Abstract
Chromosomal translocations involving band 5q31-35 occur in several hematologic disorders. A clone with a t(5; 14)(q33; q32) translocation appeared at the relapse phase in a patient with acute myelogenous leukemia who exhibited a sole chromosomal translocation, t(7; 11), at initial diagnosis. After the appearance of this clone, the leukemia progressed with marked eosinophilia, and combination chemotherapy was ineffective. Southern blot analysis showed a rearrangement of the platelet-derived growth factor receptor β (PDGFRβ) gene at 5q33 which was not observed at initial diagnosis. This translocation resulted in a chimeric transcript fusing the PDGFRβ gene on 5q33 with a novel gene, CEV14, located at 14q32. Expression of the 5′ region of the PDGFRβ cDNA, upstream of the breakpoint, was not detected. However, the 3′ region of PDGFRβ, which was transcribed as part of the CEV14-PDGFRβ fusion gene, was detected. A partial cDNA for a novel gene, CEV14, includes a leucine zipper motif and putative thyroid hormone receptor interacting domain and is expressed in a wide range of tissues. The expression of a CEV14-PDGFRβ fusion gene in association with aggressive leukemia progression suggests that this protein has oncogenic potential.
RECURRENT AND consistent chromosomal aberrations have been shown to be associated with specific types of neoplasia and particular clinical manifestations. Although tumors may present with a chromosomal abnormality, the appearance of additional karyotypic changes may be associated with its biologic progression.1 In recent years, molecular characteristics of some karyotypic aberrations have been clarified, especially those involving chromosomal translocations, inversions, and deletions.2 Specific translocations or inversions which are observed as second karyotypic changes in leukemia are likely to be involved in leukemia progression. Chronic myelogenous leukemia (CML) with a t(9; 22) translocation progress after the acquisition of a t(3; 21) translocation associated with an AML1 to EVI1 gene fusion.3,4 Additional translocations, such as t(8; 21) inv(16) or 11q23 in the course of leukemia, result in a poor prognosis.5-10
The molecular basis of the characteristic t(5; 12)(q33:p13) translocation in one subtype of myelodysplastic syndrome (MDS), chronic myelomonocytic leukemia (CMML), has been elucidated.11 This translocation joins the platelet-derived growth factor receptor β (PDGFRβ) gene on chromosome 5 which encodes a member of the receptor tyrosine kinase family,12 with the TEL gene on chromosome 12 which encodes a member of the ETS family. The translocation results in a fusion transcript linking the 5′ helix-loop-helix (HLH) domain of the TEL gene to the transmembrane and tyrosine kinase domain of PDGFRβ. The TEL gene also has been reported to form fusion transcripts with other genes such as ABL, AML1, or MN1.13-16 However, no other fusion partners for PDGFRβ have yet been identified.
Translocations involving in 14q32 are typically associated with lymphoid neoplasm and deregulate some proto-oncogenes, such as BCL-2, BCL-1, or PAX5, by juxtaposition with regulatory elements of the IgH gene.17-19 In childhood acute lymphoblastic leukemia (ALL) involving an (5; 14) translocation with clinical manifestations of eosinophilia, the interleukin-3 (IL-3) gene is overexpressed by juxtaposition with the IgH gene.20,21 However, there are some cases of leukemia in which chromosomal translocations involve 14q32 but rearrangements of the IgH gene are not observed.22 Thus, rearrangement of other genes may be involved in those cases.
We have previously reported a case of AML with t(7; 11) which exhibited leukemia progression and marked eosinophilia after the appearance of additional chromosome 5 abnormalities.23 In the terminal stage, cytogenetic studies of leukemia cells showed t(5; 14)(q33:q32),t(7; 11)(p15; p15) abnormalities, and enhanced expression of IL-5 mRNA was detected by reverse transcriptase-polymerase chain reaction (RT-PCR).
In this report, we show that a novel t(5; 14)(q33:q32) translocation occurred resulting in the fusion of PDGFRβ to a novel gene, CEV14, which may be involved in clonal evolution of leukemia and eosinophilia.
MATERIALS AND METHODS
Southern blotting.Genomic DNA was prepared from mononuclear cells of the patient's peripheral blood at first diagnosis and in relapse phase with informed consent, and digested with appropriate restriction enzymes as described.24 The resulting DNA was electrophoresed in 0.7% agarose gels and transferred to Nylon filters (Amersham Japan, Tokyo, Japan). The PDGFRβ cDNA probe P1 is a 216-bp fragment spanning nucleotides 1827 to 2042 and was amplified by RT-PCR reaction from human bone marrow (BM) stroma cDNA using primers 1827PF (5′-GTGGTGAGCACACTGCGTCTG-3′) and 2022PR (5′-GTAACGTGGCTTCTTCTGCCA-3′). Probe P1 was labeled with 32P-dCTP by the random priming method and Southern hybridization was performed as described.25 DNA sequence analysis of the resulting product confirmed the identity of the PDGFRβ cDNA.
RNA preparation and Northern blotting.Total RNA was isolated from each cell line and leukemia cells from the patient's peripheral blood by the guanidium thiocyanate method.26 Human BM stroma was collected as adherent cells from primary human BM culture which was obtained from a normal volunteer. MEG-A2 is a human immature megakaryoblastic cell line established in our laboratory.27 Poly(A)+ RNA was purified using oligo(dT)-Latex (Daiichikagaku, Tokyo, Japan).28 Multiple tissue RNA filters containing 2 μg of poly(A)+ RNA from different human tissues were purchased from Clontech (Human MTN; Clontech, Palo Alto, CA). Northern blotting analysis was performed as described previously.28 The 1.1-kb PvuII fragment of PDGFRβ cDNA (P2) and the 1.8-kb PvuII fragment of CEV14 cDNA (C1) were used as hybridization probes.
cDNA cloning and sequencing.Two micrograms of poly(A)+ RNA were purified from 100 μg of total RNA from the patient's leukemia cells at relapse phase, using oligo(dT)-Latex. Double-stranded cDNA was synthesized by the method of Gubler Hoffman with minor modifications.25 After attachment of an EcoRI-Not I adapter (Pharmacia LKB, Piscataway, NJ), double-stranded cDNA was size fractionated by electrophoresis on a 1.2% agarose gel, and cDNAs longer than 800 bp were ligated to a λZAPII vector (Stratagene, La Jolla, CA). Approximately 5 × 105 plaques were screened with the P1 probe described above and C2, using standard plaque lifts. Probe C2, derived from the anchored PCR product, contains 594 bp of CEV14 cDNA. More than 1 × 106 plaques from a HL-60 cDNA library were also probed. pBluescript plasmids were excised from the λZAPII phage clones according to the manufacturer's protocol. Double-stranded sequencing of plasmids insert was performed with Sequenase Version 2.0 (Amersham Japan).
Anchored PCR and RT-PCR.Anchored PCR was adapted from the method of Frohman29 with minor modifications. Total RNA (5 μg) was treated with DNase (Pharmacia) and reverse transcribed using MMLV reverse transcriptase (GIBCO-BRL, Grand Island, NY) and PDGFRβ oligonucleotide primer 2369PR (5′-TAGATGGGTCCTCCTTTGGTG-3′). A poly(A) tail was appended using terminal transferase (GIBCO BRL) and dATP at 37°C for 15 minutes. After a single cycle of amplification (94°C for 1 minute, 50°C for 2 minutes, 72°C for 40 minutes) using primers QT (5′-TGAGCA-G A G T G A C G A G G A C T C G A G C T C A A G C T T T T T T T T T T T TTT-TTT-3′), 35 cycles of PCR (94°C for 40 seconds, 58°C for 1 minute, 72°C for 6 minutes) were performed with primer Q0 (5′-CCAGTGAGCAGAGTGACG-3′) and the internal PDGFRβ primer 2022PR. An aliquot of this reaction was amplified using nested primers 1990PR (5′-TGAGGATGAGAAGGGAGATGATGG-3′) and Q1 (5′-GAGGACTCGAGCTCAAGC-3′) under similar PCR conditions. The resulting 600-bp PCR product was cloned into a plasmid vector, pBluescript SK(−) (Stratagene), and sequenced.
To detect the 5′ region of CEV14, we performed a second course of anchored PCR using poly(A)+ RNA from HL-60 or MEG-A2 cells,28 with CEV14-specific primers, 1625CR (5′-CTTGATGCTGAATCCAATGC-3′) and 1255CREcoRI (5′-TTGAATTCTTTCTTAGTTTAGCCTCT-3′), and primer Q0. After 40 cycles of PCR, the resulting products were digested with Xho I and EcoRI, size-fractionated, and cloned into pBluescript SK(−). The CEV14+ clones were selected by Southern hybridization of plasmids with probe C1 and sequenced. PCR amplification was performed with Thermus aquaticus DNA polymerase (Promega, Madison, WI) and Taq extender (Stratagene) following the manufacturer's recommendations.
For RT-PCR, total RNA was converted to single-stranded cDNA using oligo(dT) primers and MMLV reverse transcriptase. PCR amplification was performed with Thermus aquaticus DNA polymerase (Promega) for 35 cycles using oligonucleotide primers (94°C for 40 seconds, 66°C for 1 minute, 72°C for 2 minutes). The 5′ and 3′ PDGFRβ primers are 1827PF and 2022PR, respectively. The 5′ and 3′ CEV14 primers are 1407CF (5′-CGCTGCAGCTTTCTGTCTCTCAGGAACAAG-3′) and 2189CR (5′-GCGAGGAGCCAAAAACGGATTTACATCTGT-3′), respectively. PCR reaction products were electrophoresed on 2% agarose gels and stained with ethidium bromide.
Cytogenetic analysis and fluorescent in situ hybridization (FISH) analysis.BM and peripheral blood (PB) cells cultured in vitro for 24 to 48 hours without mitogen stimulation, were subjected to chromosome analysis. Chromosome preparations were made by a routine air-drying method with hypotonic treatment and fixed with methanol-acetic acid (3:1). Chromosomes were banded by the trypsin-Giemsa method. Karyotypes were described according to the International System for Human Cytogenetic Nomenclature.30
Metaphase chromosomes were prepared from peripheral mononuclear cells of a normal volunteer. Slides were pretreated with 100 μg/mL RNaseA in 2× SSC for 1 hour and digested with 50 μg/mL pepsin in 10 mmol/L HCl for 10 minutes at 37°C. After fixation, the metaphase chromosomes slides were denatured at 70°C in 70% formamide, 2× SSC for 2 minutes, and dehydrated in a series of cold ethanol and heated at 65°C for 2 hours. The plasmid including CEV14 cDNA was labeled with biotin 16-dUTP using a nick translation kit (Boehringer Mannheim Biochemica, Mannheim, Germany) following the manufacturer's recommendations. The hybridization signal was detected by immunofluorescence using avidin-conjugated fluorescein isothiocyanate (FITC-avidin) (Boehringer Mannheim Biochemica) and biotinylated anti-avidin D (Vector Laboratories, Burlingame, CA). The specimens were viewed at × 1,000 magnification on a NIKON UFX-DX microscope (Tokyo, Japan).
RESULTS
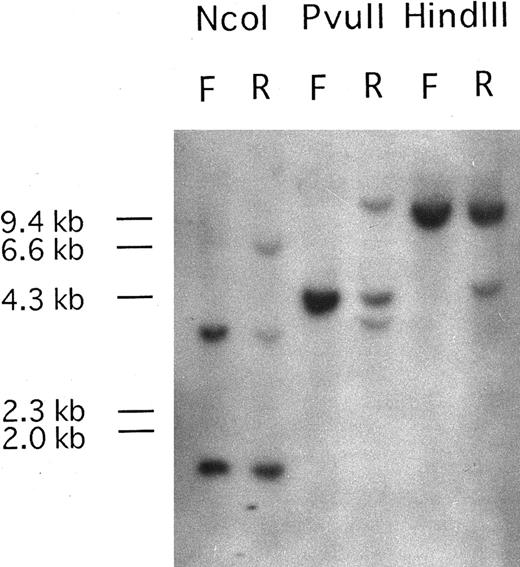
PDGFRβ is rearranged at relapse phase.Southern blots of DNA from the patient's PB mononuclear cells at first diagnosis with a t(7; 11) translocation and at relapse phase with t(5; 14)(q33; p32),t(7; 11)(p15; p15), hybridized with the PDGFRβ probe P1, are shown in Fig 1. Only genomic bands were detected at first diagnosis, but rearranged bands were observed at relapse phase. Because two rearranged bands were detected after digestion with PvuII, the break point was expected to be located near the genomic region between oligonucleotide probe 1827PF and 2022PR.
Southern blot analysis of DNA from the patient at first diagnosis (F) and at relapse phase (R) carrying the additional (5; 14)(q33; q32) translocation. Genomic DNA samples (5 μg each) were digested with Nco I, PvuII, and HindIII. Hybridization with probe Patients, a DNA fragment from PDGFRβ, shows DNA rearrangements in relapse phase. One rearranged band was detected upon Nco I and HindIII digestion and two rearranged bands were observed after PvuII digestion. The sizes of HindIII digested λ DNA markers are shown on the left of the panel.
Southern blot analysis of DNA from the patient at first diagnosis (F) and at relapse phase (R) carrying the additional (5; 14)(q33; q32) translocation. Genomic DNA samples (5 μg each) were digested with Nco I, PvuII, and HindIII. Hybridization with probe Patients, a DNA fragment from PDGFRβ, shows DNA rearrangements in relapse phase. One rearranged band was detected upon Nco I and HindIII digestion and two rearranged bands were observed after PvuII digestion. The sizes of HindIII digested λ DNA markers are shown on the left of the panel.
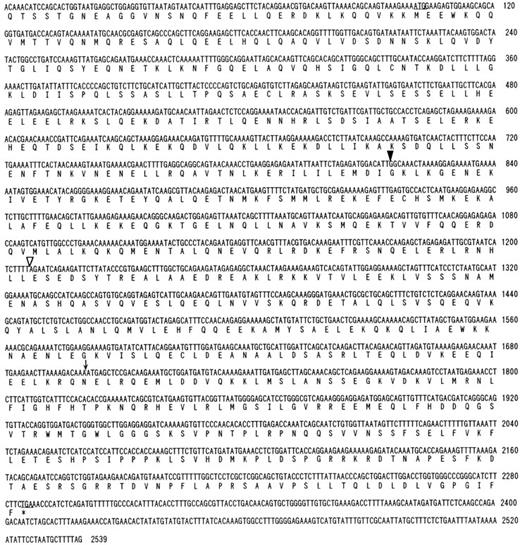
Cloning and characterization of fusion cDNAs and wild-type CEV14 cDNA.To clone the 5′ region of the putative fusion gene, we performed anchored-PCR with PDGFRβ-specific probes. The resulting 670-bp PCR product was cloned and sequenced, revealing that PDGFRβ was fused at nucleotide 1936 to a novel sequence in a contiguous open reading frame. The predicted amino acid sequence derived from the fusion gene showed no significant homology to any known protein sequences in the SWISS PROT (January 1997) or Protein Identification Resource databases (January 1997), and hence we named this novel gene CEV14 (clonal evolution related gene on chromosome 14). We then screened a cDNA library constructed from this patient's sample, with the P1 probe from PDGFRβ and C2 probe from the novel PCR product. After a series of sequential screenings, two candidate cDNAs coding for CEV14-PDGFRβ and two clones coding for CEV14 protein were isolated. Neither PDGFRβ nor PDGFRβ-CEV14 cDNAs were detected in the library made from the patient's cell. The 5′ region of one of the CEV14 cDNAs had the same 5′ end as the probe C2 which was obtaned by anchored PCR, and this clone was about 1.35 kb. Two of the other cDNA clones had the same 5′ extended ends which were about 350 bp longer than the 5′ end of C2, and those clones were about 1.7 kb without an in-frame stop codon before first ATG (Fig 2). To confirm the 5′ terminal sequence, we repeated anchored PCR with a CEV14-specific probe using RNA from the human myelomonocytic cell lines, HL-60 and MEG-A2. Two types of cDNA were isolated. One clone was identical to that detected in the patient's cells and the other cDNA consisted of 2,539-bp nucleotides with a long open reading frame with three potential translational initiation codons (ATG) at the 5′ end of the cDNA (Fig 2). Despite sequential screening of cDNA library of HL-60 with the CEV14 probe, we could not obtain extended clones and there was no in-frame stop codon at the 5′ region. The sequence surrounding the first ATG codon closely matches the consensus sequence for optimal translation initiation. We repeated a computer search with the entire CEV14 sequence and found that a partial sequence of about 800 bp in the 3′ region of CEV14 has been registered as TRIP11 (thyroid hormone receptor interacting protein 11).31 CEV14 also has a leucine zipper motif. The predicted CEV14 gene consists of an open reading frame of 2,187 bp encoding 729 amino acids. The characteristics of the CEV14 and CEV14-PDGFRβ proteins are schematically shown in Fig 3.
Partial nucleotide sequence of CEV14 cDNA and its deduced amino acid sequence. The 5′ end of each clone isolated by anchored PCR or library screening is indicated by open or closed arrows, respectively. The asterisk indicates a stop codon. The translocation breakpoint occurs following nucleotide 1701, indicated by an arrow. The predicted initiator methionine is underlined, but there is no in-frame stop codon upstream.
Partial nucleotide sequence of CEV14 cDNA and its deduced amino acid sequence. The 5′ end of each clone isolated by anchored PCR or library screening is indicated by open or closed arrows, respectively. The asterisk indicates a stop codon. The translocation breakpoint occurs following nucleotide 1701, indicated by an arrow. The predicted initiator methionine is underlined, but there is no in-frame stop codon upstream.
Schematic of the CEV14-PDGFRβ fusion protein. The nucleotide and deduced amino acids sequences near the breakpoint of the CEV14-PDGFRβ fusion are shown at the bottom of the figure. The breakpoint is indicated by an arrow. CEV14 has a leucine zipper motif 5′ of the breakpoint. TRIP indicates the predictive thyroid hormone receptor interacting domain. The PDGFRβ split tyrosine kinase domain is shown. tm indicates transmembrane domain.
Schematic of the CEV14-PDGFRβ fusion protein. The nucleotide and deduced amino acids sequences near the breakpoint of the CEV14-PDGFRβ fusion are shown at the bottom of the figure. The breakpoint is indicated by an arrow. CEV14 has a leucine zipper motif 5′ of the breakpoint. TRIP indicates the predictive thyroid hormone receptor interacting domain. The PDGFRβ split tyrosine kinase domain is shown. tm indicates transmembrane domain.
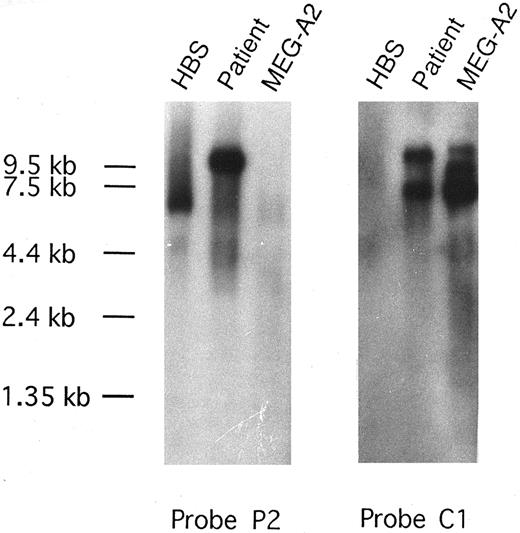
Expression of CEV14 and the CEV14-PDGFRβ fusion gene.Expression of the PDGFRβ, CEV14, and CEV14-PDGFRβ genes was examined by Northern blot analysis of RNA isolated from a blood sample of this patient, human BM stroma, and the MEG-A2 cell line (Fig 4). Wild-type 5.7-kb and 4.8-kb PDGFRβ transcripts were detected in BM stroma probed with the PDGFRβ probe P2, located 3′ of the translocation breakpoint. This probe also detects a novel 10-kb fusion transcript in the patient's sample, identical in size to that detected with probe C1. Some minor fusion products were also observed in patient's sample probed with P2. Three major CEV14 mRNA were detected in the MEG-A2 cell line with the C1 probe, while only one of these transcripts and the fusion cDNA were detected in this patient.
Northern blot analysis of the CEV14-PDGFRβ transcript. The PDGFRβ probe P2, located 3′ of the translocation breakpoint, detects wild-type 5.7-kb and 4.8-kb PDGFRβ transcripts in human BM stroma (HBS), while a novel 10-kb transcript is seen in the patient's leukemia cells with the t(5; 14) translocation. CEV14 probe C1 detects the same 10-kb transcript and one of the major CEV14 transcripts, while 10.5-kb, 9.0-kb, and 7.0-kb CEV14-specific transcripts are expressed in MEG-A2 cells.
Northern blot analysis of the CEV14-PDGFRβ transcript. The PDGFRβ probe P2, located 3′ of the translocation breakpoint, detects wild-type 5.7-kb and 4.8-kb PDGFRβ transcripts in human BM stroma (HBS), while a novel 10-kb transcript is seen in the patient's leukemia cells with the t(5; 14) translocation. CEV14 probe C1 detects the same 10-kb transcript and one of the major CEV14 transcripts, while 10.5-kb, 9.0-kb, and 7.0-kb CEV14-specific transcripts are expressed in MEG-A2 cells.
The tissue distribution of CEV14 transcripts in various human tissue were examined by Northern blot analysis (Fig 5). CEV14 was detected in all tissues and was expressed in heart, muscle, and pancreas at high levels and at weak levels in lung and liver.
Tissue distribution of CEV14 gene expression. MTN blot purchased from Clontech Laboratories was hybridized with probe C1. The RNA markers are aligned.
Tissue distribution of CEV14 gene expression. MTN blot purchased from Clontech Laboratories was hybridized with probe C1. The RNA markers are aligned.
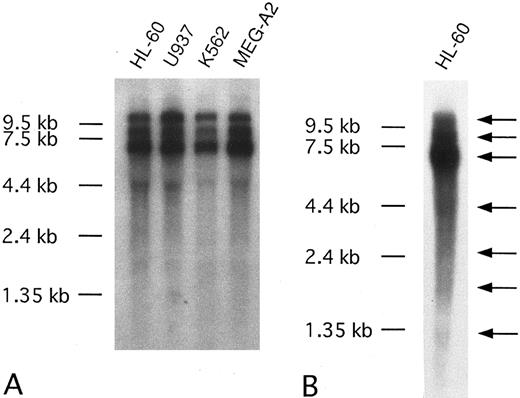
We also detected CEV14 expression in various cell lines originating from patients with hematologic malignancies with major transcripts at 10.5 kb, 9.0 kb, and 7.0 kb. There were also several minor transcripts. To detect minor transcripts, Northern blotting was performed with 20 μg of poly A+ RNA from HL-60; 4.5-kb, 2.5-kb, 1.7-kb, and 1.35-kb minor transcripts were revealed (Fig 6).
(A) Northern blot analysis of CEV14 in several kinds of leukemia cell lines. Poly(A)+ RNA (3 μg) was isolated from the following cell lines: HL-60, promyelocytic leukemia cell line; U937, monocytic leukemia cell line; K562, erythroleukemia cell line; MEG-A2, megakaryoblastic cell line. (B) Twenty micrograms of HL-60 poly(A)+ RNA was subjected to Northern blot analysis to detect minor transcripts. Probe C1 detects three major transcripts and several minor transcripts as indicated with an arrow.
(A) Northern blot analysis of CEV14 in several kinds of leukemia cell lines. Poly(A)+ RNA (3 μg) was isolated from the following cell lines: HL-60, promyelocytic leukemia cell line; U937, monocytic leukemia cell line; K562, erythroleukemia cell line; MEG-A2, megakaryoblastic cell line. (B) Twenty micrograms of HL-60 poly(A)+ RNA was subjected to Northern blot analysis to detect minor transcripts. Probe C1 detects three major transcripts and several minor transcripts as indicated with an arrow.
CEV14-PDGFRβ but not wild-type PDGFRβ is detected by RT-PCR analysis in the patient sample.RT-PCR analysis to assess expression of PDGFRβ, CEV14, CEV14-PDGFRβ, and PDGFRβ-CEV14 genes was performed in human BM stroma, MEG-A2, and patient's samples at first diagnosis and relapse phase. PDGFRβ expression was examined with primers located 5′ of the break point. PDGFRβ was detected only in human BM stroma. CEV14 was detected in all samples (Fig 7). Expression of the CEV14-PDGFRβ fusion product was detected only in the relapse phase of this patient. These data support the conclusion that the t(5:14) appeared only at relapse phase. Of note, the reciprocal PDGFRβ-CEV14 gene was not detected in any samples.
RT-PCR analysis of wild-type and chimeric transcripts. RNA samples were reverse transcribed and then amplified with 5′ and 3′ PDGFRβ primers (PDGFRβ), 5′ and 3′ CEV14 primers (CEV14), 5′ CEV14 and 3′ PDGFRβ primers (CEV14-PDGFRβ), or 5′ PDGFRβ and 3′ CEV14 primers (PDGFRβ-CEV14). PCR products were electrophoresed on 2% agarose gels and stained with ethidium bromide.
RT-PCR analysis of wild-type and chimeric transcripts. RNA samples were reverse transcribed and then amplified with 5′ and 3′ PDGFRβ primers (PDGFRβ), 5′ and 3′ CEV14 primers (CEV14), 5′ CEV14 and 3′ PDGFRβ primers (CEV14-PDGFRβ), or 5′ PDGFRβ and 3′ CEV14 primers (PDGFRβ-CEV14). PCR products were electrophoresed on 2% agarose gels and stained with ethidium bromide.
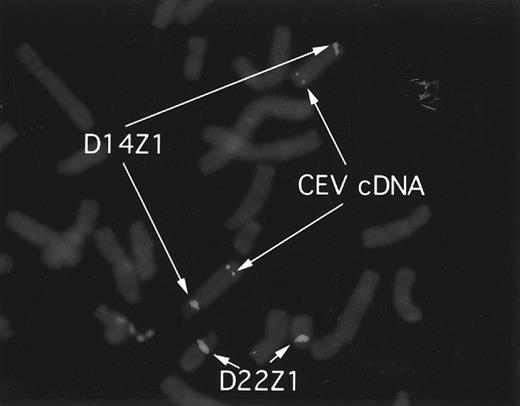
CEV14 is localized on chromosome 14q32.The identification of the reciprocal fusion gene products strongly suggests that CEV14 is located on chromosome 14q32. To confirm the location of CEV14, we performed fluorescence in situ hybridization (FISH) analysis using a 2.5-kb CEV14 cDNA probe on metaphases from normal cells (Fig 8). D14Z1/D22Z1 were used as chromosome 14 and 22 specific FISH probes, respectively. The observed hybridization signals for CEV14 were identified at 14q32.
FISH analysis and localization of CEV14. CEV14 cDNA was hybridized to metaphase chromosomes from normal human blood mononuclear cells. Chromosome 14 and chromosome 22 are visualized with D14Z1/D22Z1 probes, respectively. CEV14 is localized to 14q32.
FISH analysis and localization of CEV14. CEV14 cDNA was hybridized to metaphase chromosomes from normal human blood mononuclear cells. Chromosome 14 and chromosome 22 are visualized with D14Z1/D22Z1 probes, respectively. CEV14 is localized to 14q32.
DISCUSSION
We have obtained a partial clone of a novel gene, CEV14, which is fused to PDGFRβ in a t(5; 14)(q33:q32) translocation appearing in a case of AML with an initial t(7:11) translocation. This is the second gene partner identified as involving chromosomal rearrangements with PDGFRβ. The first identified translocation associated with PDGFRβ was detected in CMML with a t(5; 12) translocation in which TEL was fused to PDGFRβ.11
The activation of PDGFRβ promoting the cellular proliferation and the signal transduction pathway which it induces have been characterized.32,33 Receptor dimerization has been shown to be a critical step in tyrosine kinase activation of PDGFRβ and of other receptor tyrosine kinases.34 Recently, Carroll et al35 showed that TEL-PDGFRβ self-association mediated by the HLH domain of TEL resulted in constitutive activation of PDGFRβ. Similarly, in the case of the CEV14-PDGFRβ fusion described here, one hypothesis for the mechanism of transformation is that the fusion causes ectopic constitutive tyrosine kinase activation of PDGFRβ, leading to transformation via the ras signal transduction pathway. In addition to supplying an active promoter, it is possible that the leucine zipper domain in CEV14 may facilitate ligand-independent dimerization of PDGFRβ.
The 3′ region of CEV14 is homologous to TRIP11 which was isolated as one of the proteins that interacts with the thyroid hormone (T3) receptors (TRs) in the presence of T3.31 TRIP11 also showed similar ligand-dependent interactions with the retinoid X receptor (RXR). TRs are hormone-dependent transcription factors that regulate expression of a variety of specific target genes. We also identified a leucine zipper structure in CEV14 expressed in the fusion protein. The classic leucine zipper structure was first identified as a motif important for dimerization and concomitant binding to DNA36 and has since been shown to be involved in protein-protein interactions.37 Taken together, CEV14 may be a transcriptional factor or coactivator.
The total length of the cloned CEV14-PDGFRβ gene so far is approximately 5.6 kb, which is the fusion of PDGFRβ to a minor 2.5-kb CEV14 and was detected in Northern blots of RNA isolated from the patient's leukemia cells as a minor transcript. However, a major fusion gene transcript of 10 kb was detected and the remaining sequences of the CEV14 gene extending 5′ of the fusion remain obscure. Major transcripts of CEV14 were detected as 7-kb, 9-kb and 10.5-kb bands and some minor transcripts were revealed by Northern blot and cDNA cloning. Interestingly, although the normal 7-kb CEV14 band was detected in the patient's leukemia cells, other normal CEV14 transcripts were absent (Fig 4). Because CEV14 seems to be expressed constitutively in various tissues and hematopoietic cell lines, the absence of CEV14 transcripts may be a specific phenomenon in this case. Further analyses of genetic and protein structures are necessary to analyze this fusion product and the structure of CEV14 in detail.
This case is similar to childhood ALL with t(5; 14)(q31; q32) which has a rearrangement between IL-3 and IgH genes20,21 and exhibits eosinophilia. Initially, we assessed rearrangement of the IgH gene, but could not detect any rearrangements. In our case, overexpression of the IL-3 gene was not detected but overexpression of IL-5 was shown.23 Eosinophilia has been reported in recurrent chromosomal aberrations and 5q31-33 is often involved in myeloid disorders with eosinophilia. CMML with t(5; 12) has been reported to exhibit eosinophilia38-40 with similar manifestations as seen in the case reported here. Additionally, involvement of chromosome 5 has been observed in several cases with eosinophilia: t(5; 16) in AML M141 and AML M442 and t(1; 5) in infants with myeloproliferative disease,43 suggesting that PDGFRβ rearrangement may be involved in these cases as well.
There are several reports that indicate the importance of unidentified genes other than IgH for leukemia on 14q32. Hayashi et al22 reported that the IgH gene on 14q32 was not disrupted in some patients with childhood mixed lineage leukemia. Batanian et al44 suggested that t(6; 14)(q25; q32) may represent a nonrandom translocation in childhood acute mixed lineage leukemia. Although there are two proto-oncogenes (AKT-1 or ELK-2) mapped to 14q32, our results now suggest that CEV14 may be involved in translocation in other cases of leukemia.
ACKNOWLEDGMENT
We thank Makoto Kondo for chromosome analysis, Tetsuhito Kojima for helpful discussions, and Satoru Suzuki, Chika Wakamatsu, and Yoko Nakamura for their excellent technical assistance.
Address reprint requests to Akihiro Abe, MD, PhD, First Department of Internal Medicine, 65 Tsurumaicho, Showaku, Nagoya 466, Japan.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal