Abstract
Our previous study showed that the cross-linking of very late antigen (VLA)/β1 with anti-CD29 monoclonal antibody (MoAb), or interactions with extracellular matrix (ECM) proteins through VLA/β1, failed to induce T-cell costimulation via the CD3/T cell receptor (TCR) pathway for over 1 year after allogeneic bone marrow transplantation (allo-BMT), although normal CD29 and CD3 expression was observed after 3 months following allo-BMT. Molecular analysis revealed altered tyrosine phosphorylation of cellular proteins by the solid-phase cross-linking of VLA/β1 molecules in T cells from patients after allo-BMT. In T cells from early allo-BMT patients (<4 months), various sizes of highly tyrosine phosphorylated proteins were observed as high background even without the stimulation through VLA/β1 integrin. The high tyrosine phosphorylation pattern gradually disappeared and it was finally returned to normal tyrosine phosphorylation patterns by 2 years after BMT. Interestingly, poor expression of focal adhesion kinase (pp125FAK ), a VLA/β1-mediated signaling molecule, was observed within 1 year after BMT. These results suggest that these molecular defects appear to be implicated in the impaired VLA/β1-mediated signaling in T cells from patients after allo-BMT, and it could explain, in part, the persistent immunoincompetent state after allo-BMT at least 1 year.
ALLOGENEIC BONE MARROW transplantation (allo-BMT) has been shown to provide potentially curative therapy for patients with a variety of diseases including hematologic malignancies.1,2 However, one of the major obstacles in allo-BMT is prolonged T-cell dysfunction resulting in a variety of infectious complications in the months to years after hematologic engraftment.3,4 Previous studies have documented a variety of in vitro T-cell dysfunctions after allo-BMT, including impaired in vitro synthesis of B-cell immunoglobulins, poor T-cell proliferative response to mitogenic stimuli, and abnormal function of cytotoxic effector cells.5-9 In our previous studies, we showed that T cells from T-cell–depleted post–allo-BMT patients exhibited decreased proliferative response triggered by immobilized anti-CD3 plus extracellular matrix (ECM) proteins or anti-very late antigen (VLA)/CD29 monoclonal antibody (MoAb), compared with T cells from normal controls.10 Such impaired VLA/β1-mediated T-cell costimulation persisted for a prolonged period (>1 year) after allo-BMT despite normal CD3 and CD29 expression.10 After autologous BMT, impaired VLA/β1-mediated T-cell proliferation via the CD3 pathway returned to normal levels within 1 year despite no significant difference in CD3 and CD29 expression following either allo-BMT or auto-BMT.10 The adhesion of T cells from post–allo-BMT patients to a fibronectin (FN)-coated plate was normal or rather increased compared with that of normal controls even in the very early period following allo-BMT (<4 months).10 The above results strongly suggest that there are qualitative defects in the costimulatory signaling events mediated through VLA/β1 molecules. The VLA is the β1 integrin family, which consists of a common β1 subunit noncovalently associated with unique α chains to form heterodimers.11 Of VLA/β1 molecules, the expression of VLA-4 is relatively restricted to myeloid and lymphoid cells and peripheral T cells.11 We have recently shown that the ligation of VLA-4 by MoAb or its ligand induced various tyrosine phosphorylated proteins at 140, 120-105, 70, 60-65, and 50 kD.12,13 In addition, we have identified that pp140 was phospholipase C (PLCγ), pp120 was focal adhesion kinase (pp125FAK ), pp70 and pp50 were paxillin, pp45 was mitogen-activated protein (MAP) kinase, and pp60-55 proteins were pp59fyn and pp56lck, respectively.14 More recently, we have identified that pp105 is identical to a 105 kD Crk-associated substrate (Cas)-related protein (Cas-L), which is predominantly expressed in lymphoid cells, and was tyrosine phosphorylated through the ligation of VLA-4.15 As tyrosine phosphorylation of specific cellular proteins is an early and obligatory event in the activation of T cells, the above tyrosine phosphorylation events may play an important role in VLA/β1-mediated signaling. Therefore, we attempted to determine whether altered tyrosine phosphorylation is observed in lymphocytes obtained from patients following allo-BMT.
In this report, we show that the induction of tyrosine phosphorylation of several proteins was impaired in T cells from allo-BMT patients compared with that observed in normal controls. Especially poor expression of pp125FAK was observed within 1 year after allo-BMT. These results suggest that expression of pp125FAK is a key element in VLA/β1-mediated T-cell costimulation and the defective expression of pp125FAK could explain, in part, the persistent immunocompetent state for at least 1 year after allo-BMT and may be involved in susceptibility to opportunistic infections in these patients.
MATERIALS AND METHODS
Patients and samples. This study is based on the analysis of 60 patients who underwent allo-BMT over a 2-and-a-half year period at Dana-Farber Cancer Institute. Ages ranged from 18 to 56 years. The preparative regimens of allo-BMT have been described previously.16 The donor bone marrow was obtained from HLA identical siblings that were mixed lymphocyte cultures nonreactive. The harvested marrow cells were treated with anti-CD6 monoclonal antibody (MoAb) (T12, IgM) and rabbit complement before infusion to deplete mature T cells as described previously.16 None of the patients included in this analysis developed moderate or severe graft-versus-host disease (GVHD) (grade II to IV), and none were receiving any immunosuppressive therapy. Informed consent was obtained from all patients. Peripheral blood was obtained at various intervals after BMT. A total of 100 samples from allo-BMT patients were analyzed. A total of 20 samples from age- and sex-matched healthy volunteers were used as normal controls.
Reagents. Human plasma FN was obtained from GIBCO-BRL Co (Gaithersburg, MD), bovine serum albumin, Iscove's media powder, deoxycholic acid (DOC), L-leucine methyl ester hydrochloride, gentamycin, phorbol 12-myristate 13-acetate (PMA), and RPMI1640 were from Sigma Chemical Co (St Louis, MO). Fetal calf serum was obtained from BioWhittaker (Walkersville, MD). 96-Well flat-bottomed plates and nitrocellulose filters were from Costar (Cambridge, MA), 6-well plates from Becton Dickinson (Lincoln Park, NJ). Iodobeads were obtained from Pierce Chemical Co (Rockford, IL), autoradiographic film from Eastman Kodak (Rochester, NY), and [3H]thymidine and 125I were from DuPont NEN (Wilmington, DE). The enhanced chemiluminescence system kit was purchased from Amersham (Arlington Heights, IL). Goat antimouse IgG conjugated magnet beads were purchased from PerSeptive Diagnosis (Cambridge, MA), protein A-sepharose beads were from Pharmacia LKB (Uppsala, Sweden), and rabbit antimouse IgG (H + L) was from Jackson Immunoresearch Lab Inc (West Grove, PA).
Antibodies. Anti-CD2 (T11; IgG), anti-CD3 (OKT3; IgG2a), anti-CD4 (19Thy, IgG1), anti-CD8 (21Thy, IgG1), anti-CD29 (4B4; IgG1), anti-CD45RA (2H4, IgG1), anti-CD45RO (UCHL-1, IgG2), anti-CD49d (3G6; IgG1), and anti-FAK (10G2, IgG1) antibodies have been described previously.17-19 Anti-phosphotyrosine (pTyr) antibody (4G10, IgG2b) was purchased from Upstate Biotechnology Inc (UBI) (Lake Placid, NY), anti-Cas MoAb and anti-paxillin MoAb were from Transduction Laboratories (Lexington, KY), anti-p59fyn and anti-p56lck Abs were from Santa Cruz (Santa Cruz, CA), and anti-MAP kinase (ERK) MoAb was from Zymed (South San Francisco, CA).
Proliferation assays. Lymphocytes were obtained from peripheral blood mononuclear cells by depletion of contaminating monocytes by adherence to plastic plates. Further removal of monocytes was achieved by incubation with 5 mmol/L L-leucine methyl ester hydrochloride as described.20 T cells were obtained by incubating lymphocytes with anti-CD20, anti-Ia, and anti-Ig κ MoAbs (Southern Biotechnology, Birmingham, AL) using goat antimouse immunomagnetic beads as described.14 Fresh normal thymus was obtained from patients less than 3 years old who had a part of their thymus removed at corrective cardiac surgery. A portion was finely minced, and a single-cell suspension was prepared by pressing the fragments through a stainless steel mesh. Red blood cells and debris were then removed by centrifugation over Ficoll-Hypaque, and the thymocytes were washed three times as described previously.21 T-cell proliferation assays were performed as previously described.10 14 Flat-bottom 96-well microtiter plates were coated with purified anti-CD3 MoAb (0.1 μg/mL) in phosphate buffered saline (PBS). After washing the plates twice with PBS, FN (2 μg/mL), anti-CD29 (2 μg/mL) MoAb, or anti-CD49d (2 μg/mL) MoAb was added to the appropriate wells and the plates were incubated for an additional 3 hours at room temperature. T cells were cultured in triplicate wells at a concentration of 105 cells/well in RPMI1640 supplemented with human AB serum medium (10%, NABI, Miami, FL). After 3 days in culture, cells were labeled with [3H]thymidine for 18 hours, and then harvested. [3H]thymidine incorporation was measured using a beta-scintillation counter.
Tyrosine phosphorylation studies. Peripheral T cells or thymocytes were washed three times, resuspended in Iscove's serum-free medium, and stimulated with immobilized anti-CD29 MoAb (5 μg/mL) as described.16 The plates were centrifuged at 1,000 rpm for 1 minute, and cells were incubated for 30 minutes at 37°C, then solubilized in a modified RIPA buffer (1% Nonidet P-40, 0.5% DOC, NaCl [150 mmol/L], Tris-HCl [50 mmol/L], EDTA [5 mmol/L], phenylmethanesulfonyl fluoride [PMSF, 1 mmol/L], iodoacetamide [10 mmol/L], pepstatin A [1 mg/mL], sodium fluoride [10 mmol/L], sodium pyrophosphate [10 mmol/L], and sodium vanadate [0.4 mmol/L], pH 8.0). The cell lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions, transferred to a nitrocellulose membrane, and blotted using 125I-labeled anti-phosphotyrosine MoAb as described previously.14
Immunoblot. Whole extracts of peripheral T cells were separated by SDS-PAGE under reducing conditions and transferred to nitrocellulose filters. The filters were blocked with 5% dry milk in PBS and then blotted with various Abs and detected using an enhanced chemiluminescence system according to the manufacturer's procedures. Antibodies were stripped off from the membrane, which was reprobed with other antibodies.
RESULTS
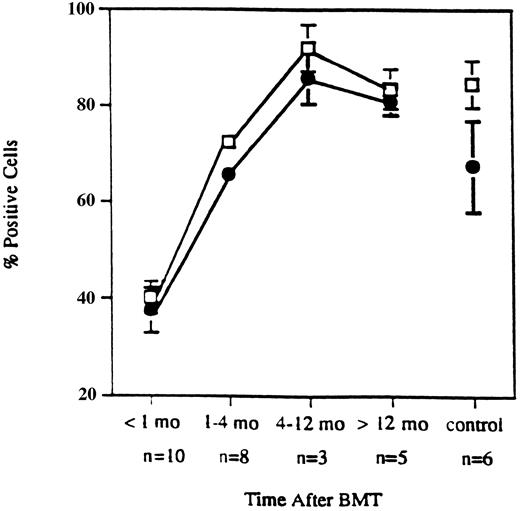
Expression of VLA-4 and VLA-4–mediated T-cell costimulation in allo-BMT patients and normals. In our previous report, we showed that recovery of CD3 and CD29 (VLAβ1) expression occurred soon after allo-BMT and achieved normal levels approximately 4 months after transplant.10 As shown in Fig 1, early recovery of CD49d (VLAα4) expression also occurred within 4 months after BMT and was similar to the recovery of CD29 expression.
Expression of CD29 and CD49d on T cells. Early recovery of CD29 (□) and CD49d (•) expression on T cells from patients after CD6-depleted allogeneic BMT. Data points represent mean values ± standard error (SEM) from samples obtained from ten patients within 1 month, eight patients during 1 through 4 months, three patients during 4 to 12 months, five patients over 12 months, and six normal controls.
Expression of CD29 and CD49d on T cells. Early recovery of CD29 (□) and CD49d (•) expression on T cells from patients after CD6-depleted allogeneic BMT. Data points represent mean values ± standard error (SEM) from samples obtained from ten patients within 1 month, eight patients during 1 through 4 months, three patients during 4 to 12 months, five patients over 12 months, and six normal controls.
In our previous report, we demonstrated prolonged impairment of VLA/β1-mediated T-cell costimulation via the CD3 pathway after allo-BMT.10 As shown in Fig 2, in normal controls, co-immobilization of anti-CD3 MoAb with FN, anti-CD29, or anti-CD49d MoAb induced marked T-cell proliferation as described previously.14 In contrast, CD3:FN–, CD3:VLA-β1–, and CD3:VLA-4–mediated T-cell proliferation was impaired in T cells from patients after allo-BMT. It should be noted that FN, anti-CD29, anti-CD49d, or anti-CD3 MoAb alone could not induce significant levels of T-cell proliferation above background (<1,000 cpm) in any normal donors or patients. As with VLA/β1-mediated T-cell proliferation, the impairment of VLA-4–mediated T-cell proliferation lasted for more than 1 year in allo-BMT patients, whereas CD3:CD2-mediated proliferation reached normal levels by 1 year.
Impaired comitogenic effects of solid phase-immobilized fibronectin (FN), anti-CD29 MoAb (4B4), or anti-CD49d MoAb (3G6) on CD3-dependent T-cell proliferation after allo-BMT. Purified lymphocytes obtained from patients after T-cell–depleted allo-BMT (<4 mo, n = 4; 4-12 mo, n = 5; <12 mo, n = 5), and healthy volunteers (n = 20) were stimulated in a serum-free medium by immobilized anti-CD3 MoAb (0.1 μg/mL) with FN (5 μg/mL) or MoAbs (5 μg/mL) against CD2, CD29, and CD49d. After 3-day culture, proliferative response was assessed by [3H]thymidine incorporation. Results are expressed as the mean cpm of triplicate samples and SEM.
Impaired comitogenic effects of solid phase-immobilized fibronectin (FN), anti-CD29 MoAb (4B4), or anti-CD49d MoAb (3G6) on CD3-dependent T-cell proliferation after allo-BMT. Purified lymphocytes obtained from patients after T-cell–depleted allo-BMT (<4 mo, n = 4; 4-12 mo, n = 5; <12 mo, n = 5), and healthy volunteers (n = 20) were stimulated in a serum-free medium by immobilized anti-CD3 MoAb (0.1 μg/mL) with FN (5 μg/mL) or MoAbs (5 μg/mL) against CD2, CD29, and CD49d. After 3-day culture, proliferative response was assessed by [3H]thymidine incorporation. Results are expressed as the mean cpm of triplicate samples and SEM.
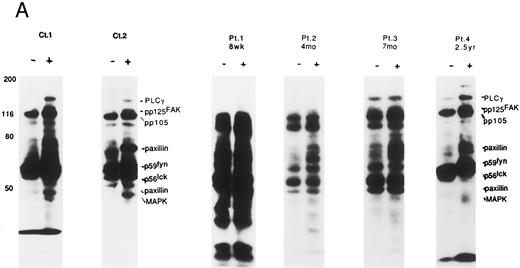
Altered tyrosine phosphorylation of signaling proteins through solid phase cross-linking of VLA in post-BMT patients' lymphocytes. We have recently shown that the solid phase cross-linking of VLA-4 using anti–VLA-β1 Ab, anti–VLA-α4 Ab, or GST-fusion proteins of the CS-1 region of FN, stimulated tyrosine phosphorylation of several proteins including PLCγ (pp140), pp125FAK (pp120), Cas-L (pp105), paxillin (pp70/pp50), p59fyn/p56lck (pp60-55), and MAP kinase (pp45).14 Therefore, we next attempted to determine whether the altered tyrosine phosphorylation patterns of the above proteins were observed in lymphocytes obtained from patients at various times after T-cell–depleted allo-BMT. As shown in Fig 3A, within 3 months after allo-BMT (PN 1), high background of tyrosine phosphorylation of various proteins was observed at both nonstimulated and stimulated lymphocytes by anti-CD29 MoAb. Moreover, during 2 months to 7 months after allo-BMT, high background of tyrosine phosphorylation gradually disappeared and some induction of tyrosine phosphorylation was observed (PN 2 and 3). It should be noted that at the very early stage after allo-BMT (PN 1 and 2), the lack of the pp120-pp140 tyrosine phosphorylated bands was observed even in the high background. A normal pattern of induction of tyrosine phosphorylation (PLCγ, pp125FAK, pp105, paxillin, Fyn, Lck, and MAP kinase) was recovered gradually until 2 years (PN 4). At 2 years after transplantation, there is clearly enhanced tyrosine phosphorylation around the 42-45 kD-sized proteins, including MAP kinase (ERK1, 2). As shown in Table 1, there are characteristics of (1) the high background, (2) little enhancement in tyrosine phosphorylation, and (3) a lack of pp120-140 in the pTyr blot of VLA/β1-stimulated T cells in post–allo-BMT patients within 4 months following the ligation of VLA/β1.
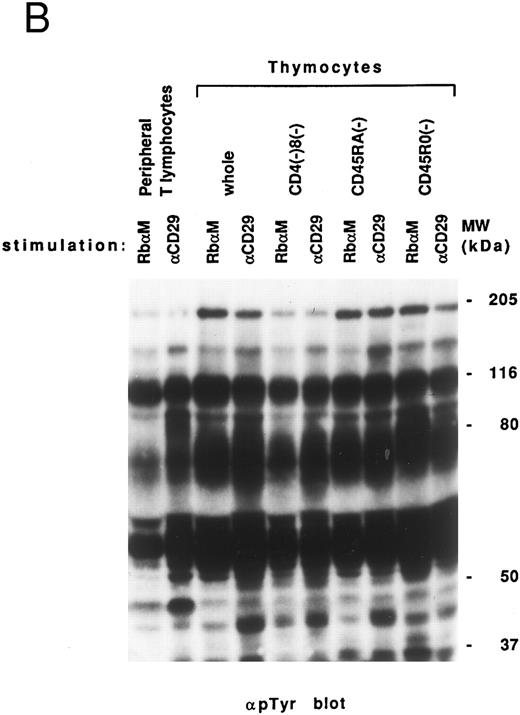
Induction of tyrosine phosphorylation through VLA/β1-stimulation. (A) Induction of several proteins' tyrosine phosphorylation in peripheral lymphocytes from two normal controls (C 1 and 2) and 4 patients (PN 1, 2, 3, and 4) 8 weeks to 2.5 years after allo-BMT. Monocyte-depleted lymphocytes were incubated on plates coated using rabbit antimouse IgG with (+) or without (−) 4B4 (5 μg/mL) for 30 minutes, and then lysed. Whole cell lysate (50 μg/lane) was immediately subjected to Western blotting with the anti-phosphotyrosine MoAb. Autoradiography was carried out for 14 to 18 hours. Normal control samples were analyzed at the same time in each experiment, which constantly demonstrated similar tyrosine phosphorylation pattern to Ct1 and Ct2 (data not shown). Representative results at each stage after allo-BMT from different membranes are shown. (B) Whole thymocytes and immunodepleted thymocytes using anti-CD4 (19Thy) plus anti-CD8 (21Thy). Anti-CD45RA (2H4) or anti-CD45RO (UCHL1) antibody were incubated on plates coated with rabbit antimouse IgG (RbαM) or rabbit antimouse IgG plus anti-CD29 antibody (4B4; 5 μg/mL) for 30 minutes, lysed, and detected by anti-pTyr blot by the same method as above.
Induction of tyrosine phosphorylation through VLA/β1-stimulation. (A) Induction of several proteins' tyrosine phosphorylation in peripheral lymphocytes from two normal controls (C 1 and 2) and 4 patients (PN 1, 2, 3, and 4) 8 weeks to 2.5 years after allo-BMT. Monocyte-depleted lymphocytes were incubated on plates coated using rabbit antimouse IgG with (+) or without (−) 4B4 (5 μg/mL) for 30 minutes, and then lysed. Whole cell lysate (50 μg/lane) was immediately subjected to Western blotting with the anti-phosphotyrosine MoAb. Autoradiography was carried out for 14 to 18 hours. Normal control samples were analyzed at the same time in each experiment, which constantly demonstrated similar tyrosine phosphorylation pattern to Ct1 and Ct2 (data not shown). Representative results at each stage after allo-BMT from different membranes are shown. (B) Whole thymocytes and immunodepleted thymocytes using anti-CD4 (19Thy) plus anti-CD8 (21Thy). Anti-CD45RA (2H4) or anti-CD45RO (UCHL1) antibody were incubated on plates coated with rabbit antimouse IgG (RbαM) or rabbit antimouse IgG plus anti-CD29 antibody (4B4; 5 μg/mL) for 30 minutes, lysed, and detected by anti-pTyr blot by the same method as above.
VLA/β1-Mediated Tyrosine Phosphorylation of Proteins in T Cells
| . | . | pp140 . | pp120 . | pp110-105 . | pp80-70 . | pp60 . | pp55 . |
|---|---|---|---|---|---|---|---|
| Patient T cells | |||||||
| <4 mo (n = 8) | − | 6 (75) | 3 (38) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| + | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| B | 2 (25) | 5 (62) | 8 (100) | 8 (100) | 8 (100) | 8 (100) | |
| 4-12 mo (n = 7) | − | 3 (43) | 1 (14) | 1 (14) | 1 (14) | 2 (28) | 0 (0) |
| + | 3 (43) | 5 (72) | 5 (72) | 6 (86) | 4 (57) | 6 (100) | |
| B | 1 (14) | 1 (14) | 1 (14) | 0 (0) | 1 (14) | 0 (0) | |
| >12 mo (n = 6) | − | 1 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| + | 5 (83) | 6 (100) | 6 (100) | 6 (100) | 6 (100) | 6 (100) | |
| B | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Normal T cells | |||||||
| (n = 3) | − | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| + | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | |
| B | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| . | . | pp140 . | pp120 . | pp110-105 . | pp80-70 . | pp60 . | pp55 . |
|---|---|---|---|---|---|---|---|
| Patient T cells | |||||||
| <4 mo (n = 8) | − | 6 (75) | 3 (38) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| + | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| B | 2 (25) | 5 (62) | 8 (100) | 8 (100) | 8 (100) | 8 (100) | |
| 4-12 mo (n = 7) | − | 3 (43) | 1 (14) | 1 (14) | 1 (14) | 2 (28) | 0 (0) |
| + | 3 (43) | 5 (72) | 5 (72) | 6 (86) | 4 (57) | 6 (100) | |
| B | 1 (14) | 1 (14) | 1 (14) | 0 (0) | 1 (14) | 0 (0) | |
| >12 mo (n = 6) | − | 1 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| + | 5 (83) | 6 (100) | 6 (100) | 6 (100) | 6 (100) | 6 (100) | |
| B | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Normal T cells | |||||||
| (n = 3) | − | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| + | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | |
| B | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Values are the number with the percentage in parentheses.
Abbreviations: −, no enhanced bands were detected; +, clearly enhanced bands were detected; B, high background of tyrosine phosphorylation with no stimulation, which persisted after VLA/β1 stimulation.
Comparison of VLA-mediated tyrosine phosphorylation pattern between lymphocytes after allo-BMT and normal thymocytes. Previously, we suggested that defective T-cell costimulation in allo-BMT patients could be a reflection of T-cell immaturity in such patients. We therefore attempted to compare the tyrosine phosphorylation pattern from allo-BMT patients to that observed in thymocytes. As shown in Fig 3B, a similar high background was observed in the whole thymocyte population, immature thymocytes (CD4-, CD8-, and CD45RA (2H4)-negative fraction) and mature CD45RO (UCHL-1)-negative fraction. It should be noted that thymocytes, in contrast to the allo-BMT patient lymphocytes, showed induced tyrosine phosphorylation of 42 to 44 kD proteins including MAP kinase (ERK1/2).
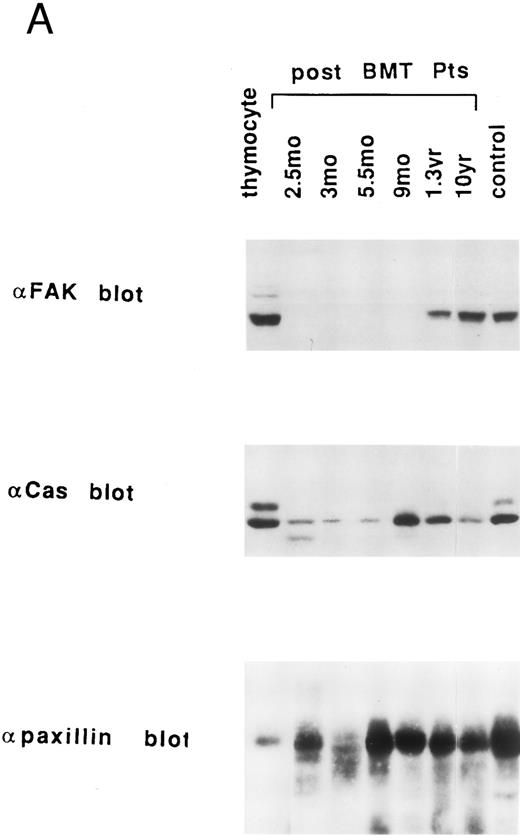
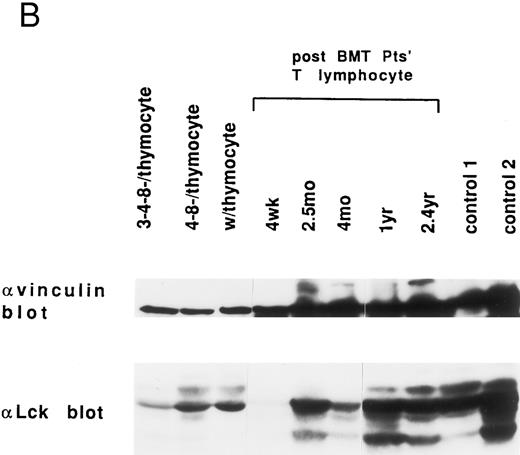
Expression of pp125FAK, Cas-L, paxillin, lck, and vinculin in lymphocytes at various times after allo-BMT. We previously reported that pp125FAK is associated with paxillin and localized to focal adhesions via paxillin.19 Moreover, our previous results suggested that tyrosine phosphorylation of pp125FAK induces recruitment of various signaling molecules to tyrosine-phosphorylated pp125FAK, which presumably results in VLA/β1-mediated cell adhesion and cell proliferation. Thus, we next attempted to examine the expression of major VLA/β1-mediated signaling molecules including pp125FAK, Cas-L (pp105), paxillin, lck and vinculin in the patients' T lymphocytes. As shown in Fig 4A, pp125FAK expression was delayed by 1 year after allo-BMT, compared with normal control's lymphocytes and human thymocytes. Cas-L (pp105) expression was also delayed, but by 9 months the expression returned to the normal level. On the other hand, normal paxillin expression was observed in T lymphocytes at every stage after allo-BMT. In addition, as shown in Fig 4B, a virtually normal level of vinculin and Lck expression (except at 4 wk) was observed at every stage of allo-BMT patients. It should be noted that reverse transcriptase-polymerase chain reaction (RT-PCR) products of pp125FAK, Cas-L, or paxillin in T lymphocytes from allo-BMT patients were detected similarly in cDNA from patients at any stage after allo-BMT (data not shown). Thus, the expression of these proteins in patients' lymphocytes is not reflected in the RT-PCR study. Table 2 summarizes results of the expression of VLA/β1-mediated signaling molecules in post-BMT patients' lymphocytes. As shown in Table 2, especially within 1 year after allo-BMT, delayed recovery of pp125FAK expression was observed in T cells.
Endogenous expression of pp125FAK, Cas-L, paxillin, Lck, and vinculin in the whole extracts of thymocytes, post-BMT patients' and healthy volunteers' lymphocytes. (A) Whole thymocytes and peripheral T lymphocytes from post-BMT patients or a normal control (50 μg/lane) were analyzed using anti-FAK MoAb. The same membrane was rehybridized with anti-Cas MoAb followed by anti-paxillin MoAb. (B) Immunodepleted cell population from normal thymocytes using anti-CD3 (OKT3) plus anti-CD4 (19Thy) plus anti-CD8 (21Thy) or anti-CD4 plus anti-CD8 MoAbs, whole thymocytes, and peripheral T lymphocytes from post-BMT patients or normal controls were lysed. Each cell lysate (50 μg/lane) was subjected to Western blotting with anti-Lck MoAb followed by anti-vinculin MoAb.
Endogenous expression of pp125FAK, Cas-L, paxillin, Lck, and vinculin in the whole extracts of thymocytes, post-BMT patients' and healthy volunteers' lymphocytes. (A) Whole thymocytes and peripheral T lymphocytes from post-BMT patients or a normal control (50 μg/lane) were analyzed using anti-FAK MoAb. The same membrane was rehybridized with anti-Cas MoAb followed by anti-paxillin MoAb. (B) Immunodepleted cell population from normal thymocytes using anti-CD3 (OKT3) plus anti-CD4 (19Thy) plus anti-CD8 (21Thy) or anti-CD4 plus anti-CD8 MoAbs, whole thymocytes, and peripheral T lymphocytes from post-BMT patients or normal controls were lysed. Each cell lysate (50 μg/lane) was subjected to Western blotting with anti-Lck MoAb followed by anti-vinculin MoAb.
Expression of Cytoplasmic Proteins in T Cells
| . | . | FAK . | CasL . | Paxillin . | Lck . | Vinculin . |
|---|---|---|---|---|---|---|
| Patient T cells | ||||||
| 0–12 mo | − | 17 (94) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| +w | 1 (6) | 5 (56) | 2 (10) | 6 (75) | 0 (0) | |
| + | 0 (0) | 4 (44) | 18 (90) | 2 (25) | 16 (100) | |
| >12 mo | − | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| +w | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| + | 7 (100) | 5 (100) | 6 (100) | 3 (100) | 7 (100) | |
| Normal T cells | − | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| +w | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| + | 5 (100) | 3 (100) | 5 (100) | 3 (100) | 6 (100) |
| . | . | FAK . | CasL . | Paxillin . | Lck . | Vinculin . |
|---|---|---|---|---|---|---|
| Patient T cells | ||||||
| 0–12 mo | − | 17 (94) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| +w | 1 (6) | 5 (56) | 2 (10) | 6 (75) | 0 (0) | |
| + | 0 (0) | 4 (44) | 18 (90) | 2 (25) | 16 (100) | |
| >12 mo | − | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| +w | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| + | 7 (100) | 5 (100) | 6 (100) | 3 (100) | 7 (100) | |
| Normal T cells | − | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| +w | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| + | 5 (100) | 3 (100) | 5 (100) | 3 (100) | 6 (100) |
Values are the number with the percentage in parentheses.
Abbreviations: −, no bands were detected; +w, faint bands (represented by Cas-L expression at 3 mo in Fig 4A) were detected; +, almost equal amounts to normal individuals.
DISCUSSION
One of the major critical problems after allo-BMT is a prolonged immunocompromised status, especially a defective defense system against opportunistic infections.4,5 This could result, in part, from immaturity of lymphocyte-mediated immunity until the immune reconstitution of the donor bone marrow is completed.22 Although previous studies revealed decreased in vitro lymphocytic function, such as proliferative responses to various mitogens and helper activity in immunoglobulin production of B cells,5-9 the mechanism of the lymphocyte dysfunction has not been clarified. Moreover, in allo-BMT patients, various immunosuppressants are generally administered, which make it difficult to analyze the accurate immunologic function after allo-BMT.
In our previous studies, we have determined T lymphocyte function of T-cell–depleted allo-BMT patients who have not received any immunosuppressive agents, and have shown that the cross-linking of VLA/β1 with anti-CD29 MoAb or interactions with ECMs through VLA/β1, failed to induce T-cell costimulation via CD3/TCR pathway for over 1 year after allo-BMT, despite normal CD29 and CD3 expression after 3 months following allo-BMT.10 In the present study, we showed that in T cells from early-stage of allo-BMT patients, various-sized proteins were highly tyrosine phosphorylated even without the stimulation through VLA/β1 integrin. The high tyrosine phosphorylation gradually disappeared from 2 to 7 months after allo-BMT, and then normal tyrosine phosphorylation patterns were observed by 2 years after allo-BMT. Finally, we demonstrated delayed FAK expression in patient T cells until 1 year after allo-BMT, whereas other VLA/β1 signaling-related molecules such as Cas-L, paxillin, vinculin, and Lck returned to normal levels from the relatively early stage after allo-BMT.
We have previously reported that cytoplasmic proteins including PLCγ, pp125FAK, Cas-L, paxillin, fyn, lck, and MAP kinase can be phosphorylated by the cross-linking of VLA/β1 in peripheral T cells from healthy donors.14 We showed altered tyrosine phosphorylation of such proteins through VLA/β1 stimulation until 2 years following allo-BMT based on the work from normal peripheral T cells. The high background of phosphorylation on the tyrosine residues of cytoplasmic proteins was observed in the patients' T cells at the early stage after allo-BMT, indicating that some of these cytoplasmic signaling molecules may be persistently activated without any in vitro stimulation. One simple explanation for the activated status of these molecules would be in vivo activation of peripheral T cells in the early stage after allo-BMT. Several investigators have shown the increased expression of HLA-DR antigens on T cells during the first 3 months post-grafting.22 23 The activating status of T cells following allo-BMT can be a reflection of clinical events including GVHD. Alternatively, increased tyrosine phosphorylation of various proteins may reflect T-cell immaturity in allo-BMT patients. We observed a similar high background of tyrosine phosphorylation pattern in normal thymocytes, as well as in T cells from allo-BMT patients at an early stage. This result could support the latter hypothesis. However, proteins of 42 to 45 kD, including MAP kinases, were tyrosine phosphorylated after VLA/β1 stimulation in normal thymocytes, but not in patient T cells at an early stage after allo-BMT. This indicates that T-cell reconstitution after BMT is not identical to the pattern observed in normal immune ontogeny. Significantly increased binding to FN or anti–VLA-4 Ab was observed in allo-BMT patient T cells at very early stage as well as in normal thymocytes (T. Sato, unpublished data). This suggests that these cells maintain a hyperphosphorylated status of cytoplasmic proteins possibly providing an excessive “inside-out” signal, which augments the avidity of VLA/β1 molecules for FN.
pp125FAK, a cytoplasmic tyrosine kinase, is localized to focal adhesions and can be tyrosine phosphorylated by the stimulation of VLA/β1 integrins.24-26 Various signaling molecules such as pp60src, pp59fyn,27,28 phosphatidylinositol 3-kinase,29 Grb2,30 and Crk31 are recruited to tyrosine phosphorylated pp125FAK. We have reported tyrosine phosphorylation of pp125FAK by VLA/β1 stimulation in the human lymphoblastoid cell line, H9.13 Maguire et al reported FAK to be synergistically tyrosine phosphorylated by CD3/TCR- and VLA/β1-mediated signals in human T cells.32 These findings indicate the involvement of pp125FAK in the VLA/β1-mediated signals in T lymphocytes. In allo-BMT patients, delayed expression of pp125FAK possibly causes, at least in part, defective VLA/β1-mediated costimulation via CD3/TCR pathway, or the highly tyrosine phosphorylated status of cytoplasmic proteins. However, even in patients more than 1 year after BMT with T cells expressing normal amounts of pp125FAK, VLA/β1-mediated T-cell costimulation via CD3 pathway was not completely improved. This suggests that regenerate T cells after allo-BMT might owe additional molecular defects in the costimulatory signaling pathway through VLA/β1.
In this report, we showed molecular defects in T cells from allo-BMT patients and delayed recoveries of pp125FAK expression and tyrosine phosphorylation patterns by VLA/β1-mediated signal. These defects are implicated in impaired T-cell proliferation via TCR/CD3 pathway and immunoincompetent state after allo-BMT; however, further studies are required to clarify the VLA/β1-mediated signaling pathway and its defects after allo-BMT until conclusion.
ACKNOWLEDGMENT
We thank Nicole D'Avirro for her technical assistance and Lisa Willis for her secretarial assistance.
Supported by National Institutes of Health Grants No. AR33713 and AI29530.
Address reprint requests to Chikao Morimoto, MD, Division of Tumor Immunology, Dana-Farber Cancer Institute, Department of Medicine, Harvard Medical School, 44 Binney St, MA 02115.

![Fig. 2. Impaired comitogenic effects of solid phase-immobilized fibronectin (FN), anti-CD29 MoAb (4B4), or anti-CD49d MoAb (3G6) on CD3-dependent T-cell proliferation after allo-BMT. Purified lymphocytes obtained from patients after T-cell–depleted allo-BMT (<4 mo, n = 4; 4-12 mo, n = 5; <12 mo, n = 5), and healthy volunteers (n = 20) were stimulated in a serum-free medium by immobilized anti-CD3 MoAb (0.1 μg/mL) with FN (5 μg/mL) or MoAbs (5 μg/mL) against CD2, CD29, and CD49d. After 3-day culture, proliferative response was assessed by [3H]thymidine incorporation. Results are expressed as the mean cpm of triplicate samples and SEM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/10/10.1182_blood.v90.10.4222/3/m_bl_0030f2.jpeg?Expires=1771038269&Signature=WAVHWK9E8kqRLR8df3rqx-BPSL70TRqUgjJ2z6O3AnhsVbDX8RYYv4d2LAGz8hQwUzP7o4bhrul6Pt~b9~UyiFTt8EUvpmVie-IoRhu3BSDpYgWtqcHeKutpj4ySjN6RjvStAxG2799P8A1P9LcvCsyoRMyMGhYna7smXHpunLLGyQggZhevdr0pAHIvwaH421OqSs6bqH~12yYSPyckZuD43HYgXmvUMnwmF~U5cYW8ABJ3eGYzz6ELwYTS2Vwns76HzEA8QcBCwREEgC0LhvvsAwB0V62kR9-RfEX-lOVR2rj96ZQ9oeUhAf7uqu6bKkE64t43Sc-TvlPqrhU4PQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal