Abstract
The proliferation and differentiation of neutrophils is regulated by granulocyte-specific colony-stimulating factor (G-CSF ). G-CSF uses a receptor of the cytokine receptor superfamily and, in common with all members of the family, induces the tyrosine phosphorylation and activation of members of the Janus protein tyrosine kinase (Jak) family. In both myeloid cells and a human fibrosarcoma cell line expressing the G-CSF receptor, G-CSF induces the tyrosine phosphorylation and activation of Jak1, Jak2, and Tyk2. In addition, G-CSF induces the tyrosine phosphorylation of the receptor and members of the signal transducers and activators of transcription (Stat) family, including Stat3, as well as Stat1 and Stat5, depending on the cells involved. Using mutant cell lines lacking various Jaks, we show here that Jak1 is critical for G-CSF–mediated Stat activation, whereas Jak2 or Tyk2 are either not required or play redundant or ancillary roles. In the absence of Jak1, G-CSF induces activation of Jak2 and Tyk2, but fails to induce receptor tyrosine phosphorylation and induces dramatically reduced levels of Stat activation. A kinase-inactive Jak2, when overexpressed in cells lacking endogenous Jak2, can suppress Jak1 activation, receptor phosphorylation, and Stat activation, suggesting competition in the receptor complex either for Jak1 binding or substrates. Because the requirement for Jak1 is very similar to that previously shown for interleukin-6 signaling, the data support the concept that the G-CSF receptor and gp130 are both structurally and functionally similar.
THE PROLIFERATION and differentiation of hematopoietic precursor cells are regulated by a family of cytokines termed colony-stimulating factors (CSFs) or interleukins (ILs). Among these factors, granulocyte colony-stimulating factor (G-CSF ) specifically stimulates the proliferation and differentiation of cells that are committed to the neutrophilic-granulocytic lineage.1,2 The various actions of G-CSF are mediated through binding to a receptor that is predominantly expressed on neutrophilic progenitor cells and mature neutrophilic granulocytes. The G-CSF receptor is a type I membrane protein that belongs to the cytokine receptor superfamily and is most closely similar to the interleukin-6 (IL-6) receptor signaling chain, gp130.2,3 Expression of the receptor in murine IL-3–dependent cell lines confers on the cells high-affinity G-CSF binding and the ability to respond to G-CSF by proliferation and, in some cell lines, differentiation.4 Like most cytokine receptors, a membrane proximal cytoplasmic domain is critical for all receptor functions. In addition, the membrane distal region of the G-CSF receptor is essential for transducing a differentiation signal.4
The G-CSF receptor, like most cytokine receptors, couples ligand binding to the induction of the tyrosine phosphorylation including members of the Janus family of protein tyrosine kinases (Jaks). For example, recent studies5-9 have indicated that G-CSF induces the tyrosine phosphorylation of Jak1 as well as Jak2, suggesting that one or both play a role in signaling transduction. However, other cytoplasmic protein tyrosine kinase, including lyn and syk,10 11 have been shown to be phosphorylated and activated in response to G-CSF. From these data it has not been possible to establish the role of the various kinases in G-CSF receptor function.
G-CSF induces the tyrosine phosphorylation of a number of cellular substrates. Tyrosine phosphorylation of the receptor is hypothesized to be critical for the subsequent recruitment of various signaling proteins to the receptor complex. In addition, G-CSF induces the tyrosine phosphorylation of members of the signal transducers and activators of transcription (Stat) family, including Stat37,12,13 and more infrequently Stat1.12
A role for Jaks in cytokine signaling was initially established through the use of cell lines that were isolated for their inability to respond to interferons (IFNs).14-16 Specifically, the U1A cell line was found to be deficient in Tyk2 and IFN signaling could be restored by introducing a functional Tyk2.17 Similarly, the U4A cell line was found to lack Jak1 and its ability to respond to IFNs was restored by introducing a functional Jak1.18 Lastly, γ2A cells lack Jak2 and can be functionally restored by introducing Jak2 into the cells.19 From these studies, an obligatory role for Jak1 and Tyk2 was shown for IFN-α/β responses, whereas Jak1 and Jak2 were both essential for IFN-γ responses. In addition, the phenotype of mice deficient in Jak3 is consistent with the hypothesis that Jak3 plays a nonredundant role in signaling through cytokine receptors that use the IL-2 receptor common γ chain.20 21
More recently,22 the cell lines lacking various Jaks have been used to assess the role of Jaks in IL-6 signaling. In contrast to the IFNs, Jak1 alone was found to be critical for IL-6 signaling although IL-6 induces the tyrosine phosphorylation of Jak1, Jak2, and Tyk2. These results have been interpreted to indicate that gp130, the signaling component of the IL-6 receptor, can bind multiple Jaks but that Jak1 is uniquely capable of phosphorylating gp130 and initiating a cellular response. In the studies presented here, we have used the Jak-deficient cell lines to examine the role of various Jaks in G-CSF signaling. Similar to IL-6, G-CSF induces the tyrosine phosphorylation and activation of Jak1, Jak2, and Tyk2, and the absence of one Jak does not preclude G-CSF–induced tyrosine phosphorylation of the remaining Jaks. However, in the absence of Jak1, G-CSF does not induce receptor tyrosine phosphorylation and the induced tyrosine phosphorylation of Stat proteins is greatly reduced. However, overexpression of a dominant negative Jak2 can suppress receptor and Stat phosphorylation. The results are consistent with the hypothesis that the G-CSF receptor can associate with multiple Jaks. However, because of either location within the receptor complex or substrate specificity, Jak1 is in a unique position to phosphorylate the receptor and thereby affect Stat protein tyrosine phosphorylation. The results also show that the G-CSF receptor is not only structurally related to gp130 but is also functionally similar.
MATERIALS AND METHODS
Cytokines and antibodies.Murine recombinant IL-3 was kindly provided by R & D (Minneapolis, MN). Human recombinant G-CSF was obtained from Amgen Inc (Thousand Oaks, CA), and human IFN-γ Ib was obtained from Genentech Inc (South San Francisco, CA). Rabbit polyclonal antisera against human G-CSF receptor, Tyk2, Jak1, Jak2, and Stat1 were purchased from Santa Cruz (Santa Cruz, CA), and mouse monoclonal antiphosphotyrosine antibody (4G10) was purchased from UBI (Lake Placid, NY). Rabbit polyclonal antisera against Stat3 and Stat5 have been previously described.23
Cell cultures.Murine NFS-60 cells and FDC-P1 cells expressing murine G-CSF receptors4 were maintained in RPMI 1640 medium containing 10% heat-inactivated fetal calf serum and 5 mmol/L L-glutamine and supplemented with murine IL-3 (25 U/mL). Human fibrosarcoma cells, 2fTGH, U1A, U4A, and γ2A were described previously.14-16 U1A cells lack Tyk2 protein,17 U4A cells lack Jak1 protein,18 and γ2A cells lack Jak2 protein.19 All cells were grown in Dulbecco's modification of Eagle's medium (DMEM) supplemented with 10% heat-inactivated fetal calf serum and 5 mmol/L L-glutamine.
Plasmid and DNA transfections.Expression plasmids for the human G-CSF receptor have been previously described.24 The murine Jak1 and Jak2 expression constructs have been described previously.25 Kinase-negative versions of Jak proteins were generated by mutating the lysine (K833) to glutamine in murine Jak1 (Jak1KE) and the lysine (K882) to glutamine in murine Jak2 (Jak2KE). 2fTGH cells, U1 cells, and U4 cells were transfected with the G-CSF receptor expression plasmid and neomycin-resistant gene (pSVNeo). γ2A cells were transfected with the G-CSF receptor expression plasmid and hygromycin-resistant gene. DNA transfections using LipofectAmine (Life Technologies, Gaithersburg, MD) were performed according to the manufacturer's protocols. Cells were selected with 700 μg/mL G418 or 200 μg/mL hygromycin B. Drug-resistant clones were singly separated and tested for the expression of G-CSF receptors by Western blotting following by immunprecipitation with anti–G-CSF receptor antibodies. Jak1 and Jak1 KE constructs were transfected to U4A cells already carrying G-CSF receptors, and Jak2 and Jak2KE constructs were transfected to γ2A cells expressing G-CSF receptors. Cells stably expressing these constructs were obtained by cotransfection with puromycin-resistance gene. After selection with 1 μg/mL puromycin, the expression of these constructs was tested by Western blotting after immunoprecipitation with anti-Jak1 or anti-Jak2 antisera.
Growth factor stimulation, immunoprecipitation, and Western blotting.Tyrosine phosphorylation was analyzed essentially as described. In brief, 1 × 107 NFS-60 cells or FDC-P1 cells carrying G-CSF receptors were washed twice with RPMI 1640 medium containing 10% fetal calf serum and incubated at 37°C for 10 to 14 hours for factor starvation. 2fTGH cells (5 to 7 × 106) and their derivatives were washed twice with DMEM medium and incubated at 37°C for 10 to 14 hours. Cells were then stimulated with 250 U/mL mouse IL-3, 50 ng/mL of human G-CSF, or 200 ng/mL (6 × 103 U/mL) of human IFN-γ for 6 minutes to see Jak activation or for 15 minutes to see the phosphorylation of G-CSF receptor or Stat proteins, and then harvested by centrifugation. The cells were subsequently lysed in 0.1 mL of lysis buffer (1% Triton X-100, 50 mmol/L NaCl, 30 mmol/L Na4P2O7 , 50 mmol/L NaF, 0.1 mmol/L Na3VO4 , 5 mmol/L EDTA, 10 μmol/L phenylmethyl sulfonyl fluoride, 10 mmol/L Tris [pH 7.6]). Lysates were cleared by centrifugation for 15 minutes at 14,000 rpm, and the supernatants were incubated in the presence of the designated sera or antibodies for 2 hours. Immune complexes were precipitated with protein A-Sepharose (Sigma, St Louis, MO) and extensively washed in lysis buffer. Proteins were then eluted with sample buffer for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Eluted proteins were separated on a 7.5% polyacrylamide gel in the presence of SDS and transferred to nitrocellulose. Filters were probed with designated sera or antibodies and visualized with the ECL detection system (Amersham, Arlington Heights, IL) as directed by the manufacturer.
RESULTS
We initially examined the ability of G-CSF to induce tyrosine phosphorylation of Jaks in various hematopoietic cell lines expressing either the endogenous G-CSF receptor or a stably transfected receptor. Cells were deprived of growth factors and were stimulated for 5 minutes with G-CSF or IL-3 for comparison. As shown in Fig 1, G-CSF induced the tyrosine phosphorylation of Jak2 in NFS-60 cells, which express the endogenous receptor, and in FDC-P1 or DA-3 cells stably transfected with the murine or human G-CSF receptor, respectively. The extent of Jak2 tyrosine phosphorylation was comparable among the cell lines and similar to that seen in response to IL-3. However, in contrast to IL-3, G-CSF also induced a lower, but consistently detectable, level of Jak1 and Tyk2 tyrosine phosphorylation. No detectable tyrosine phosphorylation of Jak3 was induced by G-CSF in FDC-P1 cells expressing the G-CSFR (Fig 1) or in the other cell lines (data not shown). These results are consistent with those of previous studies.5-9
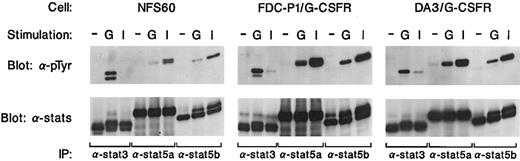
Tyrosine phosphorylation of Jaks in murine myeloid precursor cell lines. Two different murine myeloid precursor cell lines (FDC-P1, DA3) expressing wild type G-CSF receptor or NFS60 cells, which express the endogenous G-CSF receptor, were either unstimulated or stimulated with IL-3 (I) or G-CSF (G) for 5 minutes. Cells were lysed and incubated with antisera against either Jak1, Jak2, Jak3, or Tyk2, and immunoprecipitates were analyzed by Western blotting with a monoclonal antibody against phosphotyrosine (4G10).
Tyrosine phosphorylation of Jaks in murine myeloid precursor cell lines. Two different murine myeloid precursor cell lines (FDC-P1, DA3) expressing wild type G-CSF receptor or NFS60 cells, which express the endogenous G-CSF receptor, were either unstimulated or stimulated with IL-3 (I) or G-CSF (G) for 5 minutes. Cells were lysed and incubated with antisera against either Jak1, Jak2, Jak3, or Tyk2, and immunoprecipitates were analyzed by Western blotting with a monoclonal antibody against phosphotyrosine (4G10).
Cytokine stimulation also induces the tyrosine phosphorylation of members of the Stat family of transcription factors. As shown in Fig 2, G-CSF induced the tyrosine phosphorylation of Stat3 in all the cell lines examined. In contrast, IL-3 did not detectably induce Stat3 tyrosine phosphorylation in NFS-60 cells. G-CSF also detectably induced tyrosine phosphorylation of Stat5a and Stat5b. IL-3 also induced the tyrosine phosphorylation of Stat5, although this was consistently much stronger than that seen with G-CSF. It should also be noted that G-CSF–induced tyrosine phosphorylation of Stat5a or Stat5b was consistently less in NFS-60 cells compared with the FDC-P1 or DA-3 cells. The differences may be related to the observation that fewer receptors are expressed on NFS-60 cells than on the receptor-transfected, FDC-P1, or DA-3 cells. Lastly, G-CSF did not detectably induce the tyrosine phosphorylation of Stat1, Stat4, or Stat6 in any of the cell lines examined (data not shown).
Stat3 and Stat5 activation by G-CSF. Murine myeloid precursor cells expressing wild-type G-CSF receptor (FDC-P1; DA3) or the endogenous G-CSF receptor (NFS60) were either stimulated (−) or stimulated with G-CSF (G) or IL-3 (I) for 5 minutes. Cell lysate was incubated with antisera against Stat3, Stat5a, or Stat5b and the immunoprecipitates were analyzed by Western blotting with a monoclonal antibody against phosphotyrosine (4G10).
Stat3 and Stat5 activation by G-CSF. Murine myeloid precursor cells expressing wild-type G-CSF receptor (FDC-P1; DA3) or the endogenous G-CSF receptor (NFS60) were either stimulated (−) or stimulated with G-CSF (G) or IL-3 (I) for 5 minutes. Cell lysate was incubated with antisera against Stat3, Stat5a, or Stat5b and the immunoprecipitates were analyzed by Western blotting with a monoclonal antibody against phosphotyrosine (4G10).
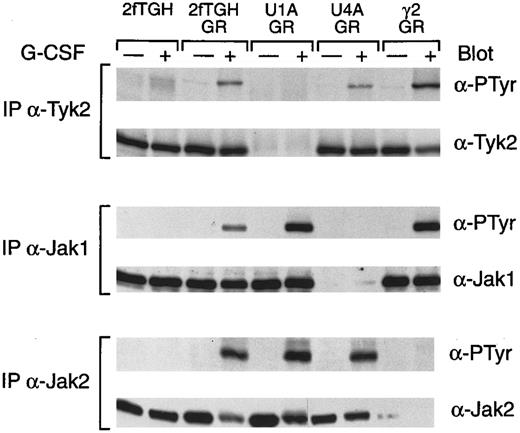
To assess the role of the various Jaks in G-CSF receptor function, we used a series of cell lines that were isolated in experiments to obtain mutants in IFN signaling and that lack individual Jaks.14-16 The cells were transfected with the G-CSF receptor and stably expressing clonal cell lines were isolated. Clonal lines expressing comparable levels of the receptor were identified and used in subsequent experiments. We initially examined the ability of G-CSF to induce the tyrosine phosphorylation of the various Jaks (Fig 3). G-CSF did not induce the tyrosine phosphorylation of any of the Jaks in the parental cell line 2fTGH. In contrast, parental cells transfected with the G-CSF receptor responded to G-CSF with inducible tyrosine phosphorylation of Tyk2, Jak1, and Jak2 in a manner similar to the myeloid cell lines. Cells lacking Tyk2 (U1A cells) expressing the G-CSF receptor responded to G-CSF by the induction of tyrosine phosphorylation of Jak1 and Jak2. Similarly, cells expressing the G-CSF receptor but lacking Jak1 (U4A) responded to G-CSF by inducing the tyrosine phosphorylation of Tyk2 and Jak2. Lastly, cells expressing the receptor but lacking Jak2 (γ2A) responded to G-CSF by inducing tyrosine phosphorylation of Tyk2 and Jak1. Thus, the absence of a specific Jak does not preclude G-CSF–induced tyrosine phosphorylation of the remaining Jaks.
G-CSF–induced tyrosine phosphorylation of Jaks in the fibrosarcoma cell lines. Whole cell extracts from the indicated cells, with or without treatment for 5 minutes at 37°C with G-CSF, were immunprecipitated with anti-Tyk2, anti-Jak1, or anti-Jak2 polyclonal antibodies. Precipitated proteins were separated by SDS-PAGE, transferred to nitrocellulose, and probed with antibodies to phosphotyrosine (α-PTyr). Subsequently, filters were stripped and reprobed with Tyk2, Jak1, or Jak2 polyclonal antibodies.
G-CSF–induced tyrosine phosphorylation of Jaks in the fibrosarcoma cell lines. Whole cell extracts from the indicated cells, with or without treatment for 5 minutes at 37°C with G-CSF, were immunprecipitated with anti-Tyk2, anti-Jak1, or anti-Jak2 polyclonal antibodies. Precipitated proteins were separated by SDS-PAGE, transferred to nitrocellulose, and probed with antibodies to phosphotyrosine (α-PTyr). Subsequently, filters were stripped and reprobed with Tyk2, Jak1, or Jak2 polyclonal antibodies.
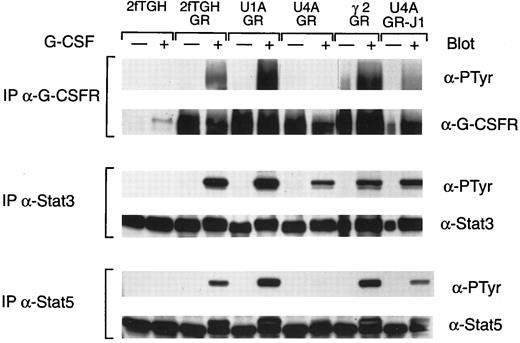
The consequences of the absence of specific Jaks on subsequent signaling events were next examined (Fig 4). One of the early events is the induced tyrosine phosphorylation of the receptor. As shown in Fig 4, G-CSF induced receptor tyrosine phosphorylation in the parental cell line expressing the G-CSF receptor (2fTGH/GR), in cells expressing the receptor but lacking Tyk2 (U1A/GR), and in cells expressing the receptor but lacking Jak2 (γ2A/GR). In contrast, the extent of G-CSF–induced receptor tyrosine phosphorylation was greatly reduced in cells expressing the receptor but that lacked Jak1 (U4A/GR), although these cells expressed levels of receptors comparable to the others. These results suggest that Jak1 is either in a unique position to phosphorylate the receptor or has a unique substrate specificity for receptor tyrosine phosphorylation.
G-CSF–induced tyrosine phosphorylation of G-CSF receptors and Stats. Whole cell extracts from the indicated cells, with or without treatment for 5 minutes at 37°C with G-CSF, were immunoprecipitated with G-CSF receptor antibody, Stat3 antiserum, and Stat5 antiserum. Precipitated proteins were separated by SDS-PAGE, transferred to nitrocellulose, and probed with antibodies to phosphotyrosine (α-PTyr). Subsequently, filters were stripped and reprobed with G-CSF receptor polyclonal antibody, Stat3 antiserum, or Stat5 antiserum.
G-CSF–induced tyrosine phosphorylation of G-CSF receptors and Stats. Whole cell extracts from the indicated cells, with or without treatment for 5 minutes at 37°C with G-CSF, were immunoprecipitated with G-CSF receptor antibody, Stat3 antiserum, and Stat5 antiserum. Precipitated proteins were separated by SDS-PAGE, transferred to nitrocellulose, and probed with antibodies to phosphotyrosine (α-PTyr). Subsequently, filters were stripped and reprobed with G-CSF receptor polyclonal antibody, Stat3 antiserum, or Stat5 antiserum.
The consequences of the absence of specific Jaks on Stat activation are also shown in Fig 4. Comparable levels of both Stat5 and Stat3 activation were seen in cells expressing the G-CSF receptor but lacking Tyk2 (U1A/GR) or Jak2 (γ2A/GR) as that seen in the parental cells expressing the receptor (2fTGH/GR). In contrast, the extent of tyrosine phosphorylation of Stat3 and Stat5 was significantly reduced in cells expressing the receptor but lacking Jak1. However, we have consistently seen a more significant reduction in Stat5 activation relative to the reduction seen in the tyrosine phosphorylation of Stat3. The possible basis for the differences is discussed below.
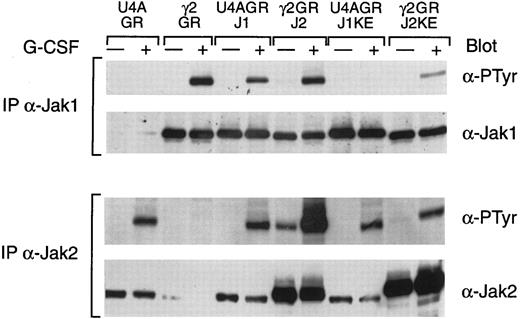
The ability of G-CSF to induce the tyrosine phosphorylation of multiple Jaks suggests the possibility that the receptor, like gp130,22 is able to associate with multiple Jaks. To assess the potential ability of different Jaks to compete for receptor function, we examined the effects of dominant negatives of Jak1 and Jak2 and compared these with the expression of the wild-type Jaks (Fig 5). Wild-type Jak1, expressed in the Jak1-deficient cells (U4A), was inducibly tyrosine phosphorylated (Fig 5) and restored G-CSF–induced receptor, Stat3, and Stat5 phosphorylation comparable to that of the parental cells. In contrast, a kinase-inactive Jak1 (J1KE) was not inducibly tyrosine phosphorylated, although G-CSF still induced levels of Jak2 tyrosine phosphorylation (Fig 5), as well as Tyk2 phosphorylation (data not shown), that were comparable to parental cells. These results indicate that Jak1 is not a substrate for either activated Jak2 or activated Tyk2 in the context of the G-CSF receptor complex.
G-CSF–induced tyrosine phosphorylation of Jaks was suppressed by a kinase-dead Jak2. Whole cell extracts from the indicated cells, with or without treatment for 5 minutes at 37°C with G-CSF, were immunoprecipitated with antisera against Jak1 or Jak2. Precipitated proteins were separated by SDS-PAGE, transferred to nitrocellulose, and probed with antibodies to phosphotyrosine (α-PTyr). Subsequently, filters were stripped and reprobed with Jak1 or Jak2 polyclonal antibodies.
G-CSF–induced tyrosine phosphorylation of Jaks was suppressed by a kinase-dead Jak2. Whole cell extracts from the indicated cells, with or without treatment for 5 minutes at 37°C with G-CSF, were immunoprecipitated with antisera against Jak1 or Jak2. Precipitated proteins were separated by SDS-PAGE, transferred to nitrocellulose, and probed with antibodies to phosphotyrosine (α-PTyr). Subsequently, filters were stripped and reprobed with Jak1 or Jak2 polyclonal antibodies.
The results obtained with either a wild-type Jak2 or a kinase-inactive Jak2 mutant (J2KE) are also shown in Fig 5. In this case, it is important to note that the introduced proteins are expressed at significantly higher levels than the endogenous Jak2 in the parental cells or in U4A cells. Wild-type Jak2, expressed in the γ2A cells, was constitutively phosphorylated at a low level that was significantly increased by G-CSF stimulation. However, expression of the wild-type Jak2 did not result in any constitutive phosphorylation of Jak1 and did not alter the extent of G-CSF–induced Jak1 tyrosine phosphorylation. However, expression of the kinase-inactive Jak2 mutant in γ2A cells dramatically reduced the G-CSF–induced tyrosine phosphorylation of Jak1. A comparable reduction of Tyk2 tyrosine phosphorylation was also observed (data not shown). Importantly, G-CSF stimulation induced the tyrosine phosphorylation of the kinase-inactive mutant, indicating that Jak2 is a substrate for a receptor-associated kinase such as Jak1 or Tyk2.
Because the kinase-inactive Jak2 mutant could suppress G-CSF–induced tyrosine phosphorylation of Jak1 and Tyk2, we also examined the effects of this mutant on subsequent phosphorylations. As shown in Fig 6, G-CSF–induced tyrosine phosphorylation of the receptor was dramatically reduced in γ2A cells expressing the kinase-inactive Jak2. Similarly there was a significant reduction in G-CSF–induced Stat3 tyrosine phosphorylation and a more dramatic reduction in the tyrosine phosphorylation of Stat5.
G-CSF–induced tyrosine phosphorylation of G-CSF receptors and Stats was suppressed by a kinase-dead Jak2. Whole cell extracts from the indicated cells, with or without treatment for 5 minutes at 37°C with G-CSF, were immunoprecipitated with G-CSF receptor antibody, Stat3 antiserum, or Stat5 antiserum, respectively. Precipitated proteins were separated by SDS-PAGE, transferred to nitrocellulose, and probed with antibodies to phosphotyrosine (α-PTyr). Subsequently, filters were stripped and reprobed with G-CSF receptor polyclonal antibody, Stat3 antiserum, or Stat5 antiserum.
G-CSF–induced tyrosine phosphorylation of G-CSF receptors and Stats was suppressed by a kinase-dead Jak2. Whole cell extracts from the indicated cells, with or without treatment for 5 minutes at 37°C with G-CSF, were immunoprecipitated with G-CSF receptor antibody, Stat3 antiserum, or Stat5 antiserum, respectively. Precipitated proteins were separated by SDS-PAGE, transferred to nitrocellulose, and probed with antibodies to phosphotyrosine (α-PTyr). Subsequently, filters were stripped and reprobed with G-CSF receptor polyclonal antibody, Stat3 antiserum, or Stat5 antiserum.
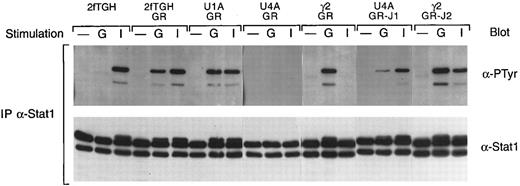
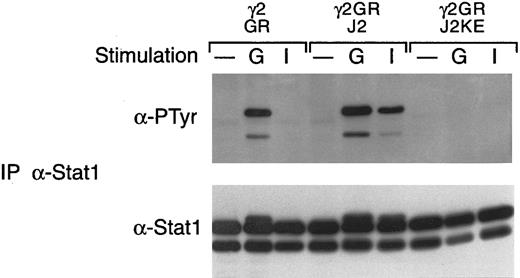
In this cell system, G-CSF consistently induces the tyrosine phosphorylation of Stat1, in contrast to the myeloid cell lines in which we have failed to observe G-CSF–induced Stat1 activation. We therefore also examined the various cell lines for the effects on Stat1 activation. As shown in Fig 7, G-CSF induces the tyrosine phosphorylation of Stat1 at levels comparable to that seen with IFN-γ in 2fTGH cells expressing the G-CSF receptor but not in the parental cells. In cells expressing the G-CSF receptor but lacking Jak1 (U4A/GR), no G-CSF–induced tyrosine phosphorylation of Stat1 was observed and IFN-γ did not induce Stat1 tyrosine phosphorylation, consistent with previous results.18 In contrast, the absence of Jak2 (γ2A/GR) or Tyk2 (U1A) had no affect on G-CSF–induced activation of Stat1. However, the absence of Jak2 did eliminate IFN-γ–induced Stat1 tyrosine phosphorylation, as predicted from previous studies.19 Introduction of wild-type Jak1 into U4A cells restored both G-CSF– and IFN-γ–induced Stat1 tyrosine phosphorylation, whereas the introduction of wild-type Jak2 into γ2A cells did not alter the G-CSF–induced tyrosine phosphorylation of Stat1 but did, as predicted, restore the IFN-γ response. The effect of the kinase-inactive Jak2 is shown in Fig 8. Consistent with the data given above, the kinase-inactive Jak2 completely suppressed G-CSF–induced tyrosine phosphorylation of Stat1.
G-CSF–induced tyrosine phosphorylation of Stat1. Whole cell extracts from the indicated cells, with or without (−) treatment for 5 minutes at 37°C with G-CSF (G) or IFN-γ (I), were immunoprecipitated with antibodies against Stat1. Precipitated proteins were separated by SDS-PAGE, transferred to nitrocellulose, and probed with antibodies to phosphotyrosine (α-PTyr). The filters were stripped and reprobed with a Stat1 polyclonal antibody.
G-CSF–induced tyrosine phosphorylation of Stat1. Whole cell extracts from the indicated cells, with or without (−) treatment for 5 minutes at 37°C with G-CSF (G) or IFN-γ (I), were immunoprecipitated with antibodies against Stat1. Precipitated proteins were separated by SDS-PAGE, transferred to nitrocellulose, and probed with antibodies to phosphotyrosine (α-PTyr). The filters were stripped and reprobed with a Stat1 polyclonal antibody.
G-CSF–induced tyrosine phosphorylation of Stat1 is suppressed by a kinase-dead Jak2. Whole cell extracts from the indicated cells, with or without (−) treatment for 5 minutes at 37°C with G-CSF (G) or IFN-γ (I), were immunoprecipitated with antibodies against Stat1. Precipitated proteins were separated by SDS-PAGE, transferred to nitrocellulose, and probed with antibodies to phosphotyrosine (α-PTyr). The filters were stripped and reprobed with a Stat1 polyclonal antibody. The cells examined included the γ2A cells expressing the G-CSF receptor (GR), the receptor with wild-type Jak2 (GR/J2), or the receptor with a kinase-inactive Jak2 mutant (GR/J2KE).
G-CSF–induced tyrosine phosphorylation of Stat1 is suppressed by a kinase-dead Jak2. Whole cell extracts from the indicated cells, with or without (−) treatment for 5 minutes at 37°C with G-CSF (G) or IFN-γ (I), were immunoprecipitated with antibodies against Stat1. Precipitated proteins were separated by SDS-PAGE, transferred to nitrocellulose, and probed with antibodies to phosphotyrosine (α-PTyr). The filters were stripped and reprobed with a Stat1 polyclonal antibody. The cells examined included the γ2A cells expressing the G-CSF receptor (GR), the receptor with wild-type Jak2 (GR/J2), or the receptor with a kinase-inactive Jak2 mutant (GR/J2KE).
DISCUSSION
Our studies show that Jak1 plays a unique, critical role in G-CSF signaling. This was, in part, unexpected because Jak2 is much more strongly tyrosine phosphorylated than Jak1. Conversely, the structural similarities between the G-CSF receptor and gp130 might have predicted that the G-CSF receptor required Jak1 in much the same manner that recent studies have demonstrated a requirement for Jak1 in gp130 signaling.22 Indeed, the results we have obtained regarding the role of the various Jaks in G-CSF signaling are virtually identical to those obtained with IL-6 using the same mutant cell lines. However, these results contrast with those obtained with the growth hormone27 or Epo receptor, in which case the absence of Jak2, but not of Jak1 or Tyk2, eliminates receptor function. Although the results with the U4A cells clearly implicate a unique role for Jak1 in G-CSF signaling, it must be emphasized that this is a transformed human cell line of nonhematopoietic orgin. It is possible that receptor associated proteins may influence, either positively or negatively, the ability of a Jak to associate with the receptor and/or to phosphorylate the receptor and therefore a comparable Jak specificity may not exist in hematopoietic cells.
Several properties of Jak1 in G-CSF signaling are suggested by the data. First, the results show that the association of Jak2 or Tyk2 with the receptor complex and their tyrosine phosphorylation are independent of Jak1. Conversely, the data demonstrate that Jak1 association with the receptor complex is not uniquely dependent on Tyk2 or Jak2. Thus, it can be hypothesized that each of the kinases is able to independently associate with the receptor complex. The ability of a kinase-negative Jak2 to suppress G-CSF signaling could also be interpreted to indicate a competition for a common site of receptor complex association. However, the observation that comparable levels of expression of wild-type Jak2, which is not capable of phosphorylation of the receptor in wild-type cells, does not block activation argues against such an interpretation.
The observation that there is a difference in the ability of G-CSF to induce the tyrosine phosphorylation of kinase-negative mutants also has implications for understanding Jak interactions. Namely, within the context of the G-CSF receptor complex, Jak1 is not a detectable substrate for either Jak2 or Tyk2 or another receptor-associated kinase. Implicit in this observation is the conclusion that, within the G-CSF receptor complex, Jak1 is solely dependent on Jak1 for tyrosine phosphorylation and activation. Conversely, a kinase-inactive Jak2 is phosphorylated in the response to G-CSF and thus is a substrate for receptor-associated kinase, which could be Jak1 or Tyk2. The dominant negative effect of the kinase-inactive Jak2 could, therefore, be due to competition, as a substrate, with Jak1.
G-CSF receptor tyrosine phosphorylation is uniquely dependent on Jak1 in a manner similar to how gp130 is dependent on Jak1 for tyrosine phosphorylation22 and the phosphorylation of the growth hormone27 or Epo receptors are dependent on Jak2. However, the basis for the specificity is not known but could be due to the substrate specificity of the kinase catalytic site or to the ability of the Jaks to associate with receptors in a manner that allows the catalytic site to have access to receptor sites. Overexpression of receptors with Jaks in insect cells provides no indication of specificity in tyrosine phosphorylation (unpublished data). However, in COS cells, Jak1 phosphorylates the G-CSF receptor approximately 10 times more efficiently than Jak2 (H. Murakami and S. Nagata, unpublished data). Therefore, the specificity seen in vivo may be a combination of catalytic specificity and specificity for association with the receptor.
The inability of the overexpression of wild-type Jak2 to suppress Jak1 function and the specificity for phosphorylation of distal regions of the receptor suggest that Jak1 and Jak2 might associate with different regions of the receptor. In this regard, the inhibition by the kinase-negative Jak2 may be due to substrate competition rather than competition for binding to the receptor. The prediction from such a model is that certain membrane proximal region mutations may disrupt Jak2 or Tyk2 receptor association and activation without affecting Jak1 association and activation. These mutants would be anticipated to retain receptor function. Conversely, mutation of the Jak1 site of association would not alter Jak2 activation but would eliminate all receptor functions. Therefore, our results clearly show the importance of assessing Jak1 activation in characterizing various G-CSF receptor mutations.
The results also address questions concerning the possible unique roles that Jaks might play in Stat tyrosine phosphorylation. First, the inducible tyrosine phosphorylation of Stat1, Stat3, and Stat5 in cells lacking either Jak2 or Tyk2 demonstrate that neither of these Jaks is uniquely required for Stat activation within the context of the G-CSF receptor complex. Whether Jak1 is capable of phosphorylating the Stats cannot be addressed from our studies, although in cotransfection studies in COS cells or in coinfections in insect cells, Jak1 is able to tyrosine phosphorylate and activate any of the Stats.25 The absence of Jak1 is associated with a reduction of Stat3 tyrosine phosphorylation and a virtual elimination of Stat1 and Stat5 phosphorylation. Because receptor tyrosine phosphorylation is critical for recruitment of Stats to the receptor complex, the results are most likely due to the unique role that Jak1 plays in receptor tyrosine phosphorylation. It is quite interesting to note that the effects are much more dramatic for Stat1 or Stat5 tyrosine phosphorylation, which is virtually eliminated, relative to Stat3. Using G-CSF receptor mutants, we have recently identified both receptor tyrosine-dependent recruitment of Stat3 as well as a tyrosine-independent, membrane distal region that can recruit Stat3. The later would, therefore, be available for recruitment of Stat3 to the receptor complex in the absence of receptor phosphorylation. Once recruited, the subsequent tyrosine phosphorylation of Stat3 may not require Jak1 but rather may be efficiently mediated by Jak2 or Tyk2.
ACKNOWLEDGMENT
The authors thank Linda Synder for excellent technical assistance.
Supported by Grants-in-Aid from the Ministry of Education, Science and Culture of Japan (S.N.), The National Cancer Institute Cancer Center Support (CORE) Grant No. P30 CA21765 (J.N.I.), by support from Amgen (J.N.I.), and by the American Lebanese Syrian Associated Charities (ALSAC). J.F. is supported by the Alma and Hal Reagan Fellowship for Cancer Research at the University of Tennessee Medical School.
Address reprint requests to James N. Ihle, PhD, Department of Biochemistry, St Jude Children's Research Hospital, Memphis, TN 38105.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal