Abstract
The relationships between dihydrofolate reductase (DHFR) levels or methotrexate membrane transport and acute lymphoblastic leukemia (ALL) immunophenotype were evaluated on 51 T-cell and 44 B-precursor ALL specimens from 90 pediatric ALL patients at diagnosis and relapse, using a fluorescent methotrexate analog (PT430) and flow cytometry assay (Matherly et al, Blood 85:500, 1995). For T-cell ALL, 35 of 45 (78%) of newly diagnosed patients' specimens exhibited elevated DHFR relative to DHFR levels in ALL blasts from methotrexate-responsive patients. For 30 of 45 diagnostic T-ALL specimens, DHFR expression was heterogeneous, with up to 3 separate subpopulations covering a 44-fold range; the DHFR-overproducing fractions comprised 10% to 88% of the total blasts. Elevated DHFR was less common in B-precursor ALL at diagnosis, being detected in only 17 of 36 specimens (47%); 11 of these samples exhibited DHFR heterogeneity. Median maximal DHFR levels were fourfold higher in T-cell than B-precursor ALL at diagnosis. Within a particular phenotypic group, there were no correlations between DHFR levels and patient prognostic features, including age, sex, chromosomal abnormalities, white blood cell counts (WBCs), and percentage of S-phase. However, for B-precursor ALL, there was a notably higher fraction of African-American than white patients with elevated DHFR. For patients (both phenotypes) with low WBCs (<50,000/μL), event-free survival times were significantly shorter for those expressing DHFR above a threshold level than for patients expressing DHFR below this level (P < .016); this relationship was not seen for patients with high WBCs (<50,000/μL). Elevated DHFR was detected in 11 of 14 relapse specimens (5 of 6 T-cell and 6 of 8 B-precursor). Two of five paired relapse specimens (both T-cell) from patients treated with methotrexate exhibited increased DHFR levels over those at diagnosis (2.5- to 5-fold); the fraction of DHFR-overproducing blasts was also increased in 4 of 5 paired relapse specimens (2 B-precursor and 2 T-cell). In contrast to the variations in DHFR, highly impaired methotrexate transport was detected in only 6 of 95 ALL specimens, including both diagnosis and relapse. There was no correlation between phenotype and impaired transport. These data provide further rationale for the use of mechanistically based prognostic factors to complement known biologic or disease-based prognostic indicators in the design of ALL therapy including methotrexate, particularly with patients presenting with low WBCs. The finding of a markedly increased frequency of DHFR overexpression in T-cell over B-precursor ALL suggests that this difference is associated with the poorer prognosis of patients with T-cell ALL treated with standard-dose antimetabolite therapy and implies that higher-dose methotrexate (≥1 g/m2) during consolidation therapy may be useful in the treatment of this disease.
THE IMPROVED PROGNOSIS for children diagnosed with acute lymphoblastic leukemia (ALL) over the last 40 years has been nothing short of impressive. Once a universally fatal disease, with modern multiagent therapies and supportive care, remissions are commonly achieved in up to 95% of ALL patients and long-term disease-free survival rates approach 70%.1 Further improvements in treatment outcome may result if patients with potentially resistant disease can be better identified who may benefit from more intensive therapy.
An assortment of patient and disease features have already been associated with ALL prognosis, including sex, age, and presenting white blood count (WBC).1-5 A major advance was the recognition that childhood ALL is heterogeneous and composed of lymphoblasts at different stages of maturation and expressing characteristic immunologic surface markers.1-7 These immunophenotypically distinct ALL subgroups are generally associated with unique biochemical, clinical, and cytogenetic features.1-7 For B-precursor ALL, age, initial WBCs, and cytogenetic abnormalities (both numerical and structural) provide bases for treatment stratification.2-4 7 Conversely, T-cell ALL, representing approximately 15% of childhood ALL cases, is associated with a lower percentage of patients with cytogenetic or other features on which to base therapy. 2,3,5,7
Patients with T-ALL continue to fare worse than those with B-precursor ALL, with long-term survival rates approximating 50%.2,3,7 This disparity between these ALL phenotypes is not unexpected given that a large number of patient or disease features of T-ALL are widely associated with poor prognosis.2,3,5 6 Patients with T-ALL tend to be older and are more commonly male than those with B-precursor ALL. Furthermore, patients with T-ALL commonly present with extramedullary disease, anterior mediastinal mass, peripheral lymph node involvement, organomegaly, and high WBCs at diagnosis.
In the current treatment of childhood ALL, methotrexate (Mtx) is a key component of both consolidation and maintenance therapies and is administered intrathecally in the prophylaxis and treatment of central nervous system (CNS) leukemia.1,8 Determinants of Mtx sensitivity and resistance described in cultured cells have been reported for leukemic blasts from children as well.9-17 Hence, ALL patients' lymphoblasts exhibit low-level dihydrofolate reductase (DHFR) gene amplification (2- to 4-fold) and increased DHFR expression at diagnosis and relapse.11 Furthermore, ALL lymphoblasts from children accumulate long chain Mtx polyglutamates (4 to 6 glutamates) after Mtx exposure.10,12,14,16,17 Accumulations were highest in hyperdiploid B-precursor ALL14,17 and other good-risk patients,16 with lower Mtx polyglutamate levels in poor-risk B-precursor ALL9 and T-cell ALL patients.9,10 14
Characteristics of B-Precursor Specimens at Diagnosis
| No. . | Paired* . | Age (yr)† . | Sex . | Race . | WBC†‡ . | %Sρ . | % Blasts† . | RD (mo)1-155 . | Therapy . | DHFR1-154 . | %1-154 . | Cytogenetics . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BP1 | 5.5 | M | AA | 35,000 | 8.7 | 100 | On Rx | −1-167 | 2.6 | 100 | 46,XY/46,XY,del(4),del(6) | |
| BP2 | 6 | F | W | 18,600 | 1.3 | 99 | On Rx | −1-167 | 2.2 | 100 | 46,XX/46,XX,t(3; 9) | |
| BP3 | 6 | M | W | 9,700 | NA | 88 | On Rx | −1-167 | 1.1 | 100 | 46,XY/47,XY,+21,−13,der(9)t(9;?), | |
| der(12)t(12;?) | ||||||||||||
| BP41-160 | 0.2 | M | W | 684,000 | NA | 87 | 6 | VcMtMpVmArCy | 3.6 | 100 | 46,XX | |
| BP51-160 | 4 | M | AA | 36,000 | 2.5 | 92 | 15 | PrVcAsMpCy | 2.8 | 100 | 47,XY,+21 | |
| BP61-160 | 3.5 | F | W | 16,500 | NA | 96 | Off Rx | PrVcAsMtMpAr | 1.9 | 100 | 46,XX | |
| BP71-160 | 0.67 | F | AA | 17,900 | 14.4 | 95 | 5 | MtVmArCy | 3.3/18.0 | 32/68 | 46,XX/46,XX,t(11; 19) | |
| BP81-160 | 4 | F | W | 5,400 | NA | 88 | Off Rx | PrVcAsMtMpAr | 2.4 | 100 | NA | |
| BP91-160 | rBP6 | 4 | F | W | 23,000 | 1.8 | 98 | 24 | PrVcAsMtMpAr | 1.8 | 100 | 46,XX/46,XX,der(13),t(1; 13) |
| BP101-160 | 3.5 | F | W | 9,800 | 4.3 | 94 | Off Rx | PrVcAsMtMpAr | 1.8/17.6 | 83/17 | 46,XX/46,XX,del(12) | |
| BP111-160 | 7.5 | M | W | 31,500 | 7.1 | 95 | 351-164 | PrVcAsMtMp | 2.1 | 100 | 46,XY/54,XY,+X,+4,+8,+10,+18, | |
| +21,+21,i(7q),i(9q),+mar | ||||||||||||
| BP121-160 | 3.5 | F | AA | 38,000 | 21.8 | 96 | 9 | PrVcAsMtMpAr | 2.5/24.6 | 78/22 | 46,XX/46,XX,t(1; 19) | |
| BP131-160 | 8 | M | AA | 4,000 | 4.7 | 99 | Off Rx | PrVcAsMtMpAr | 1.9 | 100 | 46,XY/53,XY,+X,+6,+14,+17, | |
| +18,+21,+21 | ||||||||||||
| BP141-160 | 4 | M | W | 26,500 | 15.1 | 98 | 29 | PrVcAsMtMp | 2.8/20 | 69/31 | 46,XY/45,XY,−3,−7,−12,+dic | |
| (7; 12),+der(3),t(3;?) | ||||||||||||
| BP151-160 | 6.5 | F | W | 107,000 | 16.9 | 85 | 15 | PrVcAsMtMpAr | 1.6 | 100 | 46,XX/45,XX,−12,−17,−19,del(9), | |
| +der(19),t(1; 19),+dic(12; 17) | ||||||||||||
| BP16 | 7 | M | W | 5,100 | 9.9 | 97 | On Rx | −1-167 | 1.5 | 100 | 46,XY/46,XY,i(21) | |
| BP17 | 13.5 | M | AA | 72,000 | 3.5 | 93 | 36 | PrVcAsMtMpAr | 1.7 | 100 | 46,XY/46,XY,−12,−22,+dic(12; 22) | |
| BP18 | 5 | F | AA | 5,800 | 8 | 96 | Off Rx | PrVcAsMtMpAr | 2.0/19.6 | 83/17 | 46,XX | |
| BP19 | 8 | F | AA | 38,400 | 12 | 90 | 13 | PrVcAsAdMtMpAr | 4.0 | 100 | 46,XX/46,XX,t(9; 22) | |
| BP20 | rBP1 | 8 | M | AA | 17,900 | 14 | 89 | 8 | PrVcAsAdMtMpVmAr | 2.4/12.4 | 69/31 | NA |
| BP21 | 14 | F | W | 530,000 | 6.4 | 92 | 9 | PrVcAdMtMpAr | 2.4 | 100 | 46,XX/47,XXX,t(9; 22) | |
| BP22 | 5 | F | AA | 212,000 | NA | 93 | 18 | PrVcAsMtMpAr | 2.5/25.8 | 81/19 | 46,XX/46,XX,12p+,rearr17p | |
| BP23 | 2 | F | W | 12,400 | 8.6 | 89 | On Rx | −1-167 | 2.7 | 100 | NA | |
| BP24 | 12 | M | AA | 2,400 | 13.6 | 90 | On Rx | −1-167 | 2.0 | 100 | 46,XY/26,X,+16,+18,+21/52,−Y, | |
| +X,+10,+10,+18,+18, | ||||||||||||
| +21,+21 | ||||||||||||
| BP25 | 6 | F | AA | 21,000 | NA | 88 | On Rx | −1-167 | 5.5/38.7 | 85/15 | 46,XX | |
| BP26 | 5.5 | M | AA | 35,000 | 8.7 | 93 | 9 | PrVcAsDnMtMpAr | 2.7/18.8 | 89/11 | 46,XY/46,XY,der(19),t(1; 19) | |
| BP27 | 11 | M | AA | 98,500 | 2 | 92 | On Rx | −1-167 | 1.7 | 100 | NA | |
| BP28 | 11.5 | F | W | 120,000 | NA | 86 | −1-161 | −1-161 | 3.0 | 100 | 47,XX,+21 | |
| BP29 | 8 | M | W | 47,900 | 8.9 | 94 | −1-161 | −1-161 | 1.8/13.8 | 83/17 | 47,XY,+21/49,XXY,+14,+21 | |
| BP30 | 6 | F | AA | 5,700 | 4.6 | 82 | Off Rx | PrVcAsMtMp | 1.7 | 100 | NA | |
| BP31 | 4 | M | W | 3,300 | NA | 97 | Off Rx | PrVcAsMtMpAr | 1.6 | 100 | 46,XY | |
| BP32 | 9 | M | AA | 91,000 | 2.7 | 95 | On Rx | −1-167 | 1.7 | 100 | 46,XY | |
| BP331-160 | 15 | M | W | 595,000 | 4.8 | 96 | IF | PrVcAsAd | 2.1 | 100 | 46,XY/45,XY,−Y | |
| BP341-160 | 15 | M | W | 89,800 | NA | 80 | 4 | PrVcAsMtMpAr | 1.9 | 100 | NA | |
| BP351-160 | 4.5 | F | W | 216,000 | 16.1 | 99 | 14 | PrVcAsMtMpAr | 1.3 | 100 | 46,XX | |
| BP36 | 2.5 | F | W | 172,500 | 5 | 97 | On Rx | −1-167 | 1.9/9.5 | 88/12 | 46,XX/47,XX,del(9),+21 |
| No. . | Paired* . | Age (yr)† . | Sex . | Race . | WBC†‡ . | %Sρ . | % Blasts† . | RD (mo)1-155 . | Therapy . | DHFR1-154 . | %1-154 . | Cytogenetics . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BP1 | 5.5 | M | AA | 35,000 | 8.7 | 100 | On Rx | −1-167 | 2.6 | 100 | 46,XY/46,XY,del(4),del(6) | |
| BP2 | 6 | F | W | 18,600 | 1.3 | 99 | On Rx | −1-167 | 2.2 | 100 | 46,XX/46,XX,t(3; 9) | |
| BP3 | 6 | M | W | 9,700 | NA | 88 | On Rx | −1-167 | 1.1 | 100 | 46,XY/47,XY,+21,−13,der(9)t(9;?), | |
| der(12)t(12;?) | ||||||||||||
| BP41-160 | 0.2 | M | W | 684,000 | NA | 87 | 6 | VcMtMpVmArCy | 3.6 | 100 | 46,XX | |
| BP51-160 | 4 | M | AA | 36,000 | 2.5 | 92 | 15 | PrVcAsMpCy | 2.8 | 100 | 47,XY,+21 | |
| BP61-160 | 3.5 | F | W | 16,500 | NA | 96 | Off Rx | PrVcAsMtMpAr | 1.9 | 100 | 46,XX | |
| BP71-160 | 0.67 | F | AA | 17,900 | 14.4 | 95 | 5 | MtVmArCy | 3.3/18.0 | 32/68 | 46,XX/46,XX,t(11; 19) | |
| BP81-160 | 4 | F | W | 5,400 | NA | 88 | Off Rx | PrVcAsMtMpAr | 2.4 | 100 | NA | |
| BP91-160 | rBP6 | 4 | F | W | 23,000 | 1.8 | 98 | 24 | PrVcAsMtMpAr | 1.8 | 100 | 46,XX/46,XX,der(13),t(1; 13) |
| BP101-160 | 3.5 | F | W | 9,800 | 4.3 | 94 | Off Rx | PrVcAsMtMpAr | 1.8/17.6 | 83/17 | 46,XX/46,XX,del(12) | |
| BP111-160 | 7.5 | M | W | 31,500 | 7.1 | 95 | 351-164 | PrVcAsMtMp | 2.1 | 100 | 46,XY/54,XY,+X,+4,+8,+10,+18, | |
| +21,+21,i(7q),i(9q),+mar | ||||||||||||
| BP121-160 | 3.5 | F | AA | 38,000 | 21.8 | 96 | 9 | PrVcAsMtMpAr | 2.5/24.6 | 78/22 | 46,XX/46,XX,t(1; 19) | |
| BP131-160 | 8 | M | AA | 4,000 | 4.7 | 99 | Off Rx | PrVcAsMtMpAr | 1.9 | 100 | 46,XY/53,XY,+X,+6,+14,+17, | |
| +18,+21,+21 | ||||||||||||
| BP141-160 | 4 | M | W | 26,500 | 15.1 | 98 | 29 | PrVcAsMtMp | 2.8/20 | 69/31 | 46,XY/45,XY,−3,−7,−12,+dic | |
| (7; 12),+der(3),t(3;?) | ||||||||||||
| BP151-160 | 6.5 | F | W | 107,000 | 16.9 | 85 | 15 | PrVcAsMtMpAr | 1.6 | 100 | 46,XX/45,XX,−12,−17,−19,del(9), | |
| +der(19),t(1; 19),+dic(12; 17) | ||||||||||||
| BP16 | 7 | M | W | 5,100 | 9.9 | 97 | On Rx | −1-167 | 1.5 | 100 | 46,XY/46,XY,i(21) | |
| BP17 | 13.5 | M | AA | 72,000 | 3.5 | 93 | 36 | PrVcAsMtMpAr | 1.7 | 100 | 46,XY/46,XY,−12,−22,+dic(12; 22) | |
| BP18 | 5 | F | AA | 5,800 | 8 | 96 | Off Rx | PrVcAsMtMpAr | 2.0/19.6 | 83/17 | 46,XX | |
| BP19 | 8 | F | AA | 38,400 | 12 | 90 | 13 | PrVcAsAdMtMpAr | 4.0 | 100 | 46,XX/46,XX,t(9; 22) | |
| BP20 | rBP1 | 8 | M | AA | 17,900 | 14 | 89 | 8 | PrVcAsAdMtMpVmAr | 2.4/12.4 | 69/31 | NA |
| BP21 | 14 | F | W | 530,000 | 6.4 | 92 | 9 | PrVcAdMtMpAr | 2.4 | 100 | 46,XX/47,XXX,t(9; 22) | |
| BP22 | 5 | F | AA | 212,000 | NA | 93 | 18 | PrVcAsMtMpAr | 2.5/25.8 | 81/19 | 46,XX/46,XX,12p+,rearr17p | |
| BP23 | 2 | F | W | 12,400 | 8.6 | 89 | On Rx | −1-167 | 2.7 | 100 | NA | |
| BP24 | 12 | M | AA | 2,400 | 13.6 | 90 | On Rx | −1-167 | 2.0 | 100 | 46,XY/26,X,+16,+18,+21/52,−Y, | |
| +X,+10,+10,+18,+18, | ||||||||||||
| +21,+21 | ||||||||||||
| BP25 | 6 | F | AA | 21,000 | NA | 88 | On Rx | −1-167 | 5.5/38.7 | 85/15 | 46,XX | |
| BP26 | 5.5 | M | AA | 35,000 | 8.7 | 93 | 9 | PrVcAsDnMtMpAr | 2.7/18.8 | 89/11 | 46,XY/46,XY,der(19),t(1; 19) | |
| BP27 | 11 | M | AA | 98,500 | 2 | 92 | On Rx | −1-167 | 1.7 | 100 | NA | |
| BP28 | 11.5 | F | W | 120,000 | NA | 86 | −1-161 | −1-161 | 3.0 | 100 | 47,XX,+21 | |
| BP29 | 8 | M | W | 47,900 | 8.9 | 94 | −1-161 | −1-161 | 1.8/13.8 | 83/17 | 47,XY,+21/49,XXY,+14,+21 | |
| BP30 | 6 | F | AA | 5,700 | 4.6 | 82 | Off Rx | PrVcAsMtMp | 1.7 | 100 | NA | |
| BP31 | 4 | M | W | 3,300 | NA | 97 | Off Rx | PrVcAsMtMpAr | 1.6 | 100 | 46,XY | |
| BP32 | 9 | M | AA | 91,000 | 2.7 | 95 | On Rx | −1-167 | 1.7 | 100 | 46,XY | |
| BP331-160 | 15 | M | W | 595,000 | 4.8 | 96 | IF | PrVcAsAd | 2.1 | 100 | 46,XY/45,XY,−Y | |
| BP341-160 | 15 | M | W | 89,800 | NA | 80 | 4 | PrVcAsMtMpAr | 1.9 | 100 | NA | |
| BP351-160 | 4.5 | F | W | 216,000 | 16.1 | 99 | 14 | PrVcAsMtMpAr | 1.3 | 100 | 46,XX | |
| BP36 | 2.5 | F | W | 172,500 | 5 | 97 | On Rx | −1-167 | 1.9/9.5 | 88/12 | 46,XX/47,XX,del(9),+21 |
Abbreviations: W, white; AA, African-American; Ad, adriamycin; Ar, cytosine arabinoside; Cy, cyclophosphamide; Dn, daunorubicin; As, L-asparaginase; Mp, 6-mercaptopurine; Mt, methotrexate; Pr, prednisone; Vc, vincristine; Vm, Vm-26; NA, not available; IF, induction failure.
Paired relapse/diagnostic specimen. Relapse specimens are summarized in Table 3.
At diagnosis.
Units for WBCs are cells per microliter.
ρ Percentage of S-phase cells as determined by propidium iodide staining and flow cytometry.
Remission duration intervals (RD) correspond to the time from diagnosis to first relapse. Patients designated On Rx are still under treatment, whereas those designated Off Rx remain in remission and off treatment at least 30 months. In our analysis, specimens 6, 8, 10, 13, 18, 30, and 31 are from patients who are currently disease-free and off treatment.
Relative DHFR contents expressed as a single or multiple subpopulations (A/B/C). Percentage of total blasts in each subpopulation is indicated under column labeled %.
On treatment with current POG B-precursor ALL protocol including both intravenous and intramuscular Mtx.
Included in prior study.13
Testicular relapse.
Down syndrome patients not treated at parent's request.
Characteristics of T-ALL Specimens at Diagnosis
| No. . | Paired* . | Age (yr)† . | Sex . | Race . | WBC†‡ . | %Sρ . | Blasts† . | RD (mo)2-155 . | Therapy . | DHFR2-154 . | % . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | 2 | F | W | 33,300 | 13 | 84 | On Rx | −2-167 | 3.0 | 100 | |
| T2 | rT1 | 13.5 | F | AA | 163,000 | 19.9 | 99 | 10 | PrVcAsAdMtMp | 3.12 | 100 |
| ArVm | |||||||||||
| T3 | 7 | F | W | 568,000 | 17.8 | 90 | On Rx | −2-167 | 2.0/6.7 | 57/43 | |
| T4 | 13 | F | W | 57,400 | 10.6 | 100 | On Rx | −2-167 | 1.8/10.3/43.6 | 44/46/10 | |
| T5 | 7 | M | W | 40,200 | 12.4 | 82 | On Rx | −2-167 | 1.6/10.3 | 60/40 | |
| T6 | 9 | F | W | 147,200 | 16.6 | 87 | On Rx | −2-167 | 2.1/10.1/42.4 | 51/33/16 | |
| T7 | 7 | M | AA | 8,800 | 16.5 | 80 | On Rx | −2-167 | 2.1/9.3 | 45/55 | |
| T82-160 | 3.5 | F | W | 72,000 | NA | 90 | 8 | PrAdVcAsMtHu | 1.7/20.6 | 84/16 | |
| T92-160 | 14 | M | AA | 37,000 | NA | 90 | 8 | VcAdAsVpCyArMt | 1.2/11.6 | 88/12 | |
| 6 | M | W | 515,000 | NA | 84 | 29 | PrVcCyAdVmAr | 2.3 | 100 | ||
| T102-160 | AsMp | ||||||||||
| T11 | 9 | F | W | 104,000 | 6.4 | 95 | On Rx | −2-167 | 1.6/16.4 | 81/19 | |
| T12 | 15 | F | W | 122,000 | 6.1 | 82 | On Rx | −2-167 | 1.6/8.7 | 72/28 | |
| T13 | rT6 | 13 | M | AA | 80,000 | 9.4 | 94 | 5 | PrVcAdAsHu | 3.0 | 100 |
| ArMt | |||||||||||
| T14 | 13 | F | W | 175,000 | 12.7 | 97 | 6 | PrVcAsAdMtAr | 1.7/15.5 | 89/11 | |
| T15 | 2 | M | W | 71,900 | 11 | 98 | On Rx | −2-167 | 3.3/34.0 | 70/30 | |
| T16 | 10.5 | M | AA | 450,000 | NA | 92 | Off Rx | PrVcCvAdVpAsMpAr | 2.2/9.6 | 79/21 | |
| T17 | 2 | M | W | 111,000 | 9.2 | 94 | On Rx | −2-167 | 2.4/18.9 | 79/21 | |
| T18 | 7 | M | W | 89,800 | 13.3 | 91 | On Rx | −2-167 | 3.4 | 100 | |
| T19 | 2 | M | W | 502,000 | 12.1 | 99 | On Rx | −2-167 | 1.7/8.8/35.9 | 44/36/20 | |
| T20 | 14 | M | W | 222,000 | 4.2 | 99 | Off Rx | PrVcCyAdVpAsMpAr | 1.8/20.1 | 79/21 | |
| T21 | 11 | M | W | 32,900 | 6.1 | 93 | On Rx | −2-167 | 1.7 | 100 | |
| T22 | 5 | M | W | 28,700 | 19.5 | 93 | On Rx | −2-167 | 1.8/22.3 | 12/88 | |
| T23 | 7 | M | W | 258,000 | 3.4 | 92 | On Rx | −2-167 | 2.2/13.1 | 87/13 | |
| T24 | 4 | F | W | 960,000 | 2.7 | 96 | On Rx | −2-167 | 1.2/4.0 | 80/20 | |
| T25 | 12 | F | W | 248,000 | 9.3 | 99 | On Rx | −2-167 | 2.2/12.7 | 88/12 | |
| T26 | 6 | M | W | 346,200 | 3.6 | 97 | On Rx | −2-167 | 1.3 | 100 | |
| T27 | 13 | M | W | 131,000 | 14.7 | 98 | On Rx | −2-167 | 1.0/2.6/10.6 | 18/52/30 | |
| T28 | 4 | M | W | 45,200 | 7.2 | 91 | On Rx | −2-167 | 1.7/9.0 | 84/16 | |
| T29 | 6.5 | M | W | 117,000 | 19.3 | 83 | On Rx | −2-167 | 3.0 | 100 | |
| T30 | rT2 | 13.5 | M | W | 176,000 | 11.9 | 96 | 12 | PrVcAdAsAr | 1.7/23.2 | 76/24 |
| T31 | 3 | M | W | 395,100 | 2.4 | 95 | On Rx | −2-167 | 3.8/38.0 | 90/10 | |
| T32 | 13 | M | W | 376,000 | 6.7 | 98 | On Rx | −2-167 | 1.0 | 100 | |
| T33 | 17 | M | W | 210,000 | 5.6 | 98 | On Rx | −2-167 | 2.7 | 100 | |
| T34 | 2 | F | AA | 1,063,700 | 6.5 | 94 | On Rx | −2-167 | 1.8 | 100 | |
| T35 | 5 | M | W | 140,000 | 9 | 93 | On Rx | −2-167 | 2.0/12.6 | 83/17 | |
| T36 | 6 | M | W | 320,000 | 1.8 | 99 | On Rx | −2-167 | 2.0 | 100 | |
| T37 | 10 | M | W | 180,500 | 0.8 | 94 | On Rx | −2-167 | 1.2 | 100 | |
| T38 | 4 | M | W | 801,000 | 7.6 | 83 | On Rx | −2-167 | 1.6/8.9 | 89/11 | |
| T39 | 7 | M | W | 400,000 | 6.3 | 96 | On Rx | −2-167 | 2.4/31.5 | 75/25 | |
| T40 | 13 | M | W | 20,000 | 2.1 | 98 | On Rx | −2-167 | 2.3/8.5 | 75/25 | |
| T41 | 3 | M | W | 118,000 | NA | 92 | On Rx | −2-167 | 2.0 | 100 | |
| T42 | 16.5 | F | AA | 168,000 | 4.9 | 95 | On Rx | −2-167 | 1.9 | 100 | |
| T43 | 18 | M | W | 214,000 | NA | 90 | On Rx | −2-167 | 1.5/7.2 | 80/20 | |
| T44 | 14 | M | W | 82,900 | 10.2 | 92 | On Rx | −2-167 | 2.4/11.2 | 75/25 | |
| T45 | 14 | M | W | 57,000 | 17.1 | 93 | On Rx | −2-167 | 2.8/14.3/50.5 | 47/30/23 |
| No. . | Paired* . | Age (yr)† . | Sex . | Race . | WBC†‡ . | %Sρ . | Blasts† . | RD (mo)2-155 . | Therapy . | DHFR2-154 . | % . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | 2 | F | W | 33,300 | 13 | 84 | On Rx | −2-167 | 3.0 | 100 | |
| T2 | rT1 | 13.5 | F | AA | 163,000 | 19.9 | 99 | 10 | PrVcAsAdMtMp | 3.12 | 100 |
| ArVm | |||||||||||
| T3 | 7 | F | W | 568,000 | 17.8 | 90 | On Rx | −2-167 | 2.0/6.7 | 57/43 | |
| T4 | 13 | F | W | 57,400 | 10.6 | 100 | On Rx | −2-167 | 1.8/10.3/43.6 | 44/46/10 | |
| T5 | 7 | M | W | 40,200 | 12.4 | 82 | On Rx | −2-167 | 1.6/10.3 | 60/40 | |
| T6 | 9 | F | W | 147,200 | 16.6 | 87 | On Rx | −2-167 | 2.1/10.1/42.4 | 51/33/16 | |
| T7 | 7 | M | AA | 8,800 | 16.5 | 80 | On Rx | −2-167 | 2.1/9.3 | 45/55 | |
| T82-160 | 3.5 | F | W | 72,000 | NA | 90 | 8 | PrAdVcAsMtHu | 1.7/20.6 | 84/16 | |
| T92-160 | 14 | M | AA | 37,000 | NA | 90 | 8 | VcAdAsVpCyArMt | 1.2/11.6 | 88/12 | |
| 6 | M | W | 515,000 | NA | 84 | 29 | PrVcCyAdVmAr | 2.3 | 100 | ||
| T102-160 | AsMp | ||||||||||
| T11 | 9 | F | W | 104,000 | 6.4 | 95 | On Rx | −2-167 | 1.6/16.4 | 81/19 | |
| T12 | 15 | F | W | 122,000 | 6.1 | 82 | On Rx | −2-167 | 1.6/8.7 | 72/28 | |
| T13 | rT6 | 13 | M | AA | 80,000 | 9.4 | 94 | 5 | PrVcAdAsHu | 3.0 | 100 |
| ArMt | |||||||||||
| T14 | 13 | F | W | 175,000 | 12.7 | 97 | 6 | PrVcAsAdMtAr | 1.7/15.5 | 89/11 | |
| T15 | 2 | M | W | 71,900 | 11 | 98 | On Rx | −2-167 | 3.3/34.0 | 70/30 | |
| T16 | 10.5 | M | AA | 450,000 | NA | 92 | Off Rx | PrVcCvAdVpAsMpAr | 2.2/9.6 | 79/21 | |
| T17 | 2 | M | W | 111,000 | 9.2 | 94 | On Rx | −2-167 | 2.4/18.9 | 79/21 | |
| T18 | 7 | M | W | 89,800 | 13.3 | 91 | On Rx | −2-167 | 3.4 | 100 | |
| T19 | 2 | M | W | 502,000 | 12.1 | 99 | On Rx | −2-167 | 1.7/8.8/35.9 | 44/36/20 | |
| T20 | 14 | M | W | 222,000 | 4.2 | 99 | Off Rx | PrVcCyAdVpAsMpAr | 1.8/20.1 | 79/21 | |
| T21 | 11 | M | W | 32,900 | 6.1 | 93 | On Rx | −2-167 | 1.7 | 100 | |
| T22 | 5 | M | W | 28,700 | 19.5 | 93 | On Rx | −2-167 | 1.8/22.3 | 12/88 | |
| T23 | 7 | M | W | 258,000 | 3.4 | 92 | On Rx | −2-167 | 2.2/13.1 | 87/13 | |
| T24 | 4 | F | W | 960,000 | 2.7 | 96 | On Rx | −2-167 | 1.2/4.0 | 80/20 | |
| T25 | 12 | F | W | 248,000 | 9.3 | 99 | On Rx | −2-167 | 2.2/12.7 | 88/12 | |
| T26 | 6 | M | W | 346,200 | 3.6 | 97 | On Rx | −2-167 | 1.3 | 100 | |
| T27 | 13 | M | W | 131,000 | 14.7 | 98 | On Rx | −2-167 | 1.0/2.6/10.6 | 18/52/30 | |
| T28 | 4 | M | W | 45,200 | 7.2 | 91 | On Rx | −2-167 | 1.7/9.0 | 84/16 | |
| T29 | 6.5 | M | W | 117,000 | 19.3 | 83 | On Rx | −2-167 | 3.0 | 100 | |
| T30 | rT2 | 13.5 | M | W | 176,000 | 11.9 | 96 | 12 | PrVcAdAsAr | 1.7/23.2 | 76/24 |
| T31 | 3 | M | W | 395,100 | 2.4 | 95 | On Rx | −2-167 | 3.8/38.0 | 90/10 | |
| T32 | 13 | M | W | 376,000 | 6.7 | 98 | On Rx | −2-167 | 1.0 | 100 | |
| T33 | 17 | M | W | 210,000 | 5.6 | 98 | On Rx | −2-167 | 2.7 | 100 | |
| T34 | 2 | F | AA | 1,063,700 | 6.5 | 94 | On Rx | −2-167 | 1.8 | 100 | |
| T35 | 5 | M | W | 140,000 | 9 | 93 | On Rx | −2-167 | 2.0/12.6 | 83/17 | |
| T36 | 6 | M | W | 320,000 | 1.8 | 99 | On Rx | −2-167 | 2.0 | 100 | |
| T37 | 10 | M | W | 180,500 | 0.8 | 94 | On Rx | −2-167 | 1.2 | 100 | |
| T38 | 4 | M | W | 801,000 | 7.6 | 83 | On Rx | −2-167 | 1.6/8.9 | 89/11 | |
| T39 | 7 | M | W | 400,000 | 6.3 | 96 | On Rx | −2-167 | 2.4/31.5 | 75/25 | |
| T40 | 13 | M | W | 20,000 | 2.1 | 98 | On Rx | −2-167 | 2.3/8.5 | 75/25 | |
| T41 | 3 | M | W | 118,000 | NA | 92 | On Rx | −2-167 | 2.0 | 100 | |
| T42 | 16.5 | F | AA | 168,000 | 4.9 | 95 | On Rx | −2-167 | 1.9 | 100 | |
| T43 | 18 | M | W | 214,000 | NA | 90 | On Rx | −2-167 | 1.5/7.2 | 80/20 | |
| T44 | 14 | M | W | 82,900 | 10.2 | 92 | On Rx | −2-167 | 2.4/11.2 | 75/25 | |
| T45 | 14 | M | W | 57,000 | 17.1 | 93 | On Rx | −2-167 | 2.8/14.3/50.5 | 47/30/23 |
Abbreviations: W, white; AA, African-American; Ar, cytosine arabinoside; Ad, adriamycin; Cy, cyclophosphamide; Dn, daunorubicin; Hu, hydroxyurea; Mp, 6-mercaptopurine, Mt, methotrexate, Pr, prednisone, Vc, vincristine; Vm, VM-26; Vp, Vp-16; NA, not available.
Paired relapse/diagnostic specimen. Relapse specimens are summarized in Table 3.
At diagnosis.
Units for WBCs are cells per microliter.
ρ Percentage of S-phase cells, as determined by propidium iodide staining and flow cytometry.
Remission duration intervals (RD) correspond to the time from diagnosis to first relapse. Patients designated On Rx are still under treatment whereas those designated Off Rx remain in remission and off treatment at least 30 months. Specimens T6 and T20 are from patients who are currently disease-free and off treatment.
Relative DHFR contents expressed as a single or multiple subpopulations (A/B/C). Percentage of total blasts in each subpopulation is indicated under column labeled %.
Patient on treatment with current POG T-ALL protocol which utilizes methotrexate (both intravenous and intramuscular) and anthracyclines.
Patients included in previous study.13
Characteristics of Relapse ALL Specimens
| No. . | Paired3-150 . | Age (yr)3-151 . | Sex . | Race . | WBC†‡ . | %Sρ . | % Blasts3-151 . | RD (mo)3-155 . | DHFR3-154 . | % . |
|---|---|---|---|---|---|---|---|---|---|---|
| rBP1 | BP20 | 8 | M | AA | 17,900 | NA | 95 | 8 | 6.9 | 100 |
| rBP2# | 15 | M | AA | 43,600 | NA | 96 | 9 | 2.4/14.8 | 75/25 | |
| rBP3# | 2 | F | W | 304,800 | NA | 75 | 5 | 1.6 | 100 | |
| rBP4# | 5 | M | W | 4,000 | NA | 90 | 36 | 4.3 | 100 | |
| rBP5# | 3.5 | M | W | 5,900 | NA | 95 | 45 | 2.0/6.9 | 60/40 | |
| rBP6# | BP9 | 4 | F | W | 23,000 | NA | 76 | 24 | 1.0 | 100 |
| rBP79# | 10 | M | W | 3,800 | NA | 80 | 19.5 | 1.5/12.3 | 16/84 | |
| rBP8 | 15.5 | M | W | 4,300 | NA | 70 | 27 | 4.9 | 100 | |
| rT1 | T2 | 14 | F | AA | 163,000 | NA | 61 | 10 | 7.9 | 100 |
| rT2 | T30 | 13.5 | M | W | 176,000 | NA | 80 | 12 | 2.1/19.6 | 22/78 |
| rT3# | 6 | M | W | 79,000 | NA | 90 | 15 | 2.1/15.1 | 59/41 | |
| rT4# | 5 | M | W | 3,800 | NA | 90 | 6.5 | 2.6 | 100 | |
| rT5# | 10 | M | W | 1,400 | NA | 91 | 17 | 1.9 | 100 | |
| rT6 | T13 | 13 | M | AA | 80,000 | NA | 82 | 5 | 12.2 | 100 |
| No. . | Paired3-150 . | Age (yr)3-151 . | Sex . | Race . | WBC†‡ . | %Sρ . | % Blasts3-151 . | RD (mo)3-155 . | DHFR3-154 . | % . |
|---|---|---|---|---|---|---|---|---|---|---|
| rBP1 | BP20 | 8 | M | AA | 17,900 | NA | 95 | 8 | 6.9 | 100 |
| rBP2# | 15 | M | AA | 43,600 | NA | 96 | 9 | 2.4/14.8 | 75/25 | |
| rBP3# | 2 | F | W | 304,800 | NA | 75 | 5 | 1.6 | 100 | |
| rBP4# | 5 | M | W | 4,000 | NA | 90 | 36 | 4.3 | 100 | |
| rBP5# | 3.5 | M | W | 5,900 | NA | 95 | 45 | 2.0/6.9 | 60/40 | |
| rBP6# | BP9 | 4 | F | W | 23,000 | NA | 76 | 24 | 1.0 | 100 |
| rBP79# | 10 | M | W | 3,800 | NA | 80 | 19.5 | 1.5/12.3 | 16/84 | |
| rBP8 | 15.5 | M | W | 4,300 | NA | 70 | 27 | 4.9 | 100 | |
| rT1 | T2 | 14 | F | AA | 163,000 | NA | 61 | 10 | 7.9 | 100 |
| rT2 | T30 | 13.5 | M | W | 176,000 | NA | 80 | 12 | 2.1/19.6 | 22/78 |
| rT3# | 6 | M | W | 79,000 | NA | 90 | 15 | 2.1/15.1 | 59/41 | |
| rT4# | 5 | M | W | 3,800 | NA | 90 | 6.5 | 2.6 | 100 | |
| rT5# | 10 | M | W | 1,400 | NA | 91 | 17 | 1.9 | 100 | |
| rT6 | T13 | 13 | M | AA | 80,000 | NA | 82 | 5 | 12.2 | 100 |
Abbreviations: W, white; AA, African-American; NA, not available.
At diagnosis.
Units for WBCs are cells per microliter.
ρ Percentage of S-phase cells, as determined by propidium iodide staining and flow cytometry.
Remission duration intervals (RD) correspond to the time from diagnosis to first relapse.
Relative DHFR contents expressed as a single or multiple subpopulations (A/B/C). Percentage of total blasts in each subpopulation is indicated under column labeled %.
# Patients included in previous study.13
Our recent report13 showed the facility by which flow cytometry, combined with a fluorescent Mtx analog [PT430; (N-(4-amino-4-deoxy-N10-methylpteroyl)-Nε-(4′-fluoresceinthiocarbamyl)-L-lysine)] can be used to assay Mtx resistance markers in childhood ALL. In this prior study of 28 patients, heterogeneous expression of both increased DHFR and impaired Mtx transport was detected both at diagnosis and relapse. In this report, we refine our earlier analysis in a significantly expanded group of ALL patients. We describe herein studies on 95 specimens (44 B-precursor and 51 T-ALL, including both diagnosis and relapse) from 90 children with ALL that explore the relationships between DHFR levels and Mtx transport, ALL immunophenotype, and response to chemotherapy including Mtx.
MATERIALS AND METHODS
Chemicals.Mtx and trimetrexate were obtained from the Drug Development Branch, National Cancer Institute (Bethesda, MD). Mtx was purified by reversed-phase high performance liquid chromatography as described previously.18 PT430 was synthesized and purified as described earlier.19 Tissue culture reagents and supplies were purchased from assorted vendors with the exception of iron-supplemented calf serum, which was purchased from Hyclone Laboratories, Inc (Logan, UT). Assorted chemicals and biochemicals were obtained from Sigma Chemical Co (St Louis, MO).
Patient specimens.Leukemia specimens (summarized in Tables 1, 2, and 3) were obtained from newly diagnosed or relapsed patients with ALL treated at the Children's Hospital of Michigan (CHM) from 1983 to 1996 or Pediatric Oncology Group (POG) centers participating in the ongoing POG 9400 ALL classification study. Twenty-seven samples (21 B-precursor and 6 T-ALL) were from our previous study.13 Patients treated at CHM were enrolled on several POG treatment protocols (POG 8036, 8205, 8493, 8602, 9005, 9006, 9086, 9203, and 9406) or a CHM T-cell leukemia protocol. Mtx was a common component of these treatment protocols (1 g/m2 admnistered intravenously during consolidation, 20 g/m2 during maintenance, and intrathecal Mtx for CNS prophylaxis) with the exception of POG 9086 (only intrathecal Mtx), CHM T-cell (30 mg/kg intravenous Mtx), POG8036 (15 mg/m2 intravenous Mtx), and POG 8493 (oral Mtx). Chemotherapy for T-ALL was typically more intensive than for B-precursor ALL and included both anthracyclines and epipodophyllotoxins, with or without Mtx. The chemotherapy administered to newly diagnosed patients is summarized in Tables 1 and 2.
Blasts were separated from bone marrow by standard Ficoll Hypaque density centrifugation and either used directly or cryopreserved in RPMI 1640/10% fetal bovine serum/10% dimethyl sulfoxide in liquid nitrogen. Specimens were documented for patient age, sex, race, presenting WBC, percentage of blasts in marrow, S-phase percentages, and immunophenotype; cytogenetic data were available for most of the B-precursor specimens. Because the majority of patients studied are still receiving chemotherapy, information relating to treatment response and/or other data on patient outcome were available for only a small cohort of patients previously treated at CHM.
PT430 assay of cellular DHFR and Mtx membrane transport.Blasts were suspended in complete RPMI 1640 containing 10% supplemented calf serum, L-glutamine, and antibiotics, and viabilities were evaluated microscopically by trypan-blue exclusion before treatment with PT430 (see below). Cultured K562 cells or leukemic blasts (1 to 2 × 106) were incubated with PT430 (20 μmol/L) for 12 to 14 hours in complete medium in the presence of 10 μmol/L thymidine and 60 μmol/L adenosine. Cells were washed with Ca2+/Mg2+-free Dulbecco's phosphate-buffered saline (DPBS) at 0°C to 4°C (3×) and resuspended into PT430-free medium at 0.5 to 1 × 106 cells/mL for 5 hours at 37°C in the absence of any additions or the presence of Mtx or trimetrexate (at 0.5 μmol/L). After additional washes (2×) with Ca2+/Mg2+-free DPBS, cells were suspended in DPBS containing 0.01% calf serum for flow cytometry. Autofluorescence controls were processed identically except that the PT430 treatment was omitted. All experiments were performed in parallel with K562 controls.
Flow cytometry analysis was performed on 10,000 cells with a Becton Dickinson FACScan flow cytometer (Becton Dickinson, San Jose, CA). Nonviable cells were excluded from the analysis by gating out propidium-iodide–stained cells.20 Spectral overlap of the green (fluorescein isothiocyanate) into the red (propidium iodide) fluorescence spectrum was electronically compensated. Excitation was at 488 nm; emission was detected at 525 nm. As validated in our earlier study,13 the ratio of nonexchangeable PT430/autofluorescence reflects relative cellular DHFR; moreover, the residual PT430 fluorescence after Mtx treatment to that with exposure to trimetrexate closely correlates with impaired Mtx transport.13
Although the majority of the samples were cryopreserved before the experiment, a number of specimens were analyzed as fresh samples (T17, T18, T19, T24, BP23, BP24, BP36, and rBP8 in Tables 1, 2, and 3) immediately after Ficoll-Hypaque centrifugation. For a few samples (samples T19, T24, and BP23), assays were performed on identical fresh and cryopreserved specimens with the same results.
Statistical analysis.Loess fits and Pearson's correlations were used to investigate the relationships between continuous variables. Categorical variables were analyzed by Pearson's χ2 and Fisher's exact tests. Groups of data were compared using t-tests or a nonparametric Mann-Whitney U test. Relationships between experimental parameters and events (relapse, death) were examined by logistic regression21 and classification trees.22,23 The former fits a function of the probability of an event to a linear function of covariates, such as age, sex, WBC, or maximal DHFR. Classification trees were used for examining the relationships between patient outcome and variables (ie, DHFR and Mtx transport) for which there existed a normal range of values. This approach looks for an optimal split that best discriminates between outcome and can subsequently be validated. In our analysis, normal DHFR (fluorescence ratios of 1.6 to 2.4) and normal Mtx transport (fluorescence ratios of 1.0 to 1.2) were initially defined by 5 patients (patients BP6, 8, 13, 30, and 31; Table 1) who have remained in first remission for 5 years or longer off therapy. Experimentally measured values in excess of these ranges were considered to be elevated or impaired, as appropriate, and, consequently, to reflect a higher risk of relapse. Once such a cutpoint was empirically identified, classification trees were used to establish its validity. Classification tree analysis determined that a fluorescence ratio of 2.6 for DHFR discriminated best between patients who relapsed and/or died and those that did not, a result that closely agrees with the maximal level of normal DHFR. By analogy, blast specimens exhibiting fluorescence ratios in excess of 1.2 were considered transport-impaired; however, because transport impairment was so infrequent, it was not possible to perform a classification tree validation of this number, as for DHFR. In separate experiments, similar values for DHFR and transport were measured in mononuclear cells from bone marrow aspirates obtained from 3 patients on maintenance chemotherapy for which no leukemia was detected (ratios of 1.5 to 1.9 and 1.0 to 1.1, respectively) and in peripheral blood lymphocytes from 2 normal individuals (ratios of 7E1.5 and 7E1.0, respectively). Event-free survival times (EFS) between groups were compared with Kaplan-Meier curves and the log rank test.24 S-plus for Windows (V.3.3; Mathsoft, Inc, Seattle, WA) and SPSS for Windows (V.7.1.2; SPSS, Inc, Chicago, IL) were the statistical software used.
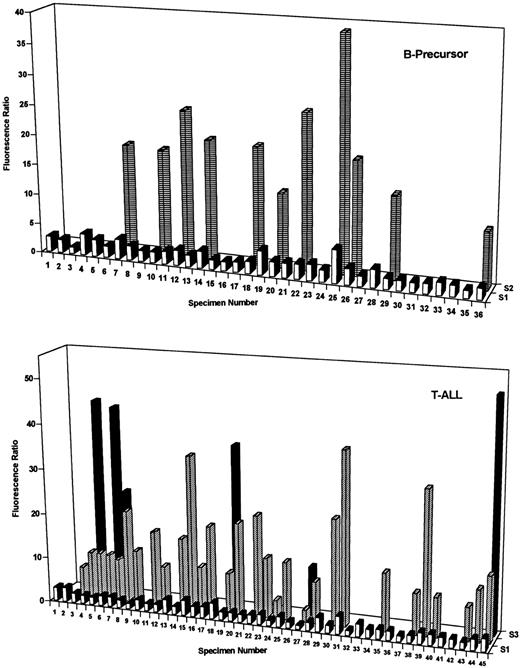
DHFR contents of B-precursor and T-cell ALL at diagnosis. DHFR levels were assayed with PT430 and flow cytometry and are plotted as relative fluorescence ratios (ie, nonexchangeable PT430/autofluorescence) versus patient numbers for one to three separate blast subpopulations (designated S1 through S3). Patient characteristics and, for heterogeneous populations, percentages for each fluorescent blast subpopulation are indicated in Tables 1 and 2. As described in Materials and Methods, normal DHFR (fluorescence ratios of 1.6 to 2.4) was defined by 5 patients (BP6, 8, 13, 30, and 31) who survived at least 5 years without relapse. Experimentally measured DHFR values in excess of this range were considered elevated.
DHFR contents of B-precursor and T-cell ALL at diagnosis. DHFR levels were assayed with PT430 and flow cytometry and are plotted as relative fluorescence ratios (ie, nonexchangeable PT430/autofluorescence) versus patient numbers for one to three separate blast subpopulations (designated S1 through S3). Patient characteristics and, for heterogeneous populations, percentages for each fluorescent blast subpopulation are indicated in Tables 1 and 2. As described in Materials and Methods, normal DHFR (fluorescence ratios of 1.6 to 2.4) was defined by 5 patients (BP6, 8, 13, 30, and 31) who survived at least 5 years without relapse. Experimentally measured DHFR values in excess of this range were considered elevated.
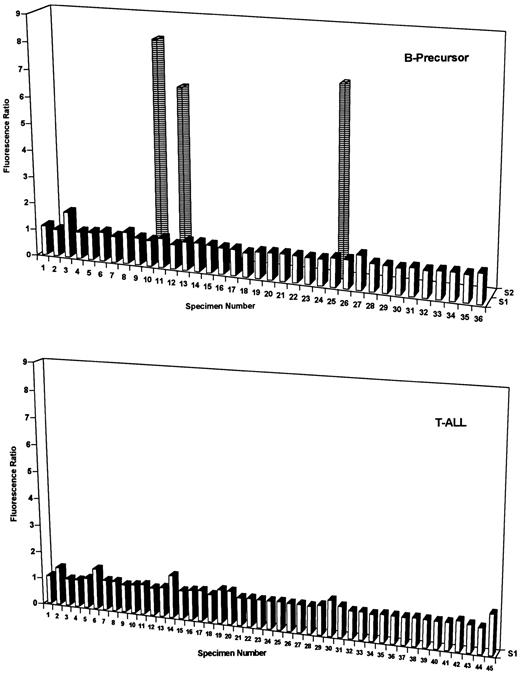
MTX transport impairment of B-precursor and T-cell ALL blasts at diagnosis. MTX transport impairment was assayed with PT430 and flow cytometry and data are plotted as relative fluorescence ratios (ie, PT430 fluorescence with MTX to trimetrexate treatments) versus patient numbers for one or two separate blast subpopulations (designated S1 and S2). The higher Mtx/trimetrexate fluorescence ratios reflect impaired Mtx transport. Specimen numbers are from Tables 1 and 2. As described in Materials and Methods, normal Mtx transport was taken as fluorescence ratios of 1 to 1.2 and ratios in excess of 1.2 were considered impaired. For the 3 B-precursor specimens (10, 12, and 25) expressing heterogeneous Mtx transport, impaired Mtx transport was detected in only a small fraction of the total blasts (14%, 22%, and 16%, respectively).
MTX transport impairment of B-precursor and T-cell ALL blasts at diagnosis. MTX transport impairment was assayed with PT430 and flow cytometry and data are plotted as relative fluorescence ratios (ie, PT430 fluorescence with MTX to trimetrexate treatments) versus patient numbers for one or two separate blast subpopulations (designated S1 and S2). The higher Mtx/trimetrexate fluorescence ratios reflect impaired Mtx transport. Specimen numbers are from Tables 1 and 2. As described in Materials and Methods, normal Mtx transport was taken as fluorescence ratios of 1 to 1.2 and ratios in excess of 1.2 were considered impaired. For the 3 B-precursor specimens (10, 12, and 25) expressing heterogeneous Mtx transport, impaired Mtx transport was detected in only a small fraction of the total blasts (14%, 22%, and 16%, respectively).
RESULTS
DHFR contents and Mtx transport were measured by PT430/flow cytometry assay13 on 44 B-precursor and 51 T-cell ALL specimens from 90 patients either at diagnosis or relapse (Tables 1, 2, and 3). Median blast percentages were consistently high and nearly identical (92.5% and 93.5%, respectively) for the B-precursor and T-cell ALL groups. Minor differences were observed between the phenotypes in median age at diagnosis (6 v 8 years for B-precursor and T-cell ALL, respectively) and median percentage of S-phase (8.0% v 9.2%, respectively). Characteristic (and more significant) differences were seen in the prevalence of males (male:female ratio of 1.3 v 2.7, respectively) and median WBCs at diagnosis (26,500/μL v 135,500/μL, respectively).
DHFR levels in ALL specimens are uniformly low compared with cultured cells.*
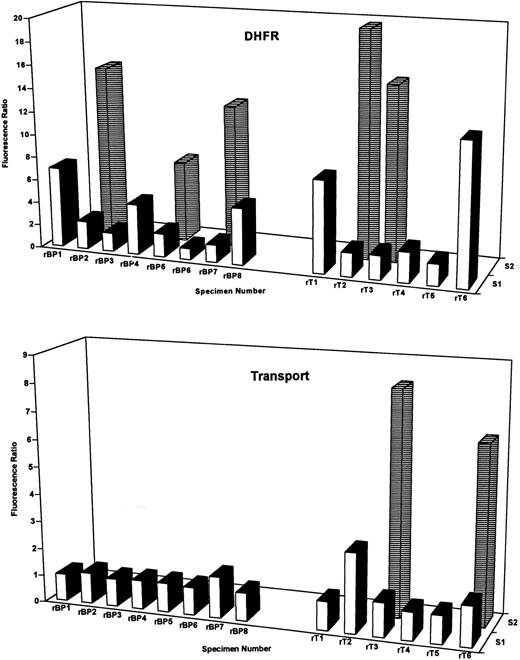
Moreover, DHFR expression in leukemic blasts is often heterogeneous with frequently 2 and occasionally 3 separate subpopulations with differing DHFR contents.13 It was notable that the frequency of heterogeneous and elevated DHFR at diagnosis was highly lineage specific. Thus, 35 of 45 diagnostic T-ALL specimens (78%) exhibited increased DHFR over levels in Mtx-responsive patients (ie, fluorescence ratio exceeding 2.4), and 30 of these were characterized by 2 or 3 subpopulations (Fig 1, lower panel, and Table 2). By contrast, elevated DHFR was detected in only 17 of 36 (47%) diagnostic B-precursor specimens (11 with 2 subpopulations; Fig 2, upper panel, and Table 1). Whereas the percentages of total cells with elevated DHFR in the heterogeneous samples were similar between the groups (range, 10% to 88%), maximal DHFR levels for T-ALL (median, 10.3; range, 1 to 50.5) and B-precursor specimens (median, 2.4; range, 1.1 to 38.7) were significantly different (P < .01 by Mann-Whitney U test).
For both immunophenotypes, impaired Mtx transport by flow cytometry assay was both infrequent and generally of low magnitude at diagnosis. For instance, the fluorescence ratios for T-ALL specimens 2, 6, 14, 30, and 45 and B-precursor specimen 3 in Fig 2 were all just slightly beyond the normal range (fluorescence ratios of 1 to 1.2), suggesting low-level transport impairment. However, 3 B-precursor specimens (10, 12, and 25 in Fig 2) exhibited significantly impaired Mtx transport, albeit in only a small fraction of blasts (14%, 22%, and 16%, respectively, of the total). For these samples, nearly identical percentages were associated with defective drug uptake and elevated DHFR (Table 1).
Within a particular phenotypic group of patients, there were no significant correlations between DHFR levels or Mtx transport at diagnosis and features such as sex, age, WBC, or percentage of S-phase (Tables 1 and 2). Furthermore, although the numbers were small, there were no apparent relationships between these resistance markers in B-precursor ALL and cytogenetic abnormalities, including hyperdiploidy (47 to 50, n = 3; >50, n = 2), hypodiploidy (<46, n = 4), or adverse chromosomal translocations [t(9; 22), n = 1; t(1; 19), n = 2]. Of particular interest was the significantly increased frequency of heterogeneous DHFR expression (7 of 16 v 4 of 20, respectively; P = .065) and maximal DHFR (median values of 3.4 and 2.2, respectively; P = .055) in African-American (AA) B-precursor patients compared with white B-precursor patients at diagnosis*; this increase predominated in AA females (5 of 7 with heterogeneous DHFR) over males (2 of 9). There were no appreciable differences in the numbers of B-precursor patients with high-risk features (ie, age and WBC) between the races.
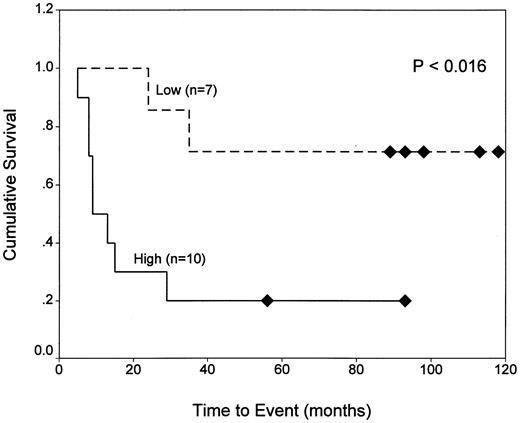
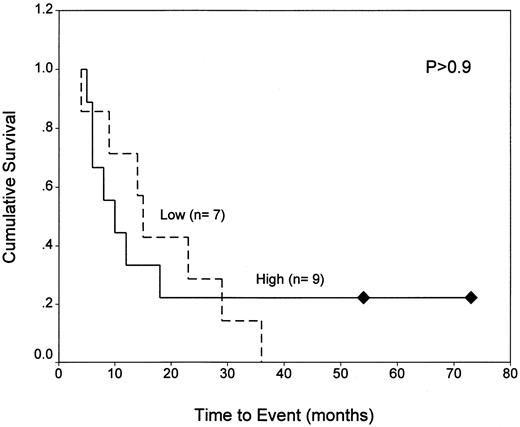
Because the majority of the patients in our analysis are still under treatment, it was not possible to correlate response to therapy with the expression of resistance markers for all but a few cases. By classification tree analysis22 23 of our 33 patients (including both immunophenotypes) for which outcome data were available, we found that the main predictors of poor outcome (ie, death or relapse) were (1) WBCs exceeding 50,000/μL and (2) DHFR fluorescence ratios exceeding 2.4 for patients with WBCs less than 50,000/μL. This is illustrated in Fig 3, which shows that, for patients with low WBCs, EFS values were significantly shorter for those expressing DHFR above this threshold level than for patients with DHFR below this level (20% v 75% at 60 months; P < .016; upper panel). This relationship was not seen among patients with high WBCs (P > .9; lower panel).
Kaplan-Meier analysis of EFS for ALL patients with normal and elevated DHFR as a function of WBC. EFS data are shown for 17 patients with WBCs less than 50,000/μL (top panel) and 16 patients with WBCs exceeding 50,000/μL (lower panel) whose blasts were characterized by high (fluorescence ratio ≥2.4) or low (ratio <2.4) DHFR levels at diagnosis. As described in Materials and Methods, normal DHFR (fluorescence ratios of 1.6 to 2.4) was defined by 5 patients (BP6, 8, 13, 30, and 31) who survived at least 5 years without relapse. Experimentally measured DHFR values in excess of this range were considered to be elevated.
Kaplan-Meier analysis of EFS for ALL patients with normal and elevated DHFR as a function of WBC. EFS data are shown for 17 patients with WBCs less than 50,000/μL (top panel) and 16 patients with WBCs exceeding 50,000/μL (lower panel) whose blasts were characterized by high (fluorescence ratio ≥2.4) or low (ratio <2.4) DHFR levels at diagnosis. As described in Materials and Methods, normal DHFR (fluorescence ratios of 1.6 to 2.4) was defined by 5 patients (BP6, 8, 13, 30, and 31) who survived at least 5 years without relapse. Experimentally measured DHFR values in excess of this range were considered to be elevated.
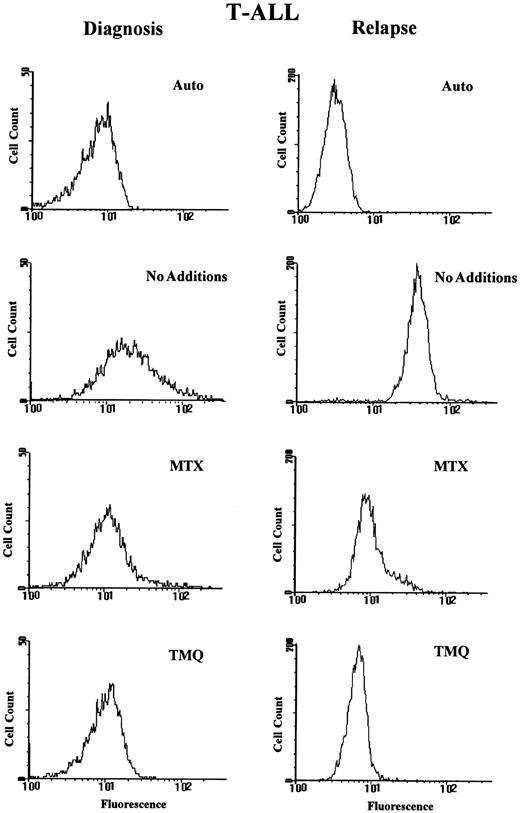
Five of our relapsed specimens (2 B-precursor and 3 T-ALL) were paired with diagnostic samples, and for 2 of these (rT1 and rT6 in Table 3) there were particularly notable changes in resistance parameters at relapse. For instance, a T-ALL patient with the t(9; 22) cytogenetic abnormality (paired specimens T13 and rT6 in Tables 2 and 3) relapsed 5 months after diagnosis and 3 courses of intravenous Mtx. At relapse, fourfold increased DHFR was detected in essentially 100% of the blasts (Fig 4). A slight (fluorescence ratio of 1.5 in 90% of the blasts) to significant (fluorescence ratio of 4.6 in 10% of the blasts) transport impairment was also detected in this sample as well. A similar, homogeneously increased DHFR population (2.5-fold) was observed for paired T-ALL specimens T2/rT1 after 10 months of chemotherapy including 3 courses of intravenous Mtx and 7 doses of intramuscular Mtx (histogram not shown). For the other 3 paired relapse specimens, there was either no change in DHFR or Mtx transport (BP9/rBP6) or a moderate increase in the fraction of DHFR-overexpressing blasts accompanying Mtx chemotherapy (BP20/rBP1 and T30/rT2).
Fluorescence histograms for paired T-cell ALL specimens at diagnosis and relapse. Paired blast samples from a patient with T-ALL (specimens T13 and rT6 in Table 2 and 3, respectively; right panel) were assayed for DHFR and MTX transport with PT430 and flow cytometry as described in Materials and Methods. Fluorescence histograms are shown for blasts treated with PT430 (20 μmol/L) and suspended in drug-free medium (No Additions) or in the presence of Mtx (0.5 μmol/L) or trimetrexate (TMQ; 0.5 μmol/L). Data are plotted as computer-generated histograms after the analysis of 10,000 cells. The abscissa is a logarithmic scale of the relative fluorescence intensity in a given fluorescence intensity channel.
Fluorescence histograms for paired T-cell ALL specimens at diagnosis and relapse. Paired blast samples from a patient with T-ALL (specimens T13 and rT6 in Table 2 and 3, respectively; right panel) were assayed for DHFR and MTX transport with PT430 and flow cytometry as described in Materials and Methods. Fluorescence histograms are shown for blasts treated with PT430 (20 μmol/L) and suspended in drug-free medium (No Additions) or in the presence of Mtx (0.5 μmol/L) or trimetrexate (TMQ; 0.5 μmol/L). Data are plotted as computer-generated histograms after the analysis of 10,000 cells. The abscissa is a logarithmic scale of the relative fluorescence intensity in a given fluorescence intensity channel.
Altogether, increases in DHFR beyond the normal range (exceeding the normal DHFR fluorescence ratio of 2.4) were detected in 11 of 14 relapse specimens including 5 of 6 T-cell and 6 of 8 B-precursor ALL (Fig 5); these changes appeared as either a uniform cell population (samples rBP4, rBP8, rT1, and rT6) or as a lymphoblast subpopulation of variable size (25% to 84% of the total; rBP2, rBP7, rT2, and rT3). Markedly impaired Mtx uptake was detected in 3 T-ALL specimens (rT2, rT3, and rT6), although minor transport effects were observed in other cases (for instance, rBP7 in Fig 5).
DHFR content and Mtx transport-impairment in T-ALL and B-precursor ALL blasts at relapse. DHFR (top panel) and Mtx transport-impairment (lower panel)were assayed in 8-relapsed B-precursor (designated rBP) and 6 relapsed T-cell (rT) blast specimens by PT430 and flow cytometry. Data are plotted as the relative fluorescence ratios versus patient numbers for one or two separate blast subpopulations (designated S1 and S2) as for Figs 2 and 3. Patient characteristics and percentages for the fluorescent DHFR subpopulations are noted in Table 3. The percentages of the total blasts in relapsed T-cell samples 3 and 6 with impaired MTX transport were 73% and 10%, respectively. As described in Materials and Methods, normal DHFR (fluorescence ratios of 1.6 to 2.4) and Mtx transport (ratio of 1.0 to 1.2) were defined by 5 patients (BP6, 8, 13, 30, and 31 in Table 1) who survived at least 5 years without relapse. Experimentally measured parameters in excess of these ranges were considered elevated or impaired, as appropriate.
DHFR content and Mtx transport-impairment in T-ALL and B-precursor ALL blasts at relapse. DHFR (top panel) and Mtx transport-impairment (lower panel)were assayed in 8-relapsed B-precursor (designated rBP) and 6 relapsed T-cell (rT) blast specimens by PT430 and flow cytometry. Data are plotted as the relative fluorescence ratios versus patient numbers for one or two separate blast subpopulations (designated S1 and S2) as for Figs 2 and 3. Patient characteristics and percentages for the fluorescent DHFR subpopulations are noted in Table 3. The percentages of the total blasts in relapsed T-cell samples 3 and 6 with impaired MTX transport were 73% and 10%, respectively. As described in Materials and Methods, normal DHFR (fluorescence ratios of 1.6 to 2.4) and Mtx transport (ratio of 1.0 to 1.2) were defined by 5 patients (BP6, 8, 13, 30, and 31 in Table 1) who survived at least 5 years without relapse. Experimentally measured parameters in excess of these ranges were considered elevated or impaired, as appropriate.
DISCUSSION
Although the prognosis for children with ALL has improved considerably over the last two decades, most studies show that patients with T-ALL continue to experience worse outcomes than patients with B-precursor ALL.1-3,7 These differences in clinical prognosis reflect disparate rates of remission induction and remission duration as well as frequency of extramedullary disease.1-7 Whereas T-cell ALL is characterized by features commonly associated with poor prognosis, the mechanistic bases for increased rates of treatment failure over B-precursor ALL have not been firmly established.
The most successful regimens for T-ALL use aggressive high-dose multiagent therapy, often including Mtx.1,2,7 Although previously empirically based, more recent approaches with high-dose Mtx (>1 g/m2) reflect the premise that elevated Mtx can overcome deficiencies in folylpolyglutamate synthetase or increased γ-glutamyl hydrolase activity in T-ALL, either of which could result in low levels of Mtx polyglutamate accumulation compared with B-precursor ALL.8,9,12 14
Our current study assesses the additional roles of lineage-specific differences in Mtx membrane transport and DHFR levels in clinical response to Mtx. Both of these parameters could conceivably contribute to the disparity in antileukemic drug activity between T-cell and B-precursor ALL and also decrease free intracellular substrate for Mtx polyglutamate synthesis.8 It was of interest that Mtx transport by indirect flow cytometry assay was remarkably constant between immunophenotypes. Although impaired Mtx transport was detected in a small group of patients, its frequency was independent of phenotype and stage of disease. Notably, the frequency and magnitude of impaired Mtx transport in our study was significantly less than previously suggested by Trippett et al15 for assorted leukemic samples (ALL, acute myleogenous leukemia, and chronic myelogenous leukemia) from both children and adults. These disparate results may reflect differences in patient or disease-specific characteristics, assay methodologies (ie, times of exposure to PT430), or data interpretation (different end-points for classifying a specimen as transport impaired).
As in our earlier report,13 DHFR overproduction was commonly observed in both newly diagnosed and relapsed ALL. Elevated DHFR frequently involved only a subfraction of leukemic blasts. For newly diagnosed ALL, DHFR overexpression was highly lineage-specific. Thus, 78% of our newly diagnosed T-ALL specimens, but only 47% of the B-precursor ALL samples exhibited elevated DHFR, suggesting a relationship to prognosis. Moreover, maximal DHFR levels were markedly increased in T-cell over B-precursor ALL. At relapse, virtually all patients had elevated DHFR independent of phenotype.
Although it is tempting to speculate that these differences in frequency of DHFR overexpression between T-cell and B-precursor ALL reflect inherent biologic properties of these lineages, for a particular phenotype there were no apparent correlations between either of the resistance markers and documented patient and prognostic features (age, sex, and chromosomal abnormalities). Nonetheless, it was interesting that our B-precursor group was composed of nearly equal numbers of AA and white patients and that, for these, there was a striking association between AA race and elevated DHFR. This pattern may contribute to the poorer prognosis of AA children with ALL compared with white children treated with predominantly antimetabolite therapies26,27 and is in keeping with the suggestion by Kalwinsky et al28 that metabolic differences may be involved. In recent years, this difference between white and AA children was less obvious with the use of regimens that included anthracyclines and/or epipodophyllotoxins.27
Differing DHFR expression in T-cell and B-precursor ALL may reflect distinctive growth rates or sites of clonal expansion (marrow v thymus)2,7 that influence the size the leukemic population and prevalence of drug-resistant variants at the time leukemia is diagnosed. In our study, the T-ALL population showed a slightly higher median percentage of S-phase population than the B-precursor group, as previously reported29-31; however, for individual specimens, there were no apparent correlations between this parameter and the expression of elevated DHFR. Recent studies suggest that there may be lineage-specific differences in mitotic checkpoint controls involving mutations in p5311,32,33 or downstream effectors that regulate p53 activity (ie, MDM-233,34 ) or other aspects of cell cycle progression (ie, p16INK4Aand p15INK4B35-38). Any of these could significantly affect genetic stability and promote resistance to a variety of chemotherapeutic agents, including Mtx. The demonstration that low-level gene amplification was associated with DHFR overexpression and p53 mutations in ALL11 gives credence to the concept that our findings may result from lineage differences in cell cycle control in an analogous fashion. The previous lack of significant detectable p53 mutations in T-ALL at diagnosis39-41 may simply reflect the inability of single-strand conformational polymorphism analysis and direct sequencing to identify p53 mutations in a small subset of lymphoblasts. Low levels of mutated p53 have been detected at diagnosis by allele-specific polymerase chain reaction methods.42
Because most of the patients in our analysis are still under treatment, it was possible to correlate response to therapy with the expression of resistance markers for only 33 patients. As suggested in our earlier report,13 DHFR levels again appeared to be a important determinant of response to chemotherapy with Mtx; however, upon closer examination, this effect was observed only for children considered good risks based on WBCs at diagnosis. This difference between patients with low versus high WBCs may partly reflect differences in treatment strategy between the groups, because a majority (12 of 17) of the former group were treated with less intensive chemotherapy (including antimetabolites but without anthracyclines, epidophyllotoxins, or cyclophosphamide), whereas most (11 of 16) of the patients with WBCs greater than 50,000/μL were treated with more intensive therapies (including anthracyclines, epipodophyllotoxins, or cyclophosphamide). These results in patients with low WBCs, combined with the finding that elevated DHFR was detected in most of our relapsed ALL specimens and increased in 4 of 5 paired samples after chemotherapy with Mtx, strongly argue that DHFR content is a critical determinant of both clinical response and resistance to Mtx. Most importantly, they suggest that measurements of this parameter may have value in predicting response of children with ALL to chemotherapy including Mtx. Of course, given the small number of evaluable patients, it is impossible to assess the prognostic importance of DHFR relative to other disease or patient characteristics.
In summary, our findings continue to provide a strong rationale for the use of mechanistically based factors to complement known biologic or disease-based prognostic indicators in the design of ALL therapy including Mtx. The demonstration of an increased frequency of elevated DHFR in T-cell over B-precursor ALL suggests that this difference is associated with the poorer prognosis of patients with T-ALL treated with antimetabolite therapy. Combined with earlier studies of lineage specificity for Mtx polyglutamate synthesis,9,10,14 our findings argue that high-dose Mtx may be useful in treating patients with T-ALL. High doses (3 to 5 g/m2) of Mtx have been reported to improve the outcome of children with T-ALL43,44 and may overcome deficiencies in folylpolyglutamate synthetase in T-ALL.9 Likewise, elevated doses of Mtx should be necessary for maximally inhibiting DHFR in subpopulations of blast cells with increased levels of this target enzyme. Although our results seem to imply that more intensive chemotherapy (with or without Mtx) may frequently be effective with T-cell and high-risk B-precursor ALL, potential long-term adverse sequelae (secondary malignancies and cardiomyopathy) of these treatments may render this strategy less desirable.45
ACKNOWLEDGMENT
The authors would thank Daryel Taliaferro for assistance in preparing this manuscript and Rita Russo and Christine Menig for clinical data collection. The authors are particularly grateful to Drs Andre Rosowsky and Joel Wright for providing the PT430 for this study.
Address reprint requests to Larry H. Matherly, PhD, Experimental and Clinical Therapeutics Program, Karmanos Cancer Institute, 110 E Warren Ave, Detroit, MI 48201.
Kamen et al25reported that DHFR levels in patient tumors (including leukemic blasts) were as low as 0.5% to 1% of those in cultured cells. We assayed DHFR levels in cytosolic extracts from 3 ALL specimens not included in this study (2 T-ALL and 1 B-precursor) by measuring direct binding of high specific activity 3H-Mtx in the presence of excess NADPH. DHFR contents ranged from 12.1 to 54.3 fmol/107 cells, or 0.2% to 0.9% of that in CCRF-CEM cells in culture.
This type of analysis was not possible for the T-cell ALL specimens because of the disproportionate numbers of white (n = 41) and AA (n = 7) patients in this group at diagnosis. The nearly equal numbers of AA and white patients in the B-precursor group reflects the racial make-up of patients treated at CHM in Detroit versus that at participating POG institutions that provided the majority of the T-ALL specimens for this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal