gradient (equilibration buffer containing 25, 50, 100, 150, 200, and 500 mmol/L NaCl) and active KL-CD was eluted at 50 to 100 mmol/L NaCl, whereas inactive KL-CD was eluted at 150 to 200 mmol/L NaCl. Purified KL-NC and active KL-CD (>95% purity as assessed by HPLC-gel filtration chromatography and sodium dodecyl sulfate-polyacrylamide gel electrophoresis [SDS-PAGE]) were dialyzed into phosphate-buffered saline (PBS) and depleted of endotoxin by Detoxigel (Pierce, Rockford, IL) chromatography. Endotoxin levels were less than 1 endotoxin unit per milligram of protein as determined by the Limulus Amebocyte Lysate assay (BioWhittaker, Walkersville, MD). Protein concentrations were determined using the BCA reagent (Pierce) and verified by amino acid analysis.
Peptide mapping. KL was lyophilized, resuspended in 25 mmol/L Tris, pH 8.5, and digested for 24 hours at 37°C at a 30:1 mass ratio of KL:endoproteinase Asp-N (Boeringer Mannheim, Indianapolis, IN). After digestion, a portion of the sample was incubated with 10 mmol/L dithiothreitol (DTT) for 15 minutes at 50°C to reduce disulfide bonds and then treated with 20 mmol/L iodoacetamide for 10 minutes at room temperature to alkylate the free cysteine sulfhydryls and prevent reformation of disulfides. The fully reduced and nonreduced peptide digests were resolved at 0.2 mL/min and monitored by UV absorbance at 215 nm using a 2.1 × 250 mm C18 reverse-phase HPLC column (Vydac Inc, Hesperia, CA) with an acetonitrile (ACN)/0.1% trifluoroacetic acid (TFA) gradient (5% ACN from 1 to 15 minutes, 5% to 30% ACN from 15 to 25 minutes, 30% to 40% ACN from 25 to 85 minutes, and 40% to 50% ACN from 85 to 95 minutes). For isolation and sequencing of peptides, 100 μg of nonreduced KL-NC digest was injected on the column, and the two peaks containing disulfide-linked peptides were collected. Each dipeptide peak was reduced with DTT and alkylated with iodoacetamide, and the two resulting cysteine-containing peptides were isolated by C18 chromatography. These isolated peptides were then identified by protein sequencing, which was performed by the Molecular Biology Core Facility at the Dana Farber Cancer Institute (Boston, MA).
Cell proliferation assays. The human factor-dependent megakaryocytic cell line M07e35 was maintained in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF; 0.5 ng/mL; R&D Systems, Minneapolis, MN) in supplemented RPMI-1640 + 10% calf serum (HyClone, Logan, UT) as described.2 Murine bone marrow-derived mast cells (BMMC) were established from the bone marrow of C57/Bl6 × DBA2 F1 mice (Charles River Laboratories, Wilmington, MA) and then maintained for up to 3 months in the presence of interleukin-3 (IL-3).2 For the proliferation assays, the cells were washed two times to remove their maintenance growth factor and plated into 96-well plates. KL samples were added and serially diluted and the cells were incubated at 37°C for 16 hours for the MO7e cell line and for 20 hours for BMMC. The cells were then pulsed with 2.5 μCi/mL of 3H-Thymidine for 4 hours and harvested onto glass fiber filters, and the amount of 3H-thymidine (cpm) on the filter was determined by scintillation counting. Commercial sources of human and mouse KL used for some assays were R&D Systems and Genzyme (Cambridge, MA).
Bone marrow colony assays. Bone marrow cells were flushed from the femurs of 2- to 4-month-old female BDF-1 mice. Cells were washed once and plated in triplicate in supplemented McCoy's 5a medium (sodium bicarbonate [0.045%], sodium pyruvate [1 mmol/L], minimum essential medium eagle (MEM) nonessential amino acids (0.6×), MEM essential amino acids (0.4×), L-glutamine (1.2 mmol/L), and MEM vitamins [0.6×]; JRH Biosciences, Lenexa, KS) with 10% fetal calf serum in the presence of 0.3% agar in 35-mm petri dishes plus KL-CD or KL-NC as the only exogenous source of cytokine. After 7 days of incubation at 37°C in 95% air/5% CO2 , colonies (>40 cells) were enumerated.
Mobilization of progenitors (colony-forming unit–granulocyte-macrophage [CFU-GM]) in vivo. Osmotic minipumps (model 2001; Alza Corp, Palo Alto, CA) were filled with KL-NC or KL-CD in PBS + bovine serum albumin (BSA) (0.1%) at concentrations that, according to the manufacturer's instructions, would deliver 30 or 100 μg/kg/d or diluent alone. Female BDF1 mice were anesthetized locally with lidocaine and pumps were implanted subcutaneously over the back. Six days after implantation, animals were killed using CO2 asphyxiation. Blood was collected by cardiac puncture into a heparanized syringe and spleens were removed and disaggregated between frosted ends of sterile slides. Blood cell counts were obtained with a hematology analyzer (Sysmex, Kobe, Japan). Unseparated blood and spleen cells were plated in triplicate at 1 × 105 and 2 × 104 or 5 × 104 and 1 × 104 cells/mL, respectively, in the presence of murine IL-3 (mIL-3; 5 ng/mL), mGM-CSF (2 ng/mL), and mKL (20 ng/mL) under standard CFU-GM culture conditions as described above.
Competitive binding assay. Competitive displacement of 125I–KL-NC by unlabeled KL-NC and KL-CD was measured on the MO7e cell line or murine BMMC. KL-NC was labeled with 125I to high specific activity with chloramine T3. Cells were first incubated with a mixture of metabolic inhibitors (50 mmol/L 2-deoxy-D-glucose, 15 mmol/L NaN3 , and 1 mg/mL BSA in PBS) to prevent ligand internalization. Serial dilutions of KL-NC and KL-CD were made and overlaid onto a mixture of 84% silicone oil and 16% paraffin oil followed by the addition of 125I-KL to a final concentration of 100 pmol/L. The treated cell suspension (2 × 105 cells/tube) was then added to each reaction tube which had been previously equilibrated to 37°C and incubated for 20 minutes. Cell-associated and cell-free radioactivity were separated by centrifugation through the silicon/paraffin oil mixture and each was measured by solid scintillation. The results represent the average of two independent determinations, with the inhibition constants calculated by standard methods.36
Mast cell activation. Primary cultures of murine mast cells were derived either in IL-3 (10 ng/mL) as described2 and used for priming assays in combination with IgE and antigen or were derived in the presence of KL (50 ng/mL) and IL-3 (10 ng/mL) and used for assays in which KL alone was used to activate the cells. The cells for the priming assays were washed one time and resuspended in modified Tyrode's buffer (10 mmol/L HEPES, 137 mmol/L NaCl, 2.7 mmol/L KCl, 0.4 mmol/L Na2HPO4 , 5.6 mmol/L glucose, 1.8 mmol/L CaCl2 , 1.3 mmol/L MgSO4 , and 0.5% fatty acid free BSA; Sigma Chemical Co, St Louis, MO) and incubated with a saturating concentration of ascites from the IgE anti-TNP hybridoma (IgELA2, ATCC #TIB 142) for 60 minutes at 37°C. Cells were then washed and resuspended at a concentration of 1 × 106 cells/mL and 100 μL was plated into 96-well V-bottom plates. KL was then added and the plates were incubated for 10 minutes at 37°C. TNP-BSA, prepared as described,37 was added to a final concentration of 10 ng/mL and the cells were incubated at 37°C for an additional 10 minutes. Plates were then centrifuged for 5 minutes at 150g and 20 to 30 μL of supernatant was removed to assay for β-hexosaminidase using standard methods38 as a measure of degranulation. Controls were run in each experiment to determine the unstimulated (no KL or TNP-BSA added) as well as the total hexoseaminidase content of the cells corresponding to 100% release where cells were lysed by the addition of 1% Triton-X 100. A standard curve was derived by setting the unstimulated as 0% release and the Triton-X 100 sample as 100% release.
For experiments in which KL alone was used to activate the mast cells, KL/IL-3–cultured cells were used on days 4 through 7 after feeding when cell densities had plateaued, reaching 1 to 4 × 106 cells/mL, and c-kit expression was the highest (data not shown). The cells were washed two times and resuspended in modified Tyrode's buffer and plated into 96-well V-bottom plates for activation. Plates were equilibrated at 37°C for 15 minutes and then KL-NC or KL-CD was added and plates were incubated at 37°C for an additional 20 minutes before β-hexoseaminidase release was determined as described above.
Mouse ear cutaneous anaphylaxis. Activation of mast cells in vivo by KL-CD and KL-NC was measured by intradermal injection into the ear with modifications of a method described previously.30 Two- to 4-month-old CD-1 mice were injected via the tail vein with 100 μL of Evan's Blue dye (5 mg/mL) in PBS. One hour later, the animals were lightly anesthetized by exposure to ether for 45 to 60 seconds and 20 μL of KL-CD or KL-NC at various concentrations was injected into the right ear, whereas PBS as the diluent control was injected into the left ear. Thirty minutes later, the animals were killed and the ears were scored visually on a scale (−, +,++,+++, or ++++) based on the blue color of the ear. The scorer was blinded to the treatment of each group of mice. A score of + was a very mild blueing that was detectable when compared with the control left ear. Scores of ++ indicated a readily detectable blueing but not very intense coloration. A score of +++ was distinctly stronger than ++, and a score of ++++ was the maximum blueing that was seen at the highest doses of KL that were tested. Little coloration of the control (left) ear was seen at all of the doses tested.
RESULTS
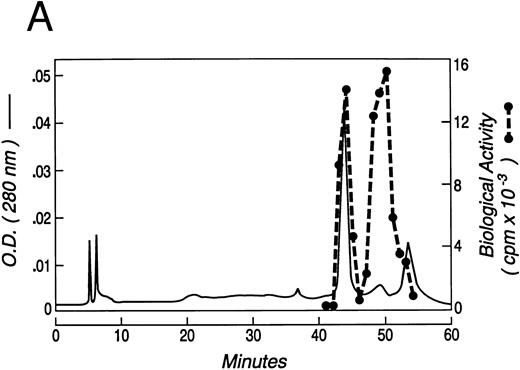
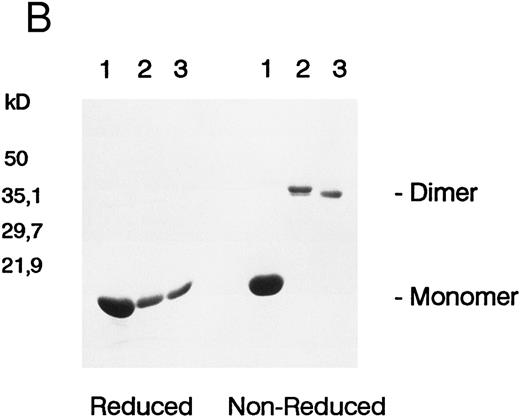
Recombinant murine KL was expressed containing amino acids 1-164 of the sequence of native soluble KL-1 plus an additional N-terminal methionine required for bacterial expression. The recombinant KL was solubilized from E coli inclusion bodies, refolded by dialysis, and purified by reverse-phase HPLC. As seen in Fig 1A, which is representative of four different refolded preparations of KL, two main peaks of growth factor activity, eluting at 43 minutes (peak 1) and 49 minutes (peak 2), and a small amount of activity in a third peak eluting at 54 minutes (peak 3), from a C18 reverse-phase column. As determined by SDS-PAGE under reduced conditions, peaks 1, 2, and 3 all contained a protein with an apparent molecular weight of 18 kD (Fig 1B), which is the appropriate size for KL based on the cDNA sequence. However, when examined by SDS-PAGE under nonreduced conditions, the KL in peaks 2 and 3 migrated with apparent molecular weights of 36 kD, whereas KL from peak 1 remained approximately 18 kD. The molecular weights of nonreduced KL from peaks A and B were confirmed to be 18,440 and 36,860 Daltons, respectively, by laser desorption/time of flight mass spectroscopy (not shown). The difference in size under reduced and nonreduced SDS-PAGE indicates that peaks 2 and 3 contain dimeric KL linked by at least one intermolecular disulfide bond. We have therefore termed these forms KL-CD and KL-CD′, respectively, for KL-covalent dimer. Peak 1 migrates as native KL would be expected as a noncovalently associated dimer and thus is referred to as KL-NC for KL-noncovalent.
(A) Chromatogram of murine kit ligand separated on C18 reverse-phase HPLC ( — ). Proliferation of the MO7e cell line is also shown (- - -) at a dilution at which both active peaks are titrating out. (B) SDS-PAGE of fractions from the three peaks of KL protein run under reduced and nonreduced conditions.
(A) Chromatogram of murine kit ligand separated on C18 reverse-phase HPLC ( — ). Proliferation of the MO7e cell line is also shown (- - -) at a dilution at which both active peaks are titrating out. (B) SDS-PAGE of fractions from the three peaks of KL protein run under reduced and nonreduced conditions.
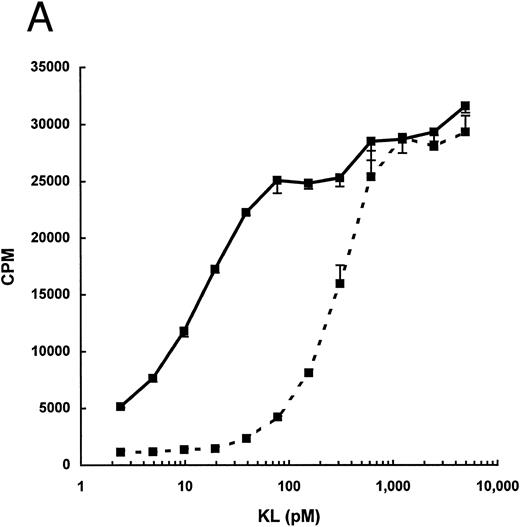
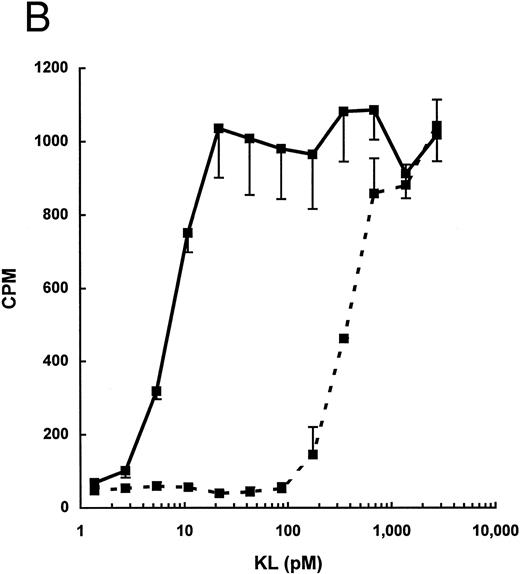
As seen in Fig 1, the total biologic activity of KL-CD (peak 2) was nearly equivalent to that of KL-NC (peak 1), yet the total protein concentration was less than 10% of peak 1. We therefore evaluated the specific activity of purified KL-CD and KL-NC by measuring their ability to stimulate 3H-thymidine incorporation by the human factor-dependent cell line MO7e (Fig 2A) as well as by murine BMMC (Fig 2B). The ED50 for KL-CD was 5 to 25 pmol/L (0.2 to 1 ng/mL, based on a predicted molecular mass of 36,734 Daltons). The specific activity of KL-NC was similar to commercially available recombinant KL from both human and mouse, ie, 140 to 280 pmol/L (approximately, 5 to 10 ng/mL, based on a molecular weight of the dimer of 36,734 Daltons). Therefore, KL-CD is 10- to 20-fold more potent than KL-NC or native KL.
Dose-response for proliferation of (A) the human megakaryocytic cell line MO7e or (B) murine BMMC. 3H-Thymidine incorporation is expressed as the mean ± standard deviation of three points and is derived from one representative experiment for each cell line/culture.
Dose-response for proliferation of (A) the human megakaryocytic cell line MO7e or (B) murine BMMC. 3H-Thymidine incorporation is expressed as the mean ± standard deviation of three points and is derived from one representative experiment for each cell line/culture.
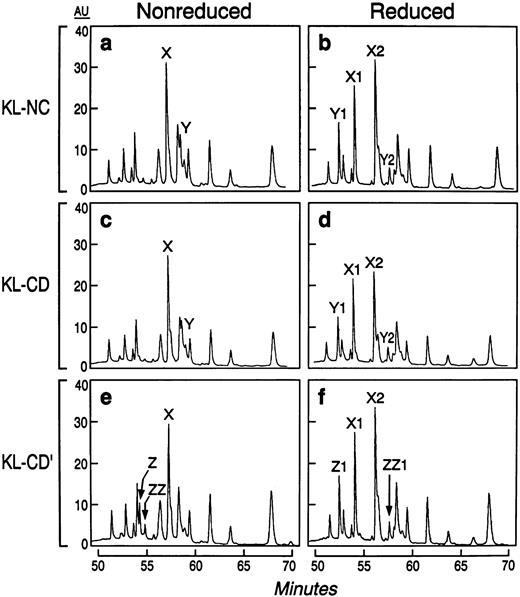
We further characterized the nature of the covalent and noncovalent dimers by examining disulfide bond pairing through peptide mapping techniques. A reverse-phase HPLC analysis of the reduced and nonreduced endoproteinase AspN-treated digests established the identity of the disulfide-bonded peptides. The maps of KL-NC and KL-CD were identical under reduced and nonreduced conditions (Fig 3), indicating that the same cysteine pairs are present in these two forms of KL. The peptide map of KL-CD′ differed in that it lacked one of the disulfide-linked peptide peaks found in KL-NC and KL-CD (labeled Y), but possessed two unique disulfide linked peptides not found in the others (labeled Z and ZZ). The cysteine-linked di-peptide peaks of KL-NC were reduced and isolated for N-terminal sequence analysis (not shown). These data show that murine KL-NC has the same two cysteine pairs found in human KL39 by exhibiting a disulfide bond between Cys4-Cys89 and Cys43-Cys138. Because KL-CD and KL-NC have identical peptide maps, KL-CD must have at least one of the cysteine pairs in an intermolecular rather than an intramolecular configuration.
Peptide maps of KL-NC, active KL-CD, and inactive KL-CD after treatment with endoproteinase AspN under reduced and nonreduced conditions. Peptides labeled X, Y, and Z under nonreduced conditions give rise to peptides X1, X2, Y1, Y2, Z, and ZZ1 after reduction, respectively.
Peptide maps of KL-NC, active KL-CD, and inactive KL-CD after treatment with endoproteinase AspN under reduced and nonreduced conditions. Peptides labeled X, Y, and Z under nonreduced conditions give rise to peptides X1, X2, Y1, Y2, Z, and ZZ1 after reduction, respectively.
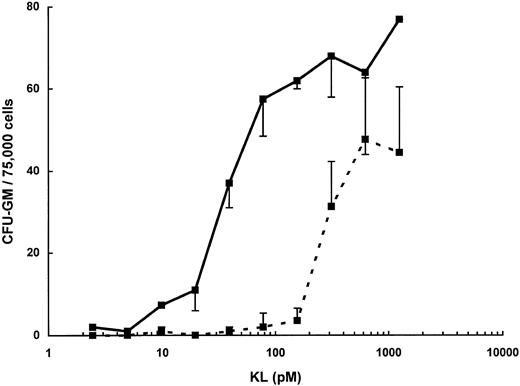
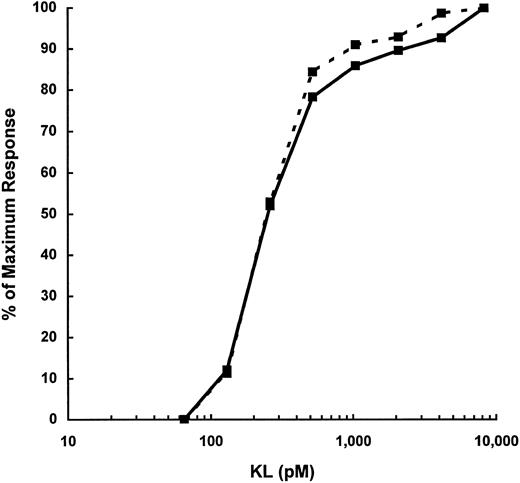
The increased potency of KL-CD relative to that of KL-NC is also seen in colony-forming assays of murine bone marrow progenitors (Fig 4). KL supports the development of CFU-GM when murine bone marrow cells are stimulated in the presence of fetal calf serum in the absence of any exogenous cytokines.40 Both KL-NC and KL-CD stimulated the growth of CFU-GM in this assay, but KL-CD was more potent, with an ED50 of 30 pmol/L and 280 pmol/L for KL-NC. Additionally, the maximum number of colonies observed with KL-CD was 30% to 40% greater than the maximum number observed with KL-NC.
CFU-GM assay of murine bone marrow cells stimulated with KL-NC (- - -) or KL-CD ( — ) for 7 days. Data are expressed as the mean number of colonies per 75,000 cells ± standard deviation from three replicate plates.
CFU-GM assay of murine bone marrow cells stimulated with KL-NC (- - -) or KL-CD ( — ) for 7 days. Data are expressed as the mean number of colonies per 75,000 cells ± standard deviation from three replicate plates.
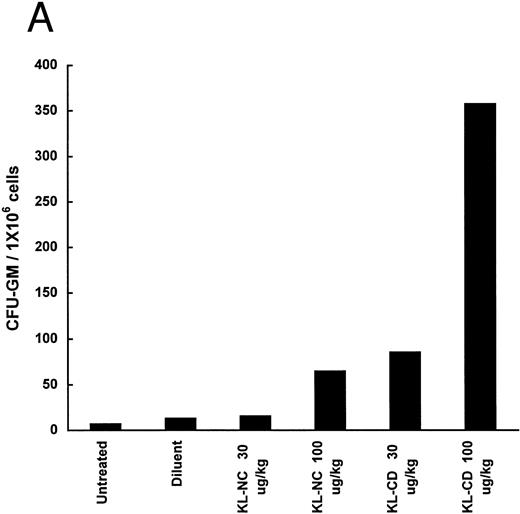
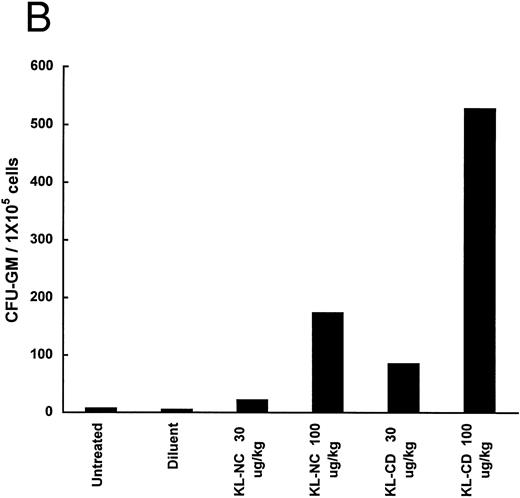
The ability of KL-NC and KL-CD to stimulate hematopoietic progenitors in vivo was examined by measuring the mobilization of progenitors to the peripheral blood and spleens of animals treated by subcutaneous continuous infusion. The number of CFU-GM per 1 × 106 or 1 × 105 cells was increased in both the blood and the spleen 6.7- and 18.6-fold, respectively, at 30 μg/kg/d of KL-CD and 18.6- and 115-fold, respectively, at 100 μg/kg/d (Fig 5). KL-NC at 30 μg/kg/d led to only 1.2- and 4.6-fold increases, respectively, and at 100 μg/kg/d gave 5.3- and 37-fold increases. There was little effect on the total number of cells in the blood or the spleens except in the spleens of animals receiving KL-CD at 100 μg/kg/d, in which there was a mean 1.9-fold increase in 6 days.
Mobilization of progenitors (CFU-GM) in mice by continuous infusion of KL-NC or KL-CD. CFU-GM were quantitated in (A) peripheral blood cells or (B) spleen cells of untreated mice or mice implanted with pumps delivering diluent (PBS + BSA), KL-NC, or KL-CD at 30 or 100 μg/kg/d for 6 days. The results are the mean of 3 plates/mice from 2 mice/group and are given as the number of CFU-GM/1 × 106 nucleated blood cells or CFU-GM/1 × 105 nucleated spleen cells plated, respectively.
Mobilization of progenitors (CFU-GM) in mice by continuous infusion of KL-NC or KL-CD. CFU-GM were quantitated in (A) peripheral blood cells or (B) spleen cells of untreated mice or mice implanted with pumps delivering diluent (PBS + BSA), KL-NC, or KL-CD at 30 or 100 μg/kg/d for 6 days. The results are the mean of 3 plates/mice from 2 mice/group and are given as the number of CFU-GM/1 × 106 nucleated blood cells or CFU-GM/1 × 105 nucleated spleen cells plated, respectively.
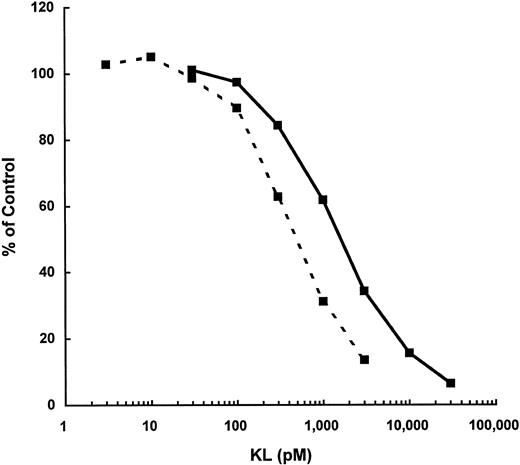
The affinity of KL-CD to the receptor, c-kit, was compared with KL-NC to see if that might explain the increased biologic activity of KL-CD. Competitive ligand displacement studies of 125I–mKL-NC were performed to compare KL-NC with KL-CD on MO7e cells. This analysis shows that KL-CD has a Ki of 1,050 versus 500 pmol/L for KL-NC on MO7e (Fig 6). A similar analysis on murine BMMC showed similar results, with a Ki of 900 pmol/L for KL-CD and 350 pmol/L for KL-NC. Therefore, the apparent affinity of KL-CD for either human or murine c-kit is twofold to threefold less than KL-NC.
Competitive displacement of 125I-KL binding to MO7e by KL-NC (- - -) or KL-CD ( — ). Data represent the mean of two replicate points at each concentration of KL.
Competitive displacement of 125I-KL binding to MO7e by KL-NC (- - -) or KL-CD ( — ). Data represent the mean of two replicate points at each concentration of KL.
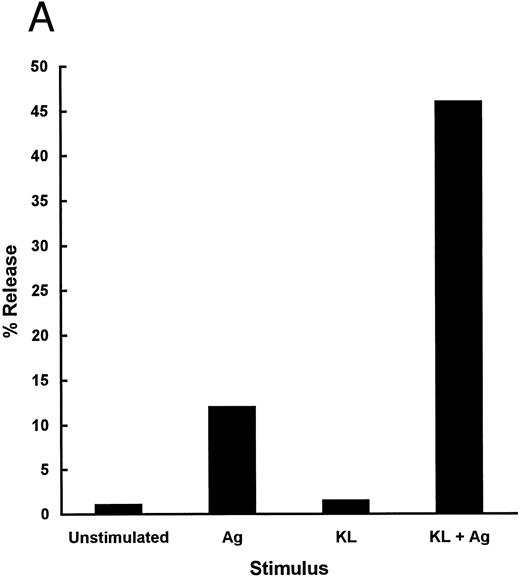
The mast cell activating potential of KL-CD was compared with KL-NC to determine whether other functional activities of KL-CD were also affected. Because IgE receptor-induced degranulation of human mast cells had been shown to be enhanced or primed by KL,27 we set up an assay using murine mast cells to compare the mast cell activating potential of KL-CD. This was performed using BMMC sensitized with IgE and then activated with antigen at 106 cells/mL. Under these conditions, 10% to 25% of the total secretory granule enzyme β-hexosaminidase was released from the cells (Fig 7A). Under these conditions, KL alone does not cause any detectable degranulation. However, the response to IgE and antigen is markedly enhanced when the cells are exposed to KL for 0 to 10 minutes before the addition of antigen. The level of degranulation is typically increased to 40% to 60% when the cells are exposed to KL for 5 minutes before the addition of antigen. KL-CD and KL-NC were compared in this assay and found to have similar ED50s of 20 to 40 pmol/L (0.7 to 1.4 ng/mL; Fig 7B). The magnitude of the priming affect was identical, with both molecules producing similar maximum degranulation at the highest concentrations tested.
(A) Priming of murine BMMC with KL before triggering with IgE + antigen. IgE-sensitized BMMC were primed for 5 minutes with control diluent or KL (100 ng/mL) and then activated with TNP-BSA. Data are expressed as the percentage of release of hexoseaminidase of two replicate points. (B) Dose-response of KL-CD ( — )and KL-NC (- - -) priming of BMMC activation. Data are expressed as a percentage of the maximal response achievable over the baseline. The baseline level of activation was 20.6% with IgE + antigen (TNP-BSA) alone and the maximal level of activation was 57.2% with KL + IgE + antigen. The data represent the mean of two points from one of three representative experiments.
(A) Priming of murine BMMC with KL before triggering with IgE + antigen. IgE-sensitized BMMC were primed for 5 minutes with control diluent or KL (100 ng/mL) and then activated with TNP-BSA. Data are expressed as the percentage of release of hexoseaminidase of two replicate points. (B) Dose-response of KL-CD ( — )and KL-NC (- - -) priming of BMMC activation. Data are expressed as a percentage of the maximal response achievable over the baseline. The baseline level of activation was 20.6% with IgE + antigen (TNP-BSA) alone and the maximal level of activation was 57.2% with KL + IgE + antigen. The data represent the mean of two points from one of three representative experiments.
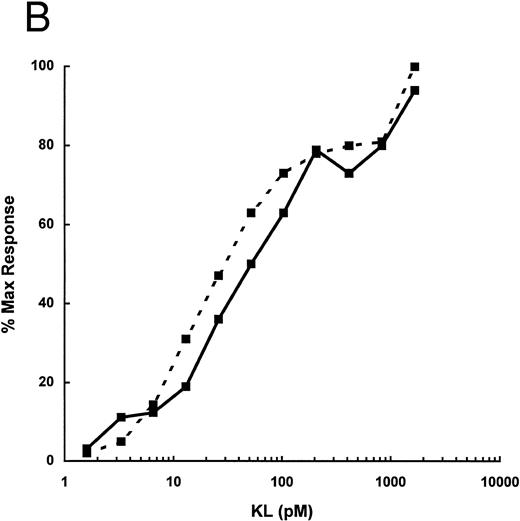
A second murine mast cell activation assay was established in which, under specific conditions, it was found that KL could lead to nearly complete degranulation of a BMMC population without the requirement for a second stimulus, such as IgE and antigen. Of particular importance is the finding that the cells are used when they are not actively growing and also important but not essential is the finding that the cells are initially derived in KL + IL-3 rather than the typical BMMC culture that is derived in IL-3 alone. KL/IL-3–derived BMMCs were stimulated with KL-NC or KL-CD and both were found to have nearly identical dose responses. Higher concentrations of KL-NC and KL-CD were required for activity in this assay, having an ED50 of 250 pmol/L, as compared with the mast cell priming assay with IgE and antigen with an ED50 of 20 to 40 pmol/L (Fig 8).
Activation of murine KL/IL-3 BMMC with KL-NC (- - -) and KL-CD ( — ) alone. Hexoseaminidase release was measured after stimulation of IL-3/KL–cultured BMMC. Data are expressed as the mean of two replicate points and are from one of three representative experiments. The maximum response in this experiment was 100% release.
Activation of murine KL/IL-3 BMMC with KL-NC (- - -) and KL-CD ( — ) alone. Hexoseaminidase release was measured after stimulation of IL-3/KL–cultured BMMC. Data are expressed as the mean of two replicate points and are from one of three representative experiments. The maximum response in this experiment was 100% release.
The in vivo mast cell degranulation activities of KL-CD and KL-NC were compared by observing the accumulation of intravenously administered dye into tissue at the site (right ears of CD-1 mice) of KL-CD or KL-NC challenge. The left ear was injected with diluent as a control. A titration of KL-CD and KL-NC from 30 to 0.1 μg/kg/site showed similar dose-response curves for the two molecules (Table 1).
Cutaneous Anaphylaxis in the Murine Ear Induced by Intradermal Injection
| Dose (μg/kg) . | KL-NC . | KL-CD . |
|---|---|---|
| 10 | ++++ | ++++ |
| 3 | ++++ | +++ |
| 1 | +++ | +++ |
| 0.1 | + | + |
| Dose (μg/kg) . | KL-NC . | KL-CD . |
|---|---|---|
| 10 | ++++ | ++++ |
| 3 | ++++ | +++ |
| 1 | +++ | +++ |
| 0.1 | + | + |
Data are expressed as the relative accumulation of Evan's blue dye in the ear due to edema formation 30 minutes after the injection of KL. Results are based on 2 to 3 animals per dose and are representative of two separate experiments.
DISCUSSION
These experiments show that KL-CD, a disulfide-linked dimer of KL, is 10- to 20-fold more potent than KL-NC in stimulating cellular proliferation, but no more potent than KL-NC in stimulating mast cell degranulation. The ability of KL-CD to stimulate hematopoiesis or mobilize progenitors in vivo is also enhanced, although the fold increase in potency is not as great as that seen in vitro. These results show that the proliferative and activation responses triggered by KL are distinct and suggest that modifications to the structure of the KL dimer differentially influences downstream signaling events mediated by c-kit.
Although a detailed tertiary structure of native KL has not been reported, the structure of a closely related cytokine, M-CSF, has been solved crystallographically.41 Based on the M-CSF crystal structure, soluble KL likely consists of four α-helices held in a defined conformation by two disulfide bonds located in positions analogous to those within M-CSF.42 Peptide mapping and SDS-PAGE showed that at least one of the disulfide pairs of KL-CD exists in an intermolecular, rather than an intramolecular, conformation. Based on the positions of the analogous cysteines in M-CSF, it is surprising that this disulfide arrangement results in a molecule that retains biologic activity, let alone has increased proliferative activity. The increased activity could be due to interaction of c-kit with amino acid residues of KL-CD that are not normally involved in the interaction of KL and the receptor. However, the increased activity of KL-CD is more likely due to the enhanced stability that the intermolecular disulfide bond confers on the KL dimer. The lower growth factor activity of KL-NC relative to KL-CD might therefore be due to dissociation of native KL-NC into monomeric subunits. The extent to which the native KL dimer dissociates into monomers under various conditions has recently been evaluated.43,44 It was shown with human KL that, at physiologic concentrations (<50 ng/mL), the majority of KL actually exists as a monomer. This is in sharp contrast to earlier studies that showed at high protein concentrations that KL is dimeric.2,14 Therefore, if only dimeric KL is responsible for the biologic activity, it is not surprising that a molecule that exists completely as a dimer would be more active than a molecule that at physiologic concentrations is only 10% to 20% dimeric.44
Even more surprising than its enhanced growth factor activity is the finding that KL-CD does not have enhanced mast cell activating properties, as assessed both in vitro and in vivo. The ability of KL to enhance mast cell secretion in response to cross-linking of IgE receptors27,28 is analogous to the priming effect of other hematopoietic growth factors on the functional responses and activation of mature leukocytes. For example, IL-3 is a growth and differentiation factor for basophil precursors and also enhances agonist-stimulated release of mediators from basophils.45 Although in vivo studies have shown that murine mast cells are fully degranulated after KL administration,30 initial in vitro studies showed that KL alone causes only a low level of mast cell secretion in the human28,46 and murine systems.29 Our second mast cell activation system using KL/IL-3–derived BMMC may be a useful model for the response to KL that is seen in vivo, because KL by itself leads to near complete degranulation of the cells. Further mechanistic studies remain to be performed to understand the basis for the different reponses seen from these two cell populations to KL alone, but preliminary results have shown that c-kit levels are significantly higher on the BMMC that respond to KL alone, in contrast to those that are only primed by KL (B.A.L. and K.H.N., data not shown). The reactivity of mast cells to KL alone in vivo could be due to a number of factors, possibly receptor number or state, but also the noncycling status of mast cells in the tissues, the type or maturation state of the cell, an effect of the tissue microenvironment, or the presence of a limiting amount of a second agonist, such as antigen and specific IgE.
The concentration at which various cytokines prime mature leukocytes and mast cells is generally much lower than the concentrations that are required for a proliferative effect. In addition to its effect on mast cell degranulation, sensitivity to low levels of KL in vitro has been observed in the chemotaxis of murine BMMC47 as well as for the activation of integrins on BMMC leading to their adhesion to fibronectin.48 49 The mechanisms that account for this difference may be related to the fact that activation responses such as degranulation, adhesion, and chemotaxis occur in a relatively short period of time, on the order of seconds to minutes. Proliferative responses to KL or other cytokines require an extended exposure to the cytokine for virtually the duration of the assay, on the order of many hours to days. KL-CD and KL-NC appear to be equipotent in mediating short-term responses such as mast cell priming. The dose-response curve for KL-CD and KL-NC for mast cell priming is similar to a proliferative dose response curve of KL-CD; however, the 10- to 20-fold lower activity of KL-NC in proliferation assays compared with its potency in the priming assay suggests that there is a deficiency in KL-NC's ability to stimulate or maintain a long-term response, except at significantly higher concentrations. Because KL-CD primes mast cells in vitro at doses equivalent to those that are required to stimulate proliferation, we have not dissociated these two activities in KL-CD. We favor the notion that we have corrected a defect in the ability of KL-NC to maintain a sustained response at low concentrations. In the experimental systems described above, KL-NC should exist primarily as a dimer in the greater than 1 μg/mL stocks that KL is stored at before addition to the assays. Therefore, in a short-term assay, the relative amounts of dimer in KL-NC and KL-CD may be very similar when the KL is first added to the assay. However, in the longer term assays, KL-NC would over time reach an equilibrium so that only a small proportion would remain as a dimer at low concentrations of KL. Because KL-CD would remain as a dimer, it would retain full activity in both short- and long-term reponses.
We50 and others51 have found that a covalent dimer of human KL (amino acid 1-165) can be formed by methods similar to those reported here, although the yield is particularly low. Alternative methods for efficiently producing a covalent dimer of human KL that has a biologic activity profile similar to that which we observed for murine KL-CD are being pursued (Nocka et al, manuscript in preparation). The ability of KL to promote the survival and proliferation of immature hematopoietic stem cells and progenitors,6 to mobilize stem cells from the bone marrow to the peripheral blood,25 and to support the differentiation of erythroid,2 granulocytic,40 and megakaryocytic progenitors52 in the presence of lineage-restricted cytokines suggests that KL could be used as a therapeutic in a variety of clinical settings. However, because doses of KL-NC that significantly stimulate hematopoiesis in vivo overlap with those doses that lead to unacceptable mast cell-related toxicity in humans, these favorable activities of KL cannot at present be safely exploited. Although low-dose KL in combination with other cytokines (ie, G-CSF ) is one potential application in which KL may safely be administered,34 53 the full potential of KL may not be realized at these doses. Although it is difficult to measure the actual concentration of KL that is reponsible for mast cell activation in vivo, our in vitro mast cell activation assay using KL/IL-3–derived BMMC suggests that higher concentrations of KL-CD are required for activation than are required to support proliferation. Therefore, KL-CD, a human analogue of KL-CD, or a molecule with similar characteristics, might have greater therapeutic potential than native KL. The activity profile of KL-CD suggests that this molecule might effectively enhance hematopoiesis at doses below the limiting toxic dose. Studies in primates and humans remain to be performed to demonstrate whether the beneficial properties of KL-CD will hold up in a clinical setting.
ACKNOWLEDGMENT
The authors thank Dr Fangming Zuo for his expert assistance with the animal studies, and Drs Malcolm Moore, George Demetri, Richard Fisher, and Tom Beck for their helpful discussions.
Address reprint requests to Karl H. Nocka, PhD, CytoMed, Inc, 840 Memorial Dr, Cambridge, MA 02139.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal