Abstract
The impairment of interleukin-2 (IL-2) production occurs very early after human immunodeficiency virus (HIV) infection as a consequence of the quantitative depletion of Th1 cells. Despite the shift in cytokine production, most individuals develop an oligoclonal expansion of major histocompatibility complex restricted, HIV-specific CD8+ cytotoxic T lymphocytes (CTL) in different organs, suggesting that other cytokines replace IL-2 in initiating the tissue infiltration of CD8+ T cells. In this study we show that IL-15, a product of monocyte-macrophages and non-T cells and which has overlapping biological activities with IL-2, is involved in local cell networks accounting for the activation and expansion of CD8+ T-cell pools in a highly affected organ, ie, the lung. IL-15 induced proliferation of T cells obtained from the lower respiratory tract of HIV-infected patients with T-cell alveolitis and severe depletion of CD4+ T cells. Lung lymphocytes were CD45R0+/CD8+ T cells spontaneously expressing activation markers (CD69 and HLA-DR) and equipped with the receptorial subunits which bind IL-15, notably the β and γ chains of the IL-2 receptor (IL-2R) and the recently identified IL-15 binding-protein termed IL-15Rα. Similar phenotypic findings were obtained after incubation of normal T cells with IL-15, which induced CD8+ T cells to express activation markers and to proliferate. The block of the IL-2Rβ/IL-2Rγ complex with specific monoclonal antibodies abolished the T-cell stimulatory activity of IL-15 while the combination of IL-15 and tumor necrosis factor-α upregulated the proliferative response of lung T lymphocytes. The hypothesis that the tissue growth of lung CD8+ lymphocytes may involve cytokines produced from cells other than T lymphocytes was confirmed by the evidence that pulmonary macrophages expressed high levels of IL-15 and that anti–IL-15 antibodies inhibited the accessory function of alveolar macrophages on mitogen-induced CD8+ T-cell proliferation. Together, these results suggest that macrophage-derived cytokines produced at sites of T-cell infiltration play a role in the activation of HIV-specific CD8+ T-cell–mediated immune response.
IN STRIKING CONTRAST to the peripheral CD4 lymphopenia, human immunodeficiency virus (HIV) may cause marked increases in the number of CD8+ T lymphocytes in peripheral blood (PB) along with CD8+ T-cell infiltration in different organs, including lung, liver, salivary glands, kidney, and bone marrow (BM).1,2 Many of the T cells infiltrating peripheral tissues are major histocompatibility complex (MHC)-restricted, antigen-driven, oligoclonal cytotoxic T lymphocytes (CTL) involved in the clearance of HIV-infected cells.3-5 The role of immune cytokines in determining the activation and accumulation of antigen-specific CTL is well established; in particular, interleukin-2 (IL-2) is regarded as an essential growth factor in initiating and promoting CTL proliferation. Nonetheless, because the number of Th1 cells (ie, the cell source of IL-2) progressively declines during the course of HIV disease, it is conceivable that other cytokines replace IL-2 in promoting the HIV-specific CTL infiltrate when a starvation of IL-2 occurs as a consequence of the low number of CD4+ T cells.2
IL-15 is a recently identified cytokine produced by monocyte-macrophages and other non-T cells that does not show homology but shares some biologic effects with IL-2.6 It induces activation and proliferation of T and natural killer (NK) cells7-9 and promotes the differentiation and growth of normal and leukemic B cells.10 IL-15 stimulates CD8+ T cells to exhibit long-term memory CTL activity against infected target cells11 and favors the recruitment of T cells at sites of inflammation in different organs, including the lung.12,13 All the above effects are mediated via binding with the β and γ chains of the IL-2 receptor (IL-2R) system.14 Interestingly, IL-15 has been shown to stimulate the proliferative activity of circulating lymphocytes in HIV-infected individuals in response to HIV-specific antigens.15 The fact that IL-15 mimics the biologic effects of IL-2 and favors migration and activation of T cells at sites of inflammation12 13 makes it an attractive candidate to investigate the mechanisms promoting host CTL response against HIV and regulating the development of CD8+ T-cell infiltrate.
The aim of this study was to investigate whether IL-15 regulates the CD8+ T-cell accumulation into extravascular tissues of HIV-infected patients. Data herein reported demonstrate that macrophages obtained from a common site of CD8+ T-cell infiltration, ie, the lung,2 bear IL-15 and that this cytokine, both alone and in cooperation with tumor necrosis factor-α (TNF-α), triggers the activation and proliferation of pulmonary T cells. The presence of the HIV genome in pulmonary cells of infected patients has been extensively proven and several data demonstrated that HIV-specific CTL responses take place in the lower respiratory tract not only in the early phases but also in the advanced phases of HIV infection.2 Locally produced IL-15 is likely to replace the requirement of IL-2 when the number of tissue CD4+ T cells declines, thereby contributing to maintain an effective CD8+ T-cell–mediated immune response against HIV.
MATERIALS AND METHODS
Study populations.Twelve patients with HIV infection were analyzed (9 male and 3 female; mean age 31.3 ± 6.3 years). According to the Centers for Disease Control criteria for HIV-related disorders, all patients showed clinical symptoms and signs of HIV infection other than, or in addition to, lymphoadenopathy. In particular, 2 patients had acquired immunodeficiency syndrome (AIDS) related complex and 10 patients had AIDS (specifically, Pneumocystis carinii pneumonia in 9 patients and P carinii pneumonia and Candida albicans in 1 patient). Bronchoalveolar lavage (BAL) was performed to obtain the specific diagnosis of opportunistic infections, according to our study protocol for HIV patients with suspected pulmonary involvement. All HIV-seropositive patients evaluated in this study showed a T-cell alveolitis, as defined by the presence of more than 30 × 103 lymphocytes/mL in the BAL fluid.
The control subjects were 7 nonsmoking healthy persons (5 men and 2 women; average age 33.5 ± 5.9 years). They had normal physical examinations, chest radiographs, lung function tests, and BAL cell numbers.
Preparation of cell suspensions.PB mononuclear cells (PBMCs) were obtained by Ficoll-Hypaque (F/H) gradient centrifugation (Pharmacia Biotech, Uppsala, Sweden). The cell suspension was initially depleted of adherent cells by incubation for 45 minutes in plastic Petri dishes at 37°C in an atmosphere of 95% air and 5% CO2 . T cells were enriched from the resulting cell suspensions by rosetting with neuraminidase-treated sheep red blood cells (SRBCs) followed by F/H gradient separations, as previously described.16 To remove the adherent monocytes, plastic dishes were sprayed with cold RPMI using a 20-mL siringe equipped with a 26-gauge needle.
After administration of local anesthesia, the BAL was performed as previously described.16 Briefly, a total of 150 to 200 mL of saline solution was injected via fiberoptic bronchoscopy in 25-mL aliquots, with immediate vacuum aspiration after each aliquot. The fluid was filtered through gauze and its volume measured: 52.1% ± 5.4% of the injected fluid was recovered. Cells recovered from the BAL were washed three times with phosphate-buffered saline (PBS) resuspended in endotoxin-tested RPMI 1640 (Sigma Chemical Co, St Louis, MO) supplemented with 20 mmol/L HEPES and L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and 10% fetal calf serum (FCS; ICN Flow, Costa Mesa, CA) and then counted. Macrophages, lymphocytes, neutrophils, and eosinophils were differentially counted in a total count of 300 cells according to morphologic criteria in cytocentrifuged smears stained with Wright-Giemsa.
Pulmonary macrophages and T cells were enriched from the entire mononuclear BAL-cell suspensions by adherence and rosetting with neuraminidase-treated SRBCs followed by F/H gradient separations, as described above. Following this multistep selection procedure, more than 95% of the above cells were viable as judged by trypan blue exclusion. Staining with CD3 (Immunotech, Marseille, France) monoclonal antibody (MoAb) showed a percentage of CD3 T cells ranging from 98% to 100% in BAL T lymphocytes, whereas more than 98% of adherent cells expressed the macrophage-associated PAM-1 antigen (kindly provided by Dr A. Mantovani, Milan, Italy).
MoAbs and cytokines.The commercially available conjugated or unconjugated MoAbs used belonged to the Becton Dickinson and Immunotech series and included: CD3, CD4, CD8, CD25 (IL-2Rα), CD45RO, CD45RA, CD69, CD122 (IL-2Rβ), HLA-DR, and isotype-matched controls. Anti-Tac (IgG2a, CD25) MoAb, which recognizes the p55 IL-2R, was a gift of Dr T. Uchiyama (Kyoto, Japan). Ascites anti-p75 IL-2R TU27 MoAb (IgG1) and Mikβ1 (IgG2) MoAbs were gifts of Dr K. Sugamura (Senday, Japan) and Dr J. Hamuro (Kawasaki, Japan) and Dr M. Tsudo (Tokyo, Japan), respectively; both MoAbs recognize the p75 chain of IL-2R (CD122). Ascites TUGh4 MoAb (IgG2b), which recognizes the p64 IL-2Rγ chain, was a gift of Dr K. Sugamura. Anti–IL-15 M110 MoAb (IgG1) was kindly provided by Dr A. Troutt (Immunex Co, Seattle, WA); anti-IL-15 M111 (IgG1) and M112 (IgG1) MoAbs were purchased from Genzyme Co (Boston, MA). Anti–IL-2PE MQ1-17H12 (IgG2) MoAb was purchased from PharMingen (San Diego, CA).
The frequency of PB and BAL cells positive for the above reagents was determined by flow cytometry, as previously described.17 Briefly, 10 × 103 cells were acquired and the analysis was determined by overlaying the histograms of the samples stained with the different reagents. Both BAL lymphocytes and pulmonary macrophages were gated in flow cytometry analysis using these two different approaches: (1) physical characteristics of cells, and (2) expression of the positivity for the T-associated CD3 and macrophage-associated PAM-1 antigens in the area of lymphocytes and pulmonary macrophages, respectively. The purity of the gates was always higher than 98% cells. Cells were scored using a FACScan analyzer (Becton Dickinson), and data were processed using the Macintosh CELLQuest software program (Becton Dickinson). The expression of cytoplasmic IL-15 and IL-2 was evaluated after permeabilization of cell membranes using 1:2 diluted PermeaFix (Ortho, Raritan, NJ) for 40 minutes. After permeabilization procedures, anti–IL-15 and anti–IL-2 MoAbs were added.
Because pulmonary cells bore membrane–IL-15, cytoplasmic–IL-15, IL-2Rβ, and IL-2Rγ subunits in a unimodal expression pattern, indicating that the entire cell population exhibits relatively homogeneous fluorescence, the percentage of positive cells does not represent the most accurate way of enumerating positive cells. For this reason, the mean fluorescence intensity (MFI) was used to compare the positivity of these specific antigens on different cell populations. To evaluate whether the shift of the positive cell peak was statistically significant, the Kolmogorov-Smirnov test for analysis of histograms was used according to the Macintosh CELLQuest software user's guide (Becton Dickinson).
For immunofluorescence analysis, control IgG1 and IgG2a and IgG2b were obtained from Becton Dickinson; control rat antiserum consisted of ascites containing an irrelevant rat IgG2b MoAb (kindly provided by A. Rosato, Padua, Italy); control rabbit antiserum consisted of rabbit IgG myeloma (purified protein) purchased from Serotec (Serotec, Oxford, UK); and goat-antirabbit IgG and goat F(ab′)2 antirat IgG were obtained from Immunotech (Marseille, France).
Recombinant IL-15 (specific activity 33.3 × 105 U/μg) was kindly provided by Dr A. Troutt (Immunex Co), recombinant IL-2 (specific activity 3.10 × 106 U/mg) by Biogen (Cambridge, MA), and TNF-α (specific activity 8.74 × 106 U/mg) by Drs V. Rollinger and J. Kempemi (Knoll AG/BASF, Ludwigshafen, Germany).
RNA extraction, cDNA synthesis, polymerase chain reaction (PCR) and amplification.Total cellular RNA was extracted using the ultraspec-II RNA isolation system (Biotec Lab, Houston, TX) from pulmonary macrophages and T cells. cDNAs were prepared as previously described.13 For the detection of mRNA for IL-15 and the α, β, and γ chains of the IL-2R and IL-15Rα the above-reported methods were used.8 13 The preparation of all stock solutions and PCR reactions was not done in the same room in which PCR-amplified products were manipulated. The pipet tips and other equipment used were exclusive for PCR preparation steps. In particular, the pipet tips were plugged and used only once. Aliquots prepared from each stock solution were discarded at the end of a single day's use. Sterile gloves were worn at all times.
IL-15 effect on the T-cell activation and proliferation.To assess the effects of IL-15 on the expression of differentiation (CD45RA, CD45R0) and activation markers (CD69, and HLA-DR) of CD8+ T cells, PBMCs and lung BAL cells at the concentration of 1 × 106 cells/mL were cultured in 24-well plates (Corning, New York, NY) for 16 hours at 37°C in 5% CO2 atmosphere with IL-15 (100 ng/mL). The frequency of PB and BAL cells positive for the above reagents was determined by flow cytometry, as described above.
To evaluate the proliferative response of lung T cells to IL-15, highly purified pulmonary T cells at the concentration of 1 × 106 cells/mL were cultured in 96 round-bottom well plates (Corning) for 72 hours at 37°C in 5% CO2 atmosphere with IL-15 (1, 10, 100 ng/mL), recombinant (r) IL-2 (10, 100, 1,000 IU/mL), and TNF-α (10, 100, 500 IU/mL). In inhibition experiments, anti-p75 IL-2R (20 μg/mL) or anti-p64 IL-2R MoAb (20 μg/mL) or control isotype-matched IgG1 were added at the beginning of the culture. The effect of p75 or p64 anti–IL-2R MoAbs on T-cell proliferation was tested in preliminary experiments using either soluble MoAbs or MoAbs cross-linked with goat-antimouse IgG, with no difference in inhibitory action. Each experiment was performed in quadruplicate. For the last 18 hours of culture, plates were pulsed with 1 μCi/well of (3H)thymidine (6.7 Ci/mmol; Du Pont, NEN, Boston, MA). Cells were then procured and the radioactivity measured with a β counter. Results are expressed as cpm or as stimulation index (SI), according to the formula: SI = Mean of cpm Quadriplicates With Stimulus/Mean of cpm Quadriplicates Without Stimulus. A SI higher than 3 was scored as positive.
Evaluation of the involvement of IL-15 in the accessory function of pulmonary macrophages.It has been previously shown that alveolar macrophages from HIV-infected patients are effective accessory cells in stimulating a T-cell mitogen-mediated response and that optimal proliferation requires macrophage-T cell contact as well as release of soluble factors.18 Because T cells accumulating in the lung of patients with HIV infection are mainly T lymphocytes expressing the CD8+ phenotype, CD8+ T cells were purified from the T-cell suspensions obtained from two control subjects by a two-step sequential depletion using magnetic separations over columns (Mini MACS; Miltenvi Biotech, Bergisch Gladbach, Germany)16 and the role of macrophagic IL-15 expression in the modulation of the proliferation of highly purified CD8+ T cells was established.
Highly purified CD8+ T cells at the concentration of 1 × 106 cells/mL were cultured in 96 round-bottom well plates for 72 hours at 37°C in 5% CO2 atmosphere with 12.5 × 103 alveolar macrophages in the presence of mitogens (ConA, 10 μg/mL; Sigma Chemical Co). In inhibition experiments, anti–IL-15 (10 μg/mL) and anti-IL-2 (10 μg/mL) or control isotype-matched IgG1 were added at the beginning of the culture. Each experiment was performed in quadruplicate. For the last 18 hours of culture, plates were pulsed with 1 μCi/well of (3H)thymidine, as reported above.
Statistical analysis.Data were analyzed with the assistance of the Statistical Analysis System. Data are expressed as mean ± SD. Values were compared using the ANOVA test. A P value <.05 was considered as significant.
RESULTS
Phenotypic analysis of T-cell subsets in the PB at the time of the BAL analysis showed that all patients had a CD4+ T-cell count <200 cells/μL; the CD8+ T-cell counts in the PB showed >400 cells/μL in 6 cases and <400 cells/μL in the remaining 6 cases. Table 1 summarizes the results of the BAL findings. Cell recovery was significantly higher in patients with AIDS with respect to control subjects. With regard to the differential count of BAL cells, CD4+ T cells were virtually absent in all BAL specimens. All patients showed a T-cell alveolitis (more than 30 × 103 lymphocytes/mL of BAL fluid). As a consequence of the decline of the CD4+ T-cell population and of the increase in the absolute number of CD8+ T cells, BAL CD4/CD8 ratio was dramatically decreased in all patients.
Bronchoalveolar Lavage Findings in 12 Patients With HIV Infection and T-Cell Alveolitis
| Patients . | Cell Recovery . | Lymphocytes . | Alveolar Macrophages . | Neutrophils . | Eosinophils . | CD3 T Cells . | CD4 T Cells . | CD8 T Cells . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | ×103 . | % . | ×103 . | % . | ×103 . | % . | ×103 . | % . | ×103 . | % . | ×103 . | % . | ×103 . | % . | ×103 . |
| 1 (ARC) | 297 | 35 | 103.9 | 65 | 193.1 | 0 | 0 | 0 | 0 | 89 | 91.6 | 1 | 1.0 | 88 | 90.6 |
| 2 (ARC) | 367 | 41 | 147.6 | 58 | 212.9 | 1 | 3.6 | 0 | 0 | 91 | 133.7 | 1 | 1.4 | 88 | 129.4 |
| 3 (AIDS) | 263 | 32 | 84.2 | 63 | 165.6 | 3 | 7.8 | 1 | 2.6 | 90 | 75.6 | 0.5 | 0.4 | 89 | 74.1 |
| 4 (AIDS) | 389 | 40 | 155.6 | 60 | 233.4 | 0 | 0 | 0 | 0 | 93 | 144.1 | 1 | 1.5 | 92 | 142.6 |
| 5 (AIDS) | 350 | 36 | 115.6 | 63 | 220.5 | 1 | 3.5 | 0 | 0 | 95 | 109.2 | 0.5 | 0.5 | 94 | 108.6 |
| 6 (AIDS) | 307 | 33 | 101.3 | 65 | 199.5 | 2 | 6.0 | 0 | 0 | 90 | 90.9 | 1 | 1.0 | 90 | 90.9 |
| 7 (AIDS) | 945 | 20 | 189.0 | 75 | 708.7 | 5 | 47.2 | 0 | 0 | 90 | 170.1 | 0.5 | 0.9 | 90 | 170.1 |
| 8 (AIDS) | 800 | 42 | 336.0 | 56 | 448.0 | 1 | 8.0 | 1 | 9.0 | 96 | 322.5 | 1 | 3.6 | 93 | 312.4 |
| 9 (AIDS) | 289 | 38 | 76.8 | 61 | 176.3 | 0 | 0 | 1 | 0.3 | 89 | 68.3 | 1 | 0.7 | 88 | 66.8 |
| 10 (AIDS) | 333 | 25 | 82.1 | 70 | 231.1 | 4 | 12.0 | 1 | 3.3 | 92 | 75.4 | 0.5 | 0.4 | 91 | 74.6 |
| 11 (AIDS) | 411 | 31 | 127.4 | 68 | 279.8 | 1 | 4.0 | 1 | 4.0 | 90 | 114.3 | 0.5 | 0.6 | 89 | 113.3 |
| 12 (AIDS) | 401 | 29 | 116.3 | 71 | 284.3 | 0 | 0 | 0 | 0 | 93 | 107.8 | 1 | 1.2 | 93 | 107.8 |
| HIV infected patients (as a group) | 429.3 ± 214.3 | 33.5 ± 6.6 | 135.1 ± 70.9 | 64.5 ± 5.6 | 279.0 ± 154.4 | 1.5 ± 1.6 | 6.6 ± 13.0 | 0.3 ± 0.3 | 1.01 ± 2.6 | 91.4 ± 2.2 | 125.2 ± 68.9 | 0.79 ± 0.3 | 1.1 ± 0.9 | 90.4 ± 2.2 | 123.2 ± 66.7 |
| Controls (n = 5) | 126.6 ± 16.4 | 6.8 ± 3.1 | 8.6 ± 3.4 | 90.8 ± 6.3 | 114.8 ± 18.8 | 0.2 ± 0.4 | 0.2 ± 0.4 | 0.1 ± 0.4 | 0.2 ± 0.2 | 87.8 ± 3.1 | 7.4 ± 2.9 | 57.6 ± 3.2 | 5.1 ± 1.9 | 30.4 ± 3.5 | 2.5 ± 1.0 |
| P | .01 | .001 | .001 | .001 | NS | NS | NS | NS | NS | NS | .001 | .001 | .01 | .001 | .001 |
| Patients . | Cell Recovery . | Lymphocytes . | Alveolar Macrophages . | Neutrophils . | Eosinophils . | CD3 T Cells . | CD4 T Cells . | CD8 T Cells . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | ×103 . | % . | ×103 . | % . | ×103 . | % . | ×103 . | % . | ×103 . | % . | ×103 . | % . | ×103 . | % . | ×103 . |
| 1 (ARC) | 297 | 35 | 103.9 | 65 | 193.1 | 0 | 0 | 0 | 0 | 89 | 91.6 | 1 | 1.0 | 88 | 90.6 |
| 2 (ARC) | 367 | 41 | 147.6 | 58 | 212.9 | 1 | 3.6 | 0 | 0 | 91 | 133.7 | 1 | 1.4 | 88 | 129.4 |
| 3 (AIDS) | 263 | 32 | 84.2 | 63 | 165.6 | 3 | 7.8 | 1 | 2.6 | 90 | 75.6 | 0.5 | 0.4 | 89 | 74.1 |
| 4 (AIDS) | 389 | 40 | 155.6 | 60 | 233.4 | 0 | 0 | 0 | 0 | 93 | 144.1 | 1 | 1.5 | 92 | 142.6 |
| 5 (AIDS) | 350 | 36 | 115.6 | 63 | 220.5 | 1 | 3.5 | 0 | 0 | 95 | 109.2 | 0.5 | 0.5 | 94 | 108.6 |
| 6 (AIDS) | 307 | 33 | 101.3 | 65 | 199.5 | 2 | 6.0 | 0 | 0 | 90 | 90.9 | 1 | 1.0 | 90 | 90.9 |
| 7 (AIDS) | 945 | 20 | 189.0 | 75 | 708.7 | 5 | 47.2 | 0 | 0 | 90 | 170.1 | 0.5 | 0.9 | 90 | 170.1 |
| 8 (AIDS) | 800 | 42 | 336.0 | 56 | 448.0 | 1 | 8.0 | 1 | 9.0 | 96 | 322.5 | 1 | 3.6 | 93 | 312.4 |
| 9 (AIDS) | 289 | 38 | 76.8 | 61 | 176.3 | 0 | 0 | 1 | 0.3 | 89 | 68.3 | 1 | 0.7 | 88 | 66.8 |
| 10 (AIDS) | 333 | 25 | 82.1 | 70 | 231.1 | 4 | 12.0 | 1 | 3.3 | 92 | 75.4 | 0.5 | 0.4 | 91 | 74.6 |
| 11 (AIDS) | 411 | 31 | 127.4 | 68 | 279.8 | 1 | 4.0 | 1 | 4.0 | 90 | 114.3 | 0.5 | 0.6 | 89 | 113.3 |
| 12 (AIDS) | 401 | 29 | 116.3 | 71 | 284.3 | 0 | 0 | 0 | 0 | 93 | 107.8 | 1 | 1.2 | 93 | 107.8 |
| HIV infected patients (as a group) | 429.3 ± 214.3 | 33.5 ± 6.6 | 135.1 ± 70.9 | 64.5 ± 5.6 | 279.0 ± 154.4 | 1.5 ± 1.6 | 6.6 ± 13.0 | 0.3 ± 0.3 | 1.01 ± 2.6 | 91.4 ± 2.2 | 125.2 ± 68.9 | 0.79 ± 0.3 | 1.1 ± 0.9 | 90.4 ± 2.2 | 123.2 ± 66.7 |
| Controls (n = 5) | 126.6 ± 16.4 | 6.8 ± 3.1 | 8.6 ± 3.4 | 90.8 ± 6.3 | 114.8 ± 18.8 | 0.2 ± 0.4 | 0.2 ± 0.4 | 0.1 ± 0.4 | 0.2 ± 0.2 | 87.8 ± 3.1 | 7.4 ± 2.9 | 57.6 ± 3.2 | 5.1 ± 1.9 | 30.4 ± 3.5 | 2.5 ± 1.0 |
| P | .01 | .001 | .001 | .001 | NS | NS | NS | NS | NS | NS | .001 | .001 | .01 | .001 | .001 |
Abbreviation: NS, not significant.
Lung CD8+ T cells from HIV-infected patients show a memory phenotype, and express activation markers and high levels of IL-15 receptor.As shown in Table 2, pulmonary T cells isolated from the lung of HIV-infected patients were primed/memory CD45R0+/CD8+ T cells that express activation markers, including CD69 and HLA-DR. Lung T cells from healthy subjects were CD45R0+/CD8+ T cells expressing low levels of activation markers. After a 12-hour exposure to IL-15, normal PB T cells shifted toward a memory phenotype. After IL-15 exposure, both lung and blood T cells from healthy subjects acquired the expression of CD69 and HLA-DR.
Phenotypic Characteristics of CD8+ T Cells in the PB and the Lung of HIV-Infected Patients and Healthy Subjects Both at Resting Conditions and After a 12-Hour Exposure to IL-15
| T-Cell Subsets . | CD45RA . | CD45R0 . | CD69 . | HLA-DR . | ||||
|---|---|---|---|---|---|---|---|---|
| . | At Resting Conditions . | After 12-h IL-15 Exposure . | At Resting Conditions . | After 12-h IL-15 Exposure . | At Resting Conditions . | After 12-h IL-15 Exposure . | At Resting Conditions . | After 12-h IL-15 Exposure . |
| . | % . | % . | % . | % . | % . | % . | % . | % . |
| HIV-infected patients | ||||||||
| PB CD8+ T cells | 38.3 ± 6.1 | 21.2 ± 5.4 | 47.1 ± 3.9 | 69.3 ± 5.2 | 5.2 ± 3.0 | 47.1 ± 7.5 | 7.4 ± 2.7 | 46.3 ± 5.3 |
| Pulmonary CD8+ T cells | 3.3 ± 1.4 | 0.1 ± 0.6 | 91.3 ± 5.3 | 98.2 ± 2.7 | 65.4 ± 5.8 | 87.3 ± 4.0 | 70.2 ± 5.2 | 93.3 ± 4.0 |
| Controls | ||||||||
| PB CD8+ T cells | 52.1 ± 4.2 | 33.1 ± 3.9 | 39.4 ± 4.2 | 66.1 ± 4.1 | 3.4 ± 1.1 | 78.6 ± 6.1 | 1.1 ± 0.3 | 69.6 ± 5.9 |
| Pulmonary CD8+ T cells | 4.1 ± 0.9 | 0.4 ± 0.4 | 84.1 ± 6.0 | 97.1 ± 2.0 | 21.4 ± 3.6 | 56.4 ± 4.6 | 22.4 ± 3.2 | 67.7 ± 7.0 |
| T-Cell Subsets . | CD45RA . | CD45R0 . | CD69 . | HLA-DR . | ||||
|---|---|---|---|---|---|---|---|---|
| . | At Resting Conditions . | After 12-h IL-15 Exposure . | At Resting Conditions . | After 12-h IL-15 Exposure . | At Resting Conditions . | After 12-h IL-15 Exposure . | At Resting Conditions . | After 12-h IL-15 Exposure . |
| . | % . | % . | % . | % . | % . | % . | % . | % . |
| HIV-infected patients | ||||||||
| PB CD8+ T cells | 38.3 ± 6.1 | 21.2 ± 5.4 | 47.1 ± 3.9 | 69.3 ± 5.2 | 5.2 ± 3.0 | 47.1 ± 7.5 | 7.4 ± 2.7 | 46.3 ± 5.3 |
| Pulmonary CD8+ T cells | 3.3 ± 1.4 | 0.1 ± 0.6 | 91.3 ± 5.3 | 98.2 ± 2.7 | 65.4 ± 5.8 | 87.3 ± 4.0 | 70.2 ± 5.2 | 93.3 ± 4.0 |
| Controls | ||||||||
| PB CD8+ T cells | 52.1 ± 4.2 | 33.1 ± 3.9 | 39.4 ± 4.2 | 66.1 ± 4.1 | 3.4 ± 1.1 | 78.6 ± 6.1 | 1.1 ± 0.3 | 69.6 ± 5.9 |
| Pulmonary CD8+ T cells | 4.1 ± 0.9 | 0.4 ± 0.4 | 84.1 ± 6.0 | 97.1 ± 2.0 | 21.4 ± 3.6 | 56.4 ± 4.6 | 22.4 ± 3.2 | 67.7 ± 7.0 |
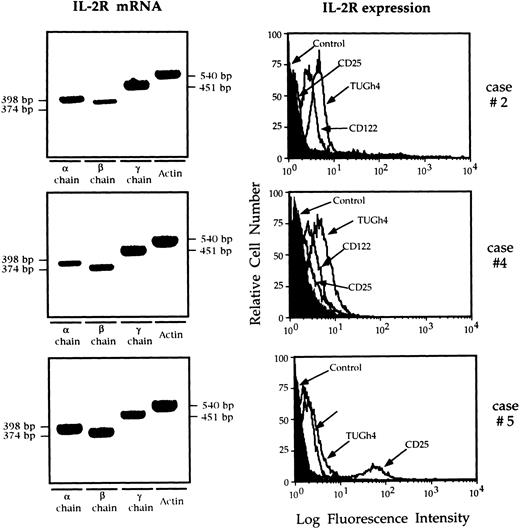
Figure 1 shows IL-2R mRNA and the overlaid histograms of BAL T cells stained with anti IL-2R chain MoAbs in three patients who show data representative for the entire study population. T cells retrieved from the lung of all nine patients with AIDS expressed α, β, and γ chain mRNA, and bore membrane IL-2Rβ and IL-2Rγ, ie, the receptor complex which binds IL-15. Two of the six patients showed a weak expression of CD25 molecule by BAL T cells (7% and 11%, respectively; case no. 5 in Fig 1). Lung T cells from control subjects did not express CD25 and CD122, while the γ chain was constitutively expressed. Interestingly, lung T lymphocytes retrieved from HIV-infected patients showed a higher IL-2Rγ expression than normal lung T cells (P < .01 at the Kolmogorov-Smirnov statistic analysis).
RT-PCR analysis of the expression of the messages for the α, β, γ IL-2R, and flow cytometry analysis of IL-2R complex expression by BAL T cells isolated from three representative patients with HIV infection. Patients' T cells expressed the messages for the α, β, and γ IL-2R chains. The limited number of T cells available from the BAL of normal subjects prevented the evaluation of the IL-2R messages. In patients with AIDS the CD122 histograms were completely shifted with respect to the control histograms, indicating that the entire T-cell population expresses the IL-2Rβ chain. The γc was constitutively expressed while the α chain was expressed by a discrete percentage of lung T cells from case no. 5 (11%).
RT-PCR analysis of the expression of the messages for the α, β, γ IL-2R, and flow cytometry analysis of IL-2R complex expression by BAL T cells isolated from three representative patients with HIV infection. Patients' T cells expressed the messages for the α, β, and γ IL-2R chains. The limited number of T cells available from the BAL of normal subjects prevented the evaluation of the IL-2R messages. In patients with AIDS the CD122 histograms were completely shifted with respect to the control histograms, indicating that the entire T-cell population expresses the IL-2Rβ chain. The γc was constitutively expressed while the α chain was expressed by a discrete percentage of lung T cells from case no. 5 (11%).
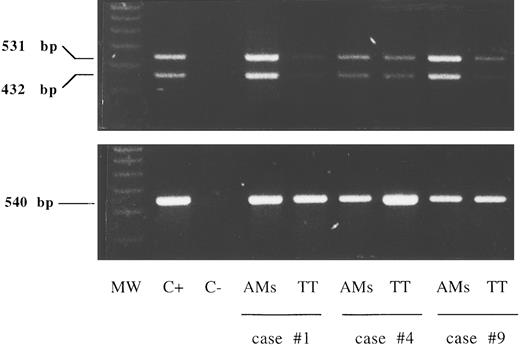
A faint expression of IL-15Rα chain mRNA was detectable by reverse transcriptase (RT)-PCR in purified lung T cells from six HIV-infected patients while alveolar macrophages bore high levels of IL-15Rα chain mRNA (Fig 2). The presence of two amplification products of 531 and 432 bp shown in the figure is consistent with the existence of two alternatively spliced mRNA isoforms characterized by the presence or absence of exon 3 (+99 bp).
RT-PCR analysis of the expression of the messages for the IL-15Rα chain and control β-actin in lung T cells and alveolar macrophages (AMs). Lane 1, molecular weight (MW) marker (molecular weight VI-digested); lane 2, positive control (C+) (PBMCs stimulated for 12 hours with phytohemagglutinin, 5 μg/mL); lane 3, the negative control (C−) (sample without cDNA); lanes 4 to 9, alveolar macrophages and lung T cells isolated from three representative patients with HIV infection (cases no. 1, 4, and 9 in Table 1). A faint expression of IL-15Rα chain mRNA was detectable by RT-PCR in purified lung T cells while alveolar macrophages bore higher levels of IL-15Rα chain mRNA at the transcript level.
RT-PCR analysis of the expression of the messages for the IL-15Rα chain and control β-actin in lung T cells and alveolar macrophages (AMs). Lane 1, molecular weight (MW) marker (molecular weight VI-digested); lane 2, positive control (C+) (PBMCs stimulated for 12 hours with phytohemagglutinin, 5 μg/mL); lane 3, the negative control (C−) (sample without cDNA); lanes 4 to 9, alveolar macrophages and lung T cells isolated from three representative patients with HIV infection (cases no. 1, 4, and 9 in Table 1). A faint expression of IL-15Rα chain mRNA was detectable by RT-PCR in purified lung T cells while alveolar macrophages bore higher levels of IL-15Rα chain mRNA at the transcript level.
Taken together, these findings suggest that lung T lymphocytes from HIV-infected patients with T-cell alveolitis are preactivated T cells expressing IL-15 receptor subunits.
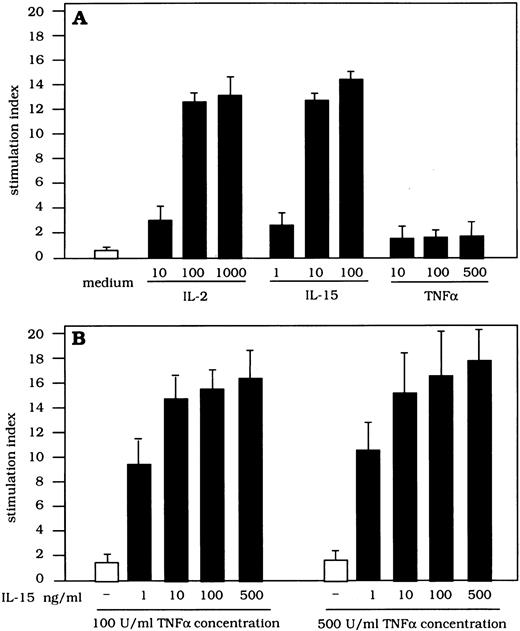
Proliferative activity of IL-15, TNF-α, and IL-2 on highly purified lung T cells.In a further set of experiments we investigated whether IL-15 may account for the proliferation of lung, preactivated CD8+ T cells (Fig 3). BAL T cells were cultured with different concentrations of IL-15, IL-2, and TNF-α. Lung T cells significantly proliferated in the presence of IL-15 (SI 11.2 ± 2.7 and 13.1 ± 4.1 with 10 and 100 ng of IL-15, respectively) and IL-2 (SI 9.2 ± 3.7 and 13.3 ± 5.7 with 100 and 1,000 UI of IL-2, respectively); on the other hand, TNF-α alone did not induce T-cell proliferation at any concentration used (SI 0.9 ± 0.2 and 1.15 ± 0.21 with 100 and 500 UI of TNF-α, respectively). IL-15 strongly upregulated the IL-2–mediated proliferative activity of lung T cells. In fact, the combined use of IL-2 (100 IU) and IL-15 (100 ng) gave rise to a significant increase in the proliferative rate of BAL T cells ranging from 46% to 54%. More interestingly, IL-15 (100 ng) effectively synergized with TNF-α in inducing the proliferative response of BAL T lymphocytes. These functional data suggest that IL-15 may cooperate with both CD4+ T-cell–derived (ie, IL-2) and macrophage-derived cytokines (ie, TNF-α) in inducing an effective proliferation of lung T cells. However, the fact that lung T cells proliferate at low levels of IL-15 (1 ng/mL) strongly suggests that we are dealing with preactivated T cells that express a functional high-affinity receptor for IL-15.
Proliferative activity of T cells retrieved from the lung of patients with HIV infection after stimulation with IL-2, IL-15, and TNF-α and with different combinations of the above cytokines. BAL T cells proliferated in the presence of IL-15 or IL-2, but not TNF-α alone. The concurrent stimulation with IL-2 (100 IU/mL) and TNF-α (100 IU/mL) significantly increases the IL-15–mediated T-cell proliferation.
Proliferative activity of T cells retrieved from the lung of patients with HIV infection after stimulation with IL-2, IL-15, and TNF-α and with different combinations of the above cytokines. BAL T cells proliferated in the presence of IL-15 or IL-2, but not TNF-α alone. The concurrent stimulation with IL-2 (100 IU/mL) and TNF-α (100 IU/mL) significantly increases the IL-15–mediated T-cell proliferation.
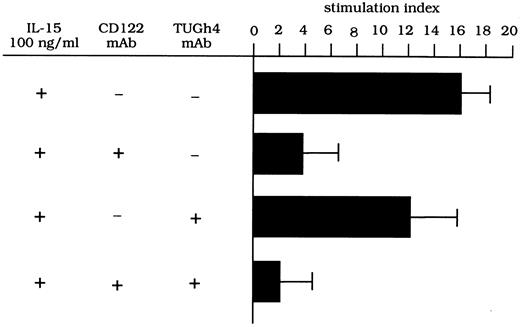
To evaluate whether the β/γ IL-2R complex has signaling functions on the proliferation of lung T lymphocytes, pulmonary T cells isolated from three HIV-infected patients were stimulated with IL-15 (100 ng) in the presence of anti–IL-2R MoAbs. As shown in Fig 4, CD122 MoAb (anti–IL-2Rβ) effectively inhibited the IL-15–driven proliferation of T cells, while the inhibitory ability of the TUGh4 (anti–IL-2Rγ) MoAb was always less than 30%. The concomitant use of anti-β and anti-γ chain MoAbs resulted in an inhibition of the proliferative activity of BAL T cells with an efficiency ranging from 90% to 96%. These data suggest that both subunits have signaling functions on the IL-15–mediated T-cell proliferation.
Proliferative activity of T cells retrieved from the lung of patients with HIV infection after stimulation with IL-15 and TNF-α and with different anti–IL-2R MoAbs. Anti-CD122 MoAb but not anti-γc MoAb inhibits the stimulatory activity of IL-15–mediated T-cell proliferation. A combination of CD122 and TUGh4 MoAbs abolished the IL-15–mediated proliferative activity.
Proliferative activity of T cells retrieved from the lung of patients with HIV infection after stimulation with IL-15 and TNF-α and with different anti–IL-2R MoAbs. Anti-CD122 MoAb but not anti-γc MoAb inhibits the stimulatory activity of IL-15–mediated T-cell proliferation. A combination of CD122 and TUGh4 MoAbs abolished the IL-15–mediated proliferative activity.
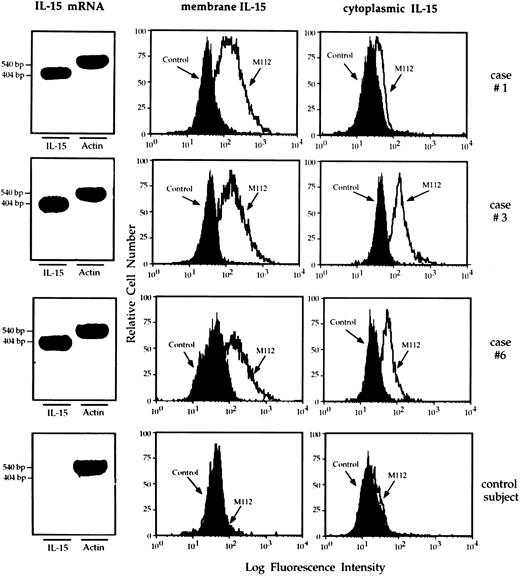
Mononuclear phagocytes from the lung of HIV-infected patients express IL-15.RT-PCR analysis showed that macrophages retrieved from the lung of patients with HIV infection showed a strong expression of mRNA IL-15 (Fig 5 shows three representative patients). Furthermore, the cells bore membrane and cytoplasmic IL-15. In particular, as demonstrated by the Kolmogorov-Smirnov analysis, lung macrophages expressed membrane and cytoplasmic IL-15 because the peak of M112 cells was significantly shifted with respect to the negative controls (percentage ranging from 57% to 88%). Consistent results were obtained using two additional MoAbs (M110 and M111) against IL-15 (data not shown). Pulmonary macrophages from healthy subjects did not show membrane or cytoplasmic IL-15 expression (Fig 5; bottom series of panels). In both healthy subjects and AIDS patients PB monocytes spontaneously expressed membrane and cytoplasmic IL-15. As previously reported,17 a percentage ranging from 40% to 53% of macrophages from patients with HIV infection expressed membrane and cytoplasmic TNF-α (mean, 44.6% ± 6.7). In contrast, less than 1% of mononuclear phagocytes isolated from the lung of healthy individuals showed cellular TNF-α.
RT-PCR analysis of the expression of IL-15 message and flow cytometry analysis of membrane and cytoplasmic expression of IL-15 by pulmonary macrophages recovered from three representative patients with HIV infection and high-intensity T-cell alveolitis and a control subject who showed a normal cell recovery (bottom series of panels). Highly purified alveolar macrophages retrieved from the patients showed a strong IL-15 message. Furthermore, they bore membrane – and cytoplasmic–IL-15, whereas alveolar macrophages retrieved from the normal individual did not.
RT-PCR analysis of the expression of IL-15 message and flow cytometry analysis of membrane and cytoplasmic expression of IL-15 by pulmonary macrophages recovered from three representative patients with HIV infection and high-intensity T-cell alveolitis and a control subject who showed a normal cell recovery (bottom series of panels). Highly purified alveolar macrophages retrieved from the patients showed a strong IL-15 message. Furthermore, they bore membrane – and cytoplasmic–IL-15, whereas alveolar macrophages retrieved from the normal individual did not.
Purified BAL T cells from HIV-infected subjects did not express IL-15 mRNA, nor was mRNA for IL-2 detectable in T cells retrieved from five of the six HIV-seropositive patients. Flow cytometry analysis showed that lung T cells isolated from the BAL of patients with AIDS having a low number of CD4+ T cells never expressed cytoplasmic IL-2 or IL-15 molecules. The limited number of T cells recovered from the BAL of normal subjects prevented the evaluation of the mRNA for IL-15 and IL-2. Nonetheless, by using anti–IL-15 and anti–IL-2 MoAbs we showed that these cells never bore membrane or cytoplasmic IL-15 while up to 15% of normal BAL T cells expressed cytoplasmic IL-2.
Expression of IL-15 on pulmonary macrophages is involved in their accessory function.Figure 6 shows the effect of blocking IL-15 with MoAbs on the accessory function of alveolar macrophages isolated from three HIV-seropositive patients with CD8+ T-cell alveolitis. The purity of the CD8+ lymphocyte populations used in the proliferation assays exceeded 99%, with virtually no detectable residual CD4+ cells (ie, the cell source of IL-2) or monocytes. Highly purified CD8+ T cells obtained using magnetic separations did not proliferate when stimulated with ConA unless accessory cells were added. However, alveolar macrophages induced a discrete proliferation of highly purified CD8+ T cells in the presence of mitogen; these data were not surprising given the known accessory function of pulmonary macrophages from HIV-infected subjects in mitogen assays.18 However, it is readily apparent that anti–IL-15 antibodies, when added to the mitogen assay at the beginning of culture, inhibited the CD8+ T-cell proliferation by roughly 50%. The presence of both anti–IL-15 and anti–IL-2 antibodies at the beginning of culture produced the same amount of inhibition. The inhibitory ability of a control MoAb was always less than 2% (data not shown).
Effect of anti–IL-15 antibodies on accessory function of pulmonary macrophages. The figure shows data on the inhibitory activity of M110 anti–IL-15 MoAb (consistent results were obtained using two additional MoAbs, ie, M111 and M112 reagents). The above-quoted antibodies, when added to the assays at the beginning of culture, significantly inhibited the mitogen-induced proliferation of highly purified CD8+ T cells.
Effect of anti–IL-15 antibodies on accessory function of pulmonary macrophages. The figure shows data on the inhibitory activity of M110 anti–IL-15 MoAb (consistent results were obtained using two additional MoAbs, ie, M111 and M112 reagents). The above-quoted antibodies, when added to the assays at the beginning of culture, significantly inhibited the mitogen-induced proliferation of highly purified CD8+ T cells.
DISCUSSION
In this study we show that IL-15 is able to induce the proliferation of pulmonary CD45R0+/CD8+ T cells retrieved from the lung of patients with advanced HIV infection and high intensity T-cell alveolitis. Furthermore, pulmonary macrophages from these patients expressed high levels of IL-15, and anti–IL-15 antibodies inhibited their accessory function. Our findings suggest that IL-15 plays a critical role in the compartmentalization of HIV-specific CTL.
Previous studies performed on lung CD8+ T-cell subsets retrieved from the BAL of patients with AIDS established that about a third of the patients develop an HIV-related T-cell alveolitis.2 The major component of the alveolitis is caused by MHC-restricted CTL which are able to recognize and attack HIV structural proteins on the surface of infected cells, while pulmonary CD56+ CTL provided with NK-like activity contribute to the resistance against opportunistic infections.2 Inasmuch as IL-15 shares two binding subunits with IL-2 and both cytokines following the binding to IL-2R initiate the progression of the cell cycle toward T-cell replication, we addressed the issue of the potential effect of both of these cytokines on pulmonary cell-mediated responses. In this regard, recent findings have pointed to the putative role of IL-2 in the mechanisms leading to the amplification of the pulmonary inflammatory process during HIV infection.19 However, the number of lung CD4+ T cells, in theory the source of IL-2, is too low (Table 1) to assume that this cytokine might act as a local growth factor for lung CTL. According to this interpretation, the mRNA for IL-2 was not detectable in lung T cells retrieved from five of the six HIV-seropositive patients, and flow cytometry analysis showed that they never expressed cytoplasmic IL-2.
On the basis of data on the local production of IL-15, the synergistic effect of IL-15 and TNF-α, and the pronounced effect of anti–IL-15 antibodies on the accessory function of pulmonary macrophages (Figs 5, 3, and 6, respectively), we suggest that the synthesis of macrophage-derived cytokines may represent an alternative step in the activation and expansion of the pulmonary CTL pool in cases where a starvation of IL-2 occurs as a consequence of the quantitative impairment of CD4+ T cells. This hypothesis is supported by previous demonstrations that alveolar macrophages of HIV-infected individuals, acting as professional antigen-presenting cells, synthesize TNF-α and that in this disease BAL T cells express high levels of TNF-R type 2 (CD120b) and bind TNF-α.17,20 Also consistent with this interpretation are data suggesting that in mice IL-15 may be more effective than IL-2 in maintaining the lymphocyte response in different organs, including the lung.21
In this scenario, we hypothesize that the in situ release of IL-2 reaches its highest levels during the early phases of the infection when the number of lung CD4+ T cells is near the normal range; as suggested by Spain et al,19 the net result of the production of IL-2 could be the activation and expansion of the CTL pool. Macrophage-derived signals are likely to replace IL-2 in patients with advanced disease and CD4+ T-cell deficit. In particular, IL-15 is a reasonable candidate because this cytokine, like IL-2, activates CD8+ T cells (22-24 and Table 2) and co-operates with TNF-α which per se is unable to induce proliferation of BAL T cells (Fig 3). Further studies are needed to establish whether macrophage-derived IL-15 may also govern the functional activity of pulmonary cytotoxic lymphocytes, as recently shown for PB NK cells and CTL.9,23,24 Preliminary data from our lab indicate that IL-12 is actively released in the lung of patients with AIDS. Because IL-12 and IL-15 have additive effects in inducing cytolytic activity,15 we are planning to examine the effects of the interactions between these two cytokines in the pulmonary microenvironment.
This report provides the first complete analysis of the expression of IL-15R subunits by lung T cells in HIV infection. Specifically, we show that BAL CD8+ CTL from patients with AIDS and T-cell alveolitis express the p75, the p64, IL-15Rα (at the transcript level) and, to some extent, the p55 chain of the IL-2R. However, lung T cells proliferated at low levels of IL-15, suggesting that we are dealing with preactivated T cells that express a functional high-affinity receptor for IL-15. Interestingly, we showed that alveolar macrophages also bear the γc of the IL-2R and, at the transcript level, IL-15Rα. These data suggest a comment on the putative role of the γc in the pulmonary cytokine network. The γc is part of the receptor system of other cytokines, such as IL-4, IL-7, IL-9, and IL-13.25-28 We demonstrated that in the lung the IL-2R β/γ complex has signaling functions, because MoAbs toward these structures blocked the IL-2– and IL-15–mediated T-cell proliferation (Fig 4). With this as a background it is postulated that other γc -related cytokines in addition to IL-2 and IL-15 could theoretically modulate the functional activities of pulmonary cells, as recently shown for IL-13 and IL-4.29 It is expected that investigations on the interactions between the different γc -related cytokines will provide new insights into the mechanisms that regulate the pathogenesis of HIV-related pulmonary involvement.
Our study did not consider the possibility that IL-15 may have a detrimental effect on HIV spreading. A number of cytokines released by macrophages, including TNF-α, IL-2, IL-6, and granulocyte-macrophage colony-stimulating factor, have been shown to upregulate viral expression in cells chronically or latently infected with HIV. In particular, IL-2, which could theoretically be used to restore the cell-mediated response in patients with pulmonary opportunistic infections, increases HIV expression operatively preventing its therapeutic use. In contrast, the magnitudine of enhancement of HIV expression by IL-15 seems to be modest and related to the mitogenic effect of IL-2.30 Because IL-15 may act as an effective surrogate of IL-2, studies on the effect of IL-15 on HIV viral replication in the pulmonary microenvironment are warranted. In particular, a complete understanding of the synergies between IL-15, other cytokines including Th2 cytokines,30 and the HIV load31 both in the PB and at pulmonary level could help in planning therapeutic interventions with combinations of cytokines. In particular, such studies should provide the rationale to support the use of IL-15 in situations in which IL-2 production is impaired.
ACKNOWLEDGMENT
The authors thank the Biogen Co (Cambridge, MA) for supplying recombinant IL-2; Drs V. Rollinger and J. Kempemi (Knoll AG/BASF, Ludwigshafen, Germany) for providing TNF-α; Dr A. Troutt from the Immunex Co (Seattle, WA) for providing recombinant IL-15 and M110 anti–IL-15 MoAb; Dr T. Uchiyama (Kyoto, Japan) for providing anti-Tac MoAb; Dr K. Sugamura (Senday, Japan) and Dr J. Hamuro (Kawasaki, Japan) and Dr M. Tsudo (Tokyo, Japan) for providing TU27 and Mikβ1 MoAbs, respectively; Dr K. Sugamura (Senday, Japan) for providing TUGh4 MoAb; Dr S. Ferrini (National Cancer Research Institute, Genoa, Italy) for providing IL-15 primers; Dr A. Mantovani (Milan, Italy) for providing PAM-1 MoAb; Dr S. Gattoni Celli (Tufts University, Boston, MA) for providing actin probe; and Martin Donach for his help in the preparation of the manuscript.
Address reprint requests to Gianpietro Semenzato, MD, Università di Padova, Dipartimento di Medicina Clinica e Sperimentale, Via Giustiniani 2, 35128 Padova, Italy.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal