CHEMOKINES have recently become the focus of intense interest and discussion. This has occurred, in part, because of an enlarging view of what chemokines do. As recently as 2 or 3 years ago a chemokine review would have begun with a discussion of the importance of chemotactic factors in controlling leukocyte function and trafficking, and would have pointed out that specificity for leukocyte subsets is what sets chemokines apart from other chemoattractants. For example, formylated peptides (eg, f-Met-Leu-Phe), complement fragments (eg, C5a), or arachidonic acid metabolites (eg, LTB4) attract neutrophils and monocytes with equal potency, whereas some chemokines (eg, interleukin-8 [IL-8]) attract neutrophils but have no discernible effects on monocytes. This discrimination has led to the frequently espoused proposition that chemokines are involved in the pathogenesis of diseases having characteristic infiltrates.
However, we now know that chemokines and their receptors are expressed by a wide variety of nonhematopoietic cells, and that chemokine function extends far beyond leukocyte physiology. Even within the world of leukocytes, the connections among chemokines, their receptors, and human immunodeficiency virus (HIV) infection broadens the previously narrow focus on chemokines as mere chemoattractants. Furthermore, a proliferation of animal models has more precisely defined the functions of chemokines in vivo. This review will attempt to describe what is understood about chemokines in light of recent discoveries.
CHEMOKINE FAMILIES
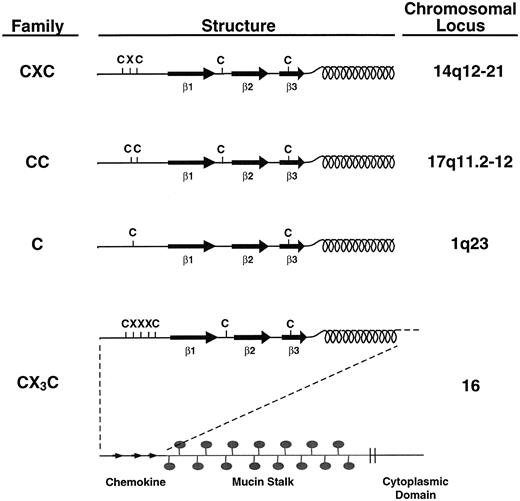
Based on genomics efforts, it has been estimated that there may be as many as 40 to 50 human chemokines. The determination that a gene encodes a chemokine depends on structural and, to a lesser extent, genetic criteria.1 Chemokines are small, with molecular weights in the range of 8 to 12 kD, but there are exceptions which involve proteins comprised of multiple domains, one of which looks like a chemokine.2 3 Chemokine domains are defined by the presence of four cysteines in highly conserved positions (Fig 1). One major chemokine subfamily is called “CXC” because the two amino acids nearest the N-termini of these proteins are separated by a single amino acid. This is in contrast to the other major subfamily which is called “CC” because these two cysteines are adjacent.
Chemokine families. Chemokines are divided into families based on structural and genetic considerations. All chemokines are structurally similar, having at least three β-pleated sheets (indicated as β1-3) and a C-terminal α-helix. Most chemokines also have at least four cysteines in conserved positions. In the CXC chemokine family, the two cysteines nearest the N-termini of family members are separated by a single (and variable) amino acid. The genes encoding these proteins cluster at human chromosome 14q12-21 (except for SDF-1α whose gene maps to chromosome 1053). In the CC chemokine family, the two cysteines nearest the N-termini of these proteins are adjacent. Their genes cluster at 17q11.2-12 (except for MIP-3β, whose gene maps to chromosome 9117 and MIP-3α/LARC which maps to chromosome 2117a). Lymphotactin is a structurally related chemokine having only one cysteine near its N-terminus and is said to belong to the C chemokine family. The CX3C chemokine (also called “fractalkine” or “neurotactin”) has a typical chemokine-like structure at its N-terminus except for the placement of three amino acids between the first two cysteines. This chemokine domain occurs at the end of a long stalk which is heavily substituted with mucin-like carbohydrates. The protein is embedded in the membrane and has a short cytoplasmic domain.
Chemokine families. Chemokines are divided into families based on structural and genetic considerations. All chemokines are structurally similar, having at least three β-pleated sheets (indicated as β1-3) and a C-terminal α-helix. Most chemokines also have at least four cysteines in conserved positions. In the CXC chemokine family, the two cysteines nearest the N-termini of family members are separated by a single (and variable) amino acid. The genes encoding these proteins cluster at human chromosome 14q12-21 (except for SDF-1α whose gene maps to chromosome 1053). In the CC chemokine family, the two cysteines nearest the N-termini of these proteins are adjacent. Their genes cluster at 17q11.2-12 (except for MIP-3β, whose gene maps to chromosome 9117 and MIP-3α/LARC which maps to chromosome 2117a). Lymphotactin is a structurally related chemokine having only one cysteine near its N-terminus and is said to belong to the C chemokine family. The CX3C chemokine (also called “fractalkine” or “neurotactin”) has a typical chemokine-like structure at its N-terminus except for the placement of three amino acids between the first two cysteines. This chemokine domain occurs at the end of a long stalk which is heavily substituted with mucin-like carbohydrates. The protein is embedded in the membrane and has a short cytoplasmic domain.
Many of the genes encoding chemokines have been mapped, and they cluster at specific loci. CC chemokine genes are grouped at 17q11.2-12 and CXC chemokine genes at 4q13 (although there are exceptions; see below). This suggests that chemokines arose by duplication and divergence from a primordial chemokine gene, with an early split into the two loci. Because mice appear to have fewer chemokines than humans (for example, there is no clear-cut murine homolog of IL-8), some of this development may have occurred relatively recently in evolutionary terms, but this has not been systematically examined. Similar clustering has been observed among genes encoding chemokine receptors.
One exception to the CC/CXC rule is lymphotactin, a potent attractant for T lymphocytes, but not monocytes.4 Although it is the right size to be a chemokine and it has several characteristic sequence signatures common to CC chemokines, it has only two cysteines. Nonetheless, these cysteines correspond to cysteines 2 and 4 of chemokines, and it has been suggested that lymphotactin belongs to a third chemokine subfamily denoted C, because of the lone cysteine in the N-terminal domain. Consistent with its new assignment, lymphotactin's gene maps to 1q. So far, lymphotactin is the only member of this putative family.
Another exception is a CX3C chemokine (also referred to as “fractalkine” or “neurotactin”) that is an integral membrane protein with a chemokine domain at its N-terminus.3 3a This domain differs from other chemokines by the presence of three amino acids intervening between the first two cysteines. Modeling studies suggest that the three-dimensional structure of chemokines (see below) can only accommodate 0, 1, or 3 amino acids between the first two cysteines, explaining the absence of a CX2C chemokine. Like lymphotactin, the gene encoding CX3C chemokine maps to a locus different from other chemokines, and so far it is the sole example of a chemokine with this structural motif.
CXC CHEMOKINES
Discussions of chemokine function tend to look like a collection of disconnected observations. It is extremely difficult to divine rules, particularly because the dose responses for given effects in vitro may not be relevant in vivo. For example, the fact that the ED50 for MCP-1's ability to attract monocytes is 10-fold lower than MCP-2's may imply that MCP-1 is a more potent chemoattractant.5 However, a 10-fold increase in MCP-2 expression in vivo would make it physiologically equipotent with MCP-1. Therefore, the following discussion merely catalogs chemokine properties without necessarily implying their physiologic importance in vivo.
ELR chemokines.The prototypic CXC chemokine is IL-8, which was purified by several groups as a monocyte-derived factor that attracts neutrophils, but not monocytes, in Boyden chamber assays.6-8 Several other CXC chemokines are also potent neutrophil chemoattractants (see Table 1), and structure/activity analyses show that this property depends on the presence of a three–amino acid motif, ELR (glutamate-leucine-arginine), between the N-terminus and the first cysteine.9,10 However, these amino acids must appear in positions close to the proteins' N-termini because platelet basic protein (PBP) and two of its N-terminally truncated derivatives, CTAPIII and β-thromboglobulin, have very weak neutrophil chemoattractant activity despite the presence of ELR. Only NAP-2, a further truncated product in which ELR appears close to the N-terminus, is an active PBP-derived neutrophil attractant.11
CXC Chemokines
| Name . | Target Cells . |
|---|---|
| ELR | |
| IL-8 | Neutrophils, T lymphocytes, basophils, angiogenesis (?endothelial cells) |
| GRO-α(MGSA) | Neutrophils, melanoma cells, (?endothelial cells) |
| GRO-β(MIP-2α) | Neutrophils, (?endothelial cells) |
| GRO-γ(MIP-2β) | Neutrophils, (?endothelial cells) |
| ENA-78 | Neutrophils |
| GCP-2 | Neutrophils |
| Platelet basic protein | |
| CTAP-III | Fibroblasts |
| β-Thromboglobulin | Fibroblasts |
| NAP-2 | Neutrophils, basophils |
| non-ELR | |
| Platelet factor 4 | Fibroblasts, endothelial cells |
| IP-10 | Activated T lymphocytes, TIL, ?endothelial cells, ?NK cells |
| MIG | Activated T lymphocytes, TIL |
| SDF-1α | T lymphocytes, CD34+ progenitors, ?B lymphocytes |
| SDF-1β | ? |
| Name . | Target Cells . |
|---|---|
| ELR | |
| IL-8 | Neutrophils, T lymphocytes, basophils, angiogenesis (?endothelial cells) |
| GRO-α(MGSA) | Neutrophils, melanoma cells, (?endothelial cells) |
| GRO-β(MIP-2α) | Neutrophils, (?endothelial cells) |
| GRO-γ(MIP-2β) | Neutrophils, (?endothelial cells) |
| ENA-78 | Neutrophils |
| GCP-2 | Neutrophils |
| Platelet basic protein | |
| CTAP-III | Fibroblasts |
| β-Thromboglobulin | Fibroblasts |
| NAP-2 | Neutrophils, basophils |
| non-ELR | |
| Platelet factor 4 | Fibroblasts, endothelial cells |
| IP-10 | Activated T lymphocytes, TIL, ?endothelial cells, ?NK cells |
| MIG | Activated T lymphocytes, TIL |
| SDF-1α | T lymphocytes, CD34+ progenitors, ?B lymphocytes |
| SDF-1β | ? |
See text for references.
IL-8 is produced by a variety of cell types including monocytes, T lymphocytes, neutrophils, fibroblasts, endothelial cells, and epithelial cells. Although subject to variable processing at the N-terminus, the most abundant form of naturally occurring IL-8 is 72 amino acids long.12,13 A 77–amino acid variant, occasionally called endothelial IL-8 because of its synthesis by these cells, is extended at the N-terminus. The longer protein is ≈10-fold less potent than the shorter protein in attracting and activating neutrophils in vitro, but has similar potency in vivo, perhaps due to proteolytic processing to the short form. The 77–amino acid form may be involved in neutrophil adherence to the endothelium as a prelude to diapedesis.14 (The long form has also been observed to inhibit neutrophil adhesion to activated endothelial cells, but this may be a vagary of the assay system.15 ) Other properties attributed to IL-8 include chemoattraction of T lymphocytes16 (but not in transendothelial assays17 ) and angiogenic activity (see below). The former is a direct effect because IL-8 receptors have been documented on CD8+, CD26− T cells,18 while the latter is controversial because of the reported absence of IL-8 receptors on endothelial cells.19 Finally, IL-8 stimulates histamine release from basophils.20 21
GRO-α was so named because of its initial description as the product of a gene differentially expressed in transformed hamster cells that had suffered loss of growth control.22 Independently, the murine homolog had been cloned in a differential screening experiment as the platelet-derived growth factor (PDGF)-inducible KC gene, and the human protein was purified as MGSA, or melanoma growth stimulatory activity, because of its mitogenic effects on melanoma cell lines.23-25 However, GRO-α was also functionally identified as a neutrophil-specific chemoattractant secreted by activated mononuclear cells along with IL-8 and having similar potency.26,27 Thus, GRO-α finds a comfortable place within the chemokine family as a neutrophil chemoattractant, but it is instructive to note that its influences have always extended beyond leukocytes. GRO-β and GRO-γ are closely related proteins that are also potent neutrophil attractants.28 (The alternate designations, MIP-2α and MIP-2β, derive from their purification as neutrophil chemoattractants by the same investigators who isolated the CC chemokines, MIP-1α and MIP-1β.29 30 )
ENA78 is an ELR-containing CXC chemokine isolated from A549 cells, which are derived from type II pneumocytes that also secrete IL-8 and GRO-γ.31 Its sequence places it closer to the GRO proteins than IL-8, and like these chemokines it specifically attracts neutrophils. Similarly, GCP-2 was purified from the conditioned medium of MG63 osteosarcoma cells along with IL-8, GRO-α, GRO-γ, and IP-10.32 It is a neutrophil-specific chemoattractant and activator, but has a specific activity ≈5- to 10-fold lower than IL-8. Like IL-8, GCP-2 attracts neutrophils when injected into rabbit skin.
As mentioned above, PBP and its processed products CTAP-III and β-TG have ELR sequences but are poor neutrophil chemoattractants because of their extended N-terminal sequences. Removal of nine N-terminal amino acids from PBP33 produces CTAP-III which stimulates glycosaminoglycan production by connective tissue cells and is a very weak mitogen for fibroblasts (ED50 ≈ 100 nmol/L).34,35 Proteolytic removal of another four N-terminal amino acids produces β-TG, which is a chemoattractant for fibroblasts.36 Cleaving another 11 N-terminal residues results in NAP-2,37 whose potency as a neutrophil chemoattractant and activator has been estimated to be 2-fold11 to 100-fold38 less than that of IL-8.
Non-ELR CXC chemokines.The ELR-containing chemokines have an apparent uniformity of function which makes it easy to think of them as a family of neutrophil chemoattractants and activators (although this raises questions of redundancy). In contrast, descriptions of non-ELR CXC chemokines are a hodgepodge of disparate activities with as yet no clear underlying theme.
For example, long before the chemokine family was recognized or named, platelet factor 4 (PF4) was the first member to purified. It is found in platelet α-granules along with PBP and its processed products, but unlike those proteins it has no ELR motif and is an extremely weak attractant for neutrophils.39 It attracts fibroblasts in vitro but is ≈30-fold less potent than β-TG.36 However, one of its most interesting properties is its inhibition of angiogenesis, which appears to occur via direct inhibition of endothelial cell proliferation.40 Although PF4's ED50 for this effect is quite high, ≈250 nmol/L, a cleavage product is 30 to 50 times more potent (see below).41
Another non-ELR CXC chemokine with antiangiogenic properties is IP-10, the product of an interferon-γ (IFN-γ)-inducible gene cloned from U937 cells.42 A variety of cell types express IP-10 in vitro including mononuclear cells, keratinocytes, fibroblasts, endothelial cells, and T lymphocytes.42 In the mouse, IFN-γ administration induces high levels of IP-10 expression in liver and kidney with lower levels in the spleen.43 Similar to other non-ELR chemokines, IP-10 is a poor neutrophil chemoattractant and activator.44 Some groups have ascribed T-lymphocyte chemoattractant properties to the protein both in vitro in Boyden chamber assays, and in vivo in severe combined immunodeficient (SCID) mice reconstituted with human peripheral blood lymphocytes (PBLs).45,46 However, others detect no such T-lymphocyte–directed activity in transendothelial models of T-cell migration.17
MIG is another IFN-γ–inducible protein isolated from macrophages. It has chemoattractant activity in vitro for tumor-infiltrating lymphocytes (TIL), and for PBLs activated by syngeneic monocytes and phytohemogglutinin (PHA).47 IP-10 also attracts TIL, and MIG and IP-10 cross-desensitize in other measures of receptor activation, suggesting that on TIL they share the same receptor.47 This receptor, CXCR3, has recently been cloned and binds IP-10 and MIG selectively in vitro.48
Finally, the genes encoding SDF-1α and SDF-1β were cloned from mouse bone marrow (BM) stromal cells (hence their designations as “stromal-derived factors”) using a signal sequence trap technique.49 Human SDF-1α is a low-potency, high-efficacy chemoattractant for T lymphocytes in vitro.50 However, perhaps relevant to its provenance, SDF-1 is also a potent chemoattractant for CD34+ hematopoietic progenitors.51 In vivo, targeted gene disruption of murine SDF-1α indicates that it is required for normal B-lymphocyte development and, surprisingly, for normal cardiac organogenesis because SDF-1α–deficient mice have nonfatal ventricular septal defects.52 Although it is a CXC chemokine, SDF-1's gene is exceptional in its location on human chromosome 10 rather than 4.53 Notably, a receptor for SDF-1α is CXCR4, and SDF-1α prevents infection of CD4/CXCR4-expressing cells by T-lymphocyte–tropic, syncytium-inducing strains of HIV-1.50 54
CC CHEMOKINES
CC chemokines are also functionally diverse. Like the CXC chemokines, their names more often reflect historical accidents of their cloning or isolation than their functions (Table 2).
CC Chemokines
| Name . | Target Cell . |
|---|---|
| MCP-1 | Monocytes, memory T lymphocytes, basophils, NK cells, hematopoietic progenitors, ?dendritic cells |
| MCP-2 | Monocytes, memory and naive T lymphocytes, eosinophils, basophils, NK cells |
| MCP-3 | Monocytes, Memory T lymphocytes, eosinophils, basophils, NK cells, dendritic cells |
| MCP-4 | Monocytes, T lymphocytes, eosinophils |
| MCP-5 (mouse only) | Monocytes, T lymphocytes, eosinophils |
| MIP-1α | Monocytes, T lymphocytes, NK cells, basophils, eosino-phils, dendritic cells, hematopoietic progenitors |
| MIP-1β | Monocytes, T lymphocytes, dendritic cells, NK cells, hematopoietic progenitors |
| MIP-1γ (mouse only) | Resting and activated T lymphocytes |
| RANTES | Memory T lymphocytes, eosinophils, basophils, NK cells, dendritic cells |
| Eotaxin | Eosinophils |
| I309 | Monocytes |
| HCC-1 | Monocytes, hematopoietic progenitors |
| TARC | T lymphocytes |
| C10 (mouse only) | ? |
| CCF18 (mouse only) | T lymphocytes, hematopoietic progenitors |
| MIP-3α/LARC | ? |
| MIP-3β | ? |
| Name . | Target Cell . |
|---|---|
| MCP-1 | Monocytes, memory T lymphocytes, basophils, NK cells, hematopoietic progenitors, ?dendritic cells |
| MCP-2 | Monocytes, memory and naive T lymphocytes, eosinophils, basophils, NK cells |
| MCP-3 | Monocytes, Memory T lymphocytes, eosinophils, basophils, NK cells, dendritic cells |
| MCP-4 | Monocytes, T lymphocytes, eosinophils |
| MCP-5 (mouse only) | Monocytes, T lymphocytes, eosinophils |
| MIP-1α | Monocytes, T lymphocytes, NK cells, basophils, eosino-phils, dendritic cells, hematopoietic progenitors |
| MIP-1β | Monocytes, T lymphocytes, dendritic cells, NK cells, hematopoietic progenitors |
| MIP-1γ (mouse only) | Resting and activated T lymphocytes |
| RANTES | Memory T lymphocytes, eosinophils, basophils, NK cells, dendritic cells |
| Eotaxin | Eosinophils |
| I309 | Monocytes |
| HCC-1 | Monocytes, hematopoietic progenitors |
| TARC | T lymphocytes |
| C10 (mouse only) | ? |
| CCF18 (mouse only) | T lymphocytes, hematopoietic progenitors |
| MIP-3α/LARC | ? |
| MIP-3β | ? |
See text for references.
MCP-1 (monocyte chemoattractant protein-1) was first purified from conditioned medium of baboon aortic smooth muscle cells in culture on the basis of its ability to attract monocytes, but not neutrophils, in vitro.55 The human version of MCP-1 (also called MCAF) was isolated soon thereafter from tumor cell lines.56,57 Its amino acid sequence turned out to be identical to the predicted amino acid sequence of the product of the human JE gene, a homolog of a PDGF-inducible gene cloned from murine 3T3 cells along with KC several years earlier.23,58 59
MCP-1 is a potent chemoattractant for monocytes in vitro, with an ED50 similar to IL-8's for neutrophils (≈500 pmol/L). As part of its migration program in monocytes, MCP-1 induces the expression of integrins required for chemotaxis.60,61 In transendothelial migration assays in vitro, MCP-1 is a similarly potent attractant for activated CD4 and CD8 memory T lymphocytes (CD45RA−/CD45RO+/CD29+/L-selectin and CD26+).62 In similar assays, MCP-1 attracts neither B lymphocytes nor natural killer (NK) cells.62 In contrast, in assays that do not involve endothelial cells, MCP-1 has been reported to attract NK cells63,64 as well as T lymphocytes.65 MCP-1 also induces granule release from NK cells and CD8+ T cells,63 and activates NK function in CD56+ cells.66 Finally, MCP-1 is a potent histamine-releasing factor for basophils,67-69 but does not attract or activate eosinophils.
MCP-2 and MCP-3 were isolated as novel monocyte chemoattractants from the conditioned medium of MG-63 osteosarcoma cells70 (although the cDNA encoding MCP-2 had been cloned earlier in a differential screen of IFN-γ–treated monocytes71 and murine MCP-3 had been cloned in another differential screen as FIC72). MCP-1 has generally been observed to be a slightly more potent and efficacious monocyte chemoattractant than MCP-2 or MCP-3.5,70,73,74 Like MCP-1, these chemokines attract CD4+ and CD8+ cells that are CD45RO+ in transendothelial assays, although MCP-2 appears to be unique in its ability to attract significant numbers of naive CD45RA+ cells.17 Again, there is some debate about MCP-2 and MCP-3 attracting NK cells with negative results in transendothelial assays,17 and positive results with acellular membrane assays.63,64,74 Like MCP-1, these chemokines activate basophils,75,76 but in contrast to MCP-1, MCP-2 and MCP-3 attract and activate eosinophils.75,77,78 MCP-3 also attracts dendritic cells.79
The newest members of this family are MCP-480 and MCP-5.81 MCP-4 shares closest amino acid similarity with MCP-3 and eotaxin, and like those proteins it attracts monocytes, T lymphocytes, and eosinophils. On the latter cell type, it completely cross-desensitizes with eotaxin, suggesting that they share the same receptor (CCR3). In terms of relative chemoattractant potency, MCP-4 is equipotent with eotaxin in attracting eosinophils, but less potent than MCP-1 in attracting monocytes or T cells.80 (However, it should be noted that MCP-4's ED50 for all three cell types is the same, ≈10-fold higher than MCP-1's ED50 for monocytes or T cells). MCP-5 has so far only been identified in the mouse, but it attracts monocytes, eosinophils, and T lymphocytes, and activates human and murine CCR2.81,82 It is expressed abundantly in the lung during allergic inflammation and neutralizing antibodies reduce the number of infiltrating eosinophils in these models.82
MIP-1α and MIP-1β were purified from lipopolysaccharide (LPS)-treated monocytic cell lines,83,84 hence their designations as “macrophage inflammatory proteins.” Some confusion has surrounded the activities of these proteins. Initial descriptions of neutrophil chemoattraction were based on using supraphysiological doses of chemokine.83 The current consensus is that MIP-1α attracts and activates monocytes more efficiently than MIP-1β, but less so than MCP-1.5,17,85 The MIP-1 proteins are also much less efficient than MCP-1 in promoting exocytosis by monocytes.5 MIP-1α may be a more important neutrophil attractant in mice.
In assays that do not involve endothelial cells, MIP-1α attracts predominantly CD8+ T cells while MIP-1β attracts CD4+ cells, although there is some overlap between subsets in response to both chemokines.86,87 In transendothelial assays, both MIP-1s attract CD4+ cells better than CD8+ cells, and MIP-1α is more potent and effective than MIP-1β for both subsets.17 MIP-1α also has the interesting property of being an inhibitor of hematopoietic stem cell proliferation (see below).88
Other cellular targets for MIP-1α include dendritic cells,79 NK cells,63,89 basophils (weakly),75,90,91 and eosinophils.75,92 MIP-1β has no activity on dendritic cells,79 and minimal activity on NK cells.89 Both MIP-1α and MIP-1β activate NK function in CD56+ cells.66
The cDNA encoding RANTES (regulated upon activation, normal Texpressed and secreted) was isolated in a T- versus B-lymphocyte differential screen, and found to be inducible by mitogens or antigen in a variety of T-cell lines and circulating lymphocytes.93 In vitro, RANTES is nearly as potent a chemoattractant for monocytes as MCP-1 but is much less effective in stimulating exocytosis.5,94 In endothelial cell–free assays, RANTES attracts CD4+, CD45R0+ T lymphocytes,94 but in transendothelial systems it attracts CD8+ cells as well as CD4+, and is the most potent CC chemokine for CD8+ chemoattraction.17 It also attracts and activates NK cells.66,89 RANTES is an important chemoattractant for eosinophils, which can also secrete it.92,95 Basophils are a RANTES target and, like MCP-1, RANTES induces histamine release.96
Eotaxin was first isolated from bronchoalveolar lavage fluid in guinea pigs induced to respond to aerosol antigen challenge with an eosinophil-rich pulmonary infiltrate.97 The purified protein was shown to be unique among guinea pig chemokines in its ability to attract eosinophils specifically when injected intradermally. It has been suggested that eotaxin works in concert with IL-5 to elicit eosinophil infiltration: IL-5 stimulates eosinophil release from the BM, and eotaxin directs intravascular eosinophils to their local destination.98 Interestingly, in guinea pigs99 and mice,100,101 eotaxin is expressed at significant constitutive levels in several organs (including lung and heart) and its expression can be further induced upon antigen challenge. Human eotaxin attracts eosinophils when injected in primate skin and appears to use some of the same receptors on eosinophils as MCP-3 and RANTES.102 103
A cDNA encoding I309 was isolated from a γδ T-cell line using subtractive hybridization from a B-lymphoblastoid line.104 Although not expressed in freshly isolated peripheral blood mononuclear cells (PBMCs), its expression is induced in αβ T-lymphocyte lines by PHA or anti-CD3. It is the likely human homolog of TCA3, the cDNA of which was identified by subtractive hybridization of murine activated T-cell clones versus B cells.105 Both chemokines are potent monocyte chemoattractants,106,107 although TCA3 has also been reported to attract neutrophils.107 Unlike many of the other CC chemokines that attract monocytes and T cells, I309 has no effects on T or NK cells63,106 and is not a histamine-releasing factor.108
HCC-1 was isolated from the plasma of patients with chronic renal failure.109 Like SDF-1α, HCC-1 is expressed constitutively in a wide variety of tissues and in normal plasma. It does not attract any leukocyte subtype, but it does induce calcium flux and enzyme release specifically from monocytes. Its structure is distantly related to that of MIP-1α and it stimulates the proliferation of CD34+ cells, but less potently than MIP-1α (see below). (This chemokine is not to be confused with an antibody called HCC-1, which identifies a subset of CD34+ cells.110 )
Like SDF-1, TARC was isolated from PHA-stimulated PBMCs by a signal sequence trap strategy.111 The acronym stands for “thymus and activation-regulated chemokine. It is constitutively expressed in thymus and in vitro it binds and attracts T lymphocytes exclusively.
Several CC chemokines in addition to MCP-5 have so far been described only in the mouse. MIP-1γ was isolated from a murine macrophage cell line and found to have structural similarities to C10 (another CC chemokine of unknown function112 ), MIP-1α, and MIP-1β.113 It has been shown to be expressed by Langerhans cells and, perhaps relevantly, it attracts both resting and activated CD4 and CD8 cells in vitro, suggesting a possible role in immune cell recruitment.114 CCF18 is another murine CC chemokine closely related to C10 which is able to attract CD4 T-cell clones, and is expressed constitutively in several myeloid cell lines (especially P388D1 and 32D).115 It has been independently isolated as MIP-Related Protein-2 and shown to have myeloid stem cell inhibitory activity similar to MIP-1α.116
Although some of these proteins have only been defined in the mouse, the question of whether their human homologs exist and have already been defined is not straightforward. This is a relevant concern because of the use of gene targeting in the mouse to infer chemokine function. Because chemokines are so closely related, simple assessments of homology by proportion of shared sequence may not be reliable. Similarly, because chemokine genes cluster, relatedness cannot be inferred by mapping. Instead, it may turn out that the issue of precise gene-to-gene interspecies homology may not be relevant. Rather, functional homology may be the major criterion for assigning a murine chemokine a role in physiology analogous to a human chemokine.
Because chemokines are so closely related in primary structure, there is great potential for using bioinformatics to isolate new chemokines. This approach, which has been pioneered in the chemokine field by the group at DNAX (Palo Alto, CA), uses computer-based homology searches to identify expressed sequence tags (ESTs) having significant homology to known chemokines. Novel ESTs are then used to search for other ESTs derived from the same cDNA, and when the process is complete, novel chemokine cDNA's may be identified. The CC chemokines MIP-3α and MIP-3β were recently identified this way.117 (MIP-3α was also cloned as LARC.117a ) Although their functions have not yet been described, MIP-3α is expressed in thymus, appendix, PBLs, and fetal liver, while MIP-3β is expressed only in thymus, lymph node, and appendix. Interestingly, the expression of both chemokines is suppressed by IL-10. The gene encoding MIP-3β maps to chromosome 9 and the gene encoding MIP-3α/LARC maps to chromosome 2, making them the first CC chemokine genes identified outside of the gene cluster at 17q11.2-12.
Genomic informatics was also used to identify the CX3C chemokine mentioned above.3,3a This is a membrane-bound protein with multiple structural domains: a chemokine portion at the N-terminus is followed by a long stretch of serine- and threonine-rich repeats that are heavily substituted with mucinlike polysaccharides; a transmembrane region then appears followed by a short cytoplasmic domain (see Fig 1). It appears that the protein may be cleaved physiologically near its membrane insertion, and that the free molecule attracts T lymphocytes and monocytes. Furthermore, the cell surface form of the CX3C chemokine enhances T-lymphocyte and monocyte adhesion to an expressing cell. The function of the mucin stalk is unclear but its structure is highly reminiscent of the C-terminal extension of murine MCP-1 which is also heavily substituted with sialylated carbohydrate.2
STRUCTURAL CONSIDERATIONS
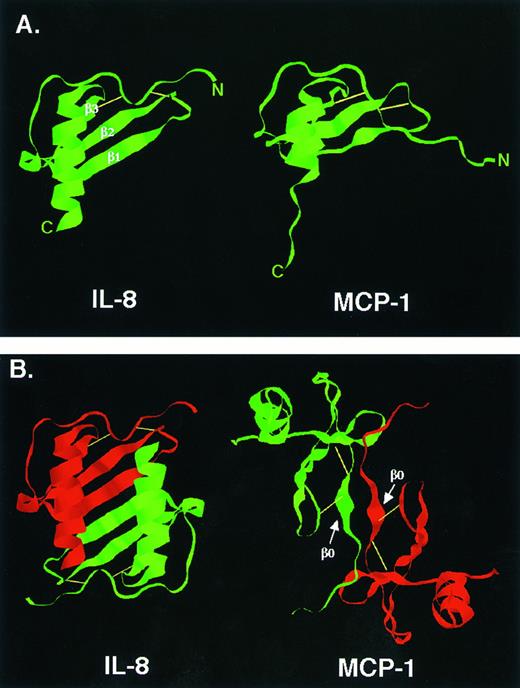
Three-dimensional structure.Because chemokine amino acid sequences are so similar, it is not surprising that their secondary and tertiary structures are also similar (see Fig 1). Several chemokine structures have been solved and all share the same basic features (Fig 2A).118-121 A relatively disordered N-terminus is anchored to the rest of the molecule by disulfide bonds involving the two N-terminal domain cysteines. This is followed by an extended loop that leads into three antiparallel β-pleated sheets (a so-called Greek key) which provide a flat base over which the C-terminal α helix extends.
Chemokine structure. (A) Three-dimensional structures of IL-8 and MCP-1 monomers. The three β-pleated sheets of IL-8 are easier to appreciate than those of MCP-1 and are indicated. (B) Three-dimensional structures of IL-8 and MCP-1 dimers. The profound difference in dimer structure is indicated. For MCP-1 and other CC chemokines, dimerization near the N-terminus involves a short, so-called β0 sheet which is indicated.119,120 Reprinted with permission from Rollins BJ: Monocyte chemoattractant protein 1: A potential regulator of monocyte recruitment in inflammatory disease. Molecular Medicine Today, vol 2, p 198, 1996.266
Chemokine structure. (A) Three-dimensional structures of IL-8 and MCP-1 monomers. The three β-pleated sheets of IL-8 are easier to appreciate than those of MCP-1 and are indicated. (B) Three-dimensional structures of IL-8 and MCP-1 dimers. The profound difference in dimer structure is indicated. For MCP-1 and other CC chemokines, dimerization near the N-terminus involves a short, so-called β0 sheet which is indicated.119,120 Reprinted with permission from Rollins BJ: Monocyte chemoattractant protein 1: A potential regulator of monocyte recruitment in inflammatory disease. Molecular Medicine Today, vol 2, p 198, 1996.266
So far, all structural analyses show that chemokines are multimers under conditions required for crystallization or NMR study. All are dimers except for PF4, which is a tetramer.122 However, the structures of the dimers differ profoundly depending on whether they are comprised of CXC or CC chemokines (Fig 2B). In the case of CXC chemokines, such as IL-8, the dimer interface occurs by solvent exclusion along the edge of the first β sheet.118 This creates an extended plane over which the two α helices from each subunit are arrayed in parallel, creating a structure reminiscent of the peptide binding groove of major histocompatibility complex (MHC) molecules. In the case of CC chemokines, such as MCP-1, the dimer interface forms primarily by interactions between short β sheets near the N-termini of the monomers.119-121 This creates an extended molecule that is more cylindrical than the compact CXC dimers.
What is the significance of the different shapes of chemokine dimers? To approach that question, one must first ask whether or not chemokine dimerization itself has any functional significance. Several observations militate against the importance of dimerization. First, physical measurements of monomer affinities by some investigators suggest that the dissociation constant (Kd) for dimer dissociation is several logs higher than the Kd for receptor binding or the ED50 for biological activity.123,124 This would indicate that chemokines are monomers at physiologically relevant concentrations. Furthermore, IL-8 variants that are incapable of forming dimers nonetheless have full biological activity on neutrophils.125 (Although this does not exclude the possibility that two nondimerizing monomers might bind to the same receptor to activate it.)
In contrast, others have used coprecipitation and crosslinking techniques to derive Kds for dimer dissociation that are in line with ED50's.126 In addition, N-terminal deletion variants of chemokines are potent inhibitors of the parent molecules from which they are derived.126-128 In some cases (eg, MCP-1) it has been shown that these variants do not bind efficiently to MCP-1 receptors, suggesting that they are not acting as competitive inhibitors. Instead, they form heterodimers with wild-type MCP-1, raising the possibility that they act as dominant negative inhibitors.126 Dimer-mediated activation of chemokine receptors would be consistent with this model, but not a necessary prerequisite.
Because most chemokines bind to cell-surface or connective-tissue components such as glycosaminoglycans, it may be that dimerization is favored when chemokines associate with these molecules.129 These interactions may make chemokine dimerization a critical process in vivo.
The role of dimerization remains controversial, but it is an important question. If it could be shown that dimer formation were an essential component of chemokine receptor activation, then the dimer interface would be a superb target for antichemokine drugs. This is an attractive notion because the dimerization surface may present a smaller target than the chemokine:receptor interface.
An interesting argument in favor of dimerization is the recent solution of the structure of macrophage migration inhibitory factor (MIF).130 This T-lymphocyte product was first isolated on the basis of its ability to inhibit macrophage migration, making it a cytokine with properties analogous, albeit inverse, to those of chemokines.131 132 The MIF monomer consists of four antiparallel β sheets over which two α helices are arrayed in parallel, creating a structure that looks very much like a CXC chemokine dimer. Because MIF has little amino acid sequence similarity with chemokines, this raises the intriguing possibility that cytokines related to cell migration have undergone convergent evolution toward a three-dimensional structure optimized for this type of interaction. This would support the notion that chemokines form dimers to recapitulate this structure.
Structure/activity relationships.Several lines of evidence point to the importance of the N-terminal domain in the function of CXC and CC chemokines. As mentioned earlier, the presence of the tripeptide ELR sequence near the N-terminus is essential for the activity of CXC chemokines that activate the receptors, CXCR1 or CXCR2.9,10 A further demonstration of the importance of this motif is that addition of ELR to the N-terminal domain of PF4 transforms it into a neutrophil chemoattractant that binds to IL-8 receptors.133 However, the context in which ELR is placed must be important because ELR-modified IP-10 remains inactive toward neutrophils. This suggests that other regions of CXC chemokines are also important for receptor activation. In fact, it has recently been shown that there is a hydrophobic pocket in IL-8 involving the loop beyond the N-terminal cysteines that is essential for binding to one of the IL-8 receptors (CXCR1).134-136
Among CC chemokines, there is no amino acid sequence motif analogous to ELR that is associated with biologic effects on monocytes or T cells. Nonetheless, N-terminal regions of CC chemokines are clearly important for their activity as demonstrated by deletion analysis and single amino acid substitutions.127,137 Similar to CXC chemokines, there are also data implicating the loop structure between the N-terminal cysteines and the first β sheet.137
The C-terminal α helices have also been shown to be important for imparting maximal biologic potency to chemokines. Part of this effect may be due to interactions between the α-helices and glycosaminoglycans. For example, IL-8's potency and efficacy are enhanced in the presence of heparan sulfate.138
CHEMOKINE RECEPTORS
One of the confounding problems in understanding chemokine physiology is the fact that chemokine receptors bind several different chemokines, and chemokines bind several different receptors. This so-called promiscuity had been apparent through cross-desensitization experiments even before chemokine receptors were cloned. For example, treating a neutrophil with IL-8 produces a calcium flux and prevents a subsequent calcium flux in response to GRO-α, whereas treatment with GRO-α first does not prevent an IL-8–induced flux. This suggested the presence of two IL-8 receptors, one of which recognizes GRO-α as well as IL-8.27 139 The literature is replete with similar experiments involving a variety of chemokines and cell types. Although the information is critical for new receptor discovery, it can be confusing. The following discussion is therefore limited to data on interactions between chemokines and cloned receptors, with the understanding that there are more receptors waiting to be cloned.
So far, all chemokine receptors are members of the 7-transmembrane spanning (7-TMS), G-protein–coupled receptor family. Although similar to many other 7-TMS receptors, chemokine receptors have some unique structural signatures such as the amino acid sequence DRYLAIV in the second intracellular loop domain.140,141 These receptors are, for the most part, coupled to Gαi proteins, making cellular responses to chemokines inhibitable by pertussis toxin. As expected, receptor activation inhibits cyclic adenosine monophosphate (cAMP) production, but other signal transduction pathways are clearly involved as well. For example, chemoattractant responses to RANTES and MCP-1 can be inhibited by wortmannin, implicating PI3 kinase activation, and by inhibitors of MAPK activation.142-144 The precise mechanisms of coupling receptor activation to complex physiological responses such as chemotaxis are still being investigated.
Receptor-ligand specificities are summarized in Table 3. Four CXC chemokine receptors have been cloned and, as implied earlier, there are two receptors that bind IL-8 with high affinities (Kd < 5 nmol/L).145,146 All of the ELR-containing CXC chemokines bind to one receptor or the other. CXCR1 (IL-8RA) binds only IL-8, whereas CXCR2 (IL-8RB) binds IL-8, GRO-α, GRO-β, GRO-γ, NAP-2, and ENA-78.139,147-151 Much of the ligand specificity resides in the N-terminal domain of these two receptors.149 152
Chemokine Receptors
| Receptor . | Ligands . |
|---|---|
| CXC Receptors | |
| CXCR1 | IL-8 |
| CXCR2 | IL-8, GRO-α, GRO-β, GRO-γ, NAP-2, ENA-78 |
| CXCR3 | IP-10, MIG |
| CXCR4 | SDF-1α |
| CC Receptors | |
| CCR1 | MIP-1α, RANTES, MCP-3 |
| CCR2 | MCP-1, MCP-3, MCP-5 |
| CCR3 | Eoxtaxin, RANTES, MCP-2, MCP-3, ?MCP-4 |
| CCR4 | MIP-1α, RANTES, MCP-1, TARC |
| CCR5 | MIP-1α, MIP-1β, RANTES |
| CCR6 | MIP-3α/LARC |
| CCR7 | MIP-3β/ELC |
| Receptor . | Ligands . |
|---|---|
| CXC Receptors | |
| CXCR1 | IL-8 |
| CXCR2 | IL-8, GRO-α, GRO-β, GRO-γ, NAP-2, ENA-78 |
| CXCR3 | IP-10, MIG |
| CXCR4 | SDF-1α |
| CC Receptors | |
| CCR1 | MIP-1α, RANTES, MCP-3 |
| CCR2 | MCP-1, MCP-3, MCP-5 |
| CCR3 | Eoxtaxin, RANTES, MCP-2, MCP-3, ?MCP-4 |
| CCR4 | MIP-1α, RANTES, MCP-1, TARC |
| CCR5 | MIP-1α, MIP-1β, RANTES |
| CCR6 | MIP-3α/LARC |
| CCR7 | MIP-3β/ELC |
Ligand assignments are based on in vitro binding assays showing Kd < 10 nmol/L. See text and “Note Added in Proof” for references.
So far, only two receptors have been cloned that bind non–ELR-containing CXC chemokines. IP-10's receptor had originally been identified as a heparan sulfate–containing proteoglycan,153 but it is now clear that it also has a specific heptahelical G-protein–coupled receptor called CXCR3 which also binds MIG.48 A receptor for SDF-1α was first cloned as LESTR, a so-called orphan chemokine receptor isolated by homology cloning based on motifs conserved in this receptor family.154 LESTR was independently cloned as fusin, the coreceptor with CD4 for laboratory-adapted, syncytium-inducing strains of HIV-1.155 Subsequent work identified SDF-1α as a ligand for LESTR/fusin, and the receptor was formally named CXCR4.50 54
The CC chemokine receptors are much more confusing because they all display overlapping specificities. Most ligands also have overlapping specificities except (so far) for eotaxin, which binds only to CCR3, and MIP-1β, which binds only to CCR5. CCR1 binds MIP-1α, RANTES, and MCP-3 with high affinities.156-158 CCR2 occurs in two forms that are the results of alternative splicing.159 CCR2A and CCR2B differ only in their C-terminal intracellular tails, hence their ligand binding specificities defined to date (MCP-1, MCP-3, and MCP-5) are identical.81,158,160 Although the mRNAs for both receptors are expressed at nearly equivalent levels in monocytic cells, the C-terminal tail of CCR2B is rich in serines and threonines (expected phosphorylation sites in G-protein–coupled receptors) and is homologous to the C-terminal tail of CCR1.159
CCR3 was cloned as a highly expressed receptor from eosinophils.102,103,161,162 Gratifyingly, this receptor binds the eosinophil chemoattractants, eotaxin, RANTES, and MCP-3, as well as MCP-2. (Eosinophils also express CCR1 and this may account for whatever effects MIP-1α has on eosinophil trafficking.162 ) Because MCP-4 cross-desensitizes eotaxin on eosinophils, CCR3 may also bind MCP-4.80 In an analogous manner, CCR4 was cloned from basophils, and its ligands include the major histamine-releasing CC chemokines, MIP-1α, RANTES, and MCP-1163 as well as TARC.163a Homology cloning resulted in the isolation of a cDNA encoding CCR5.164-166 Although it shares significant primary sequence similarity with CCR2, CCR5 binds MIP-1α, MIP-1β, and RANTES with high affinity.
In the discussion so far, it is apparent that chemokine/ligand promiscuity does not cross CC versus CXC boundaries. However, there is an exception to this rule in the Duffy RBC antigen, which is a heptahelical membrane protein that binds chemokines (as well as Plasmodium vivax).167,168 Because of this property, it is also known as DARC, the Duffy antigen receptor for chemokines. DARC binds several ELR-containing CXC and CC chemokines with high affinity.169 DARC is expressed on postcapillary endothelial cells even in Duffy-negative individuals whose RBCs selectively downregulate expression.170,171 Because DARC has not yet been shown to signal upon binding its ligands, it has been suggested that DARC may act as a chemokine sink, or as a way to present chemokines to circulating or diapedesing leukocytes. The significance of this mechanism is obscure, however, given the existence of an otherwise healthy individual carrying a truncated version of DARC.172 (Duffy is also a receptor for P vivax, and malarial invasion can be inhibited by some chemokine variants.173 )
There are several intriguing examples of DNA viruses that encode chemokine receptorlike molecules. Herpesvirus saimiri's ECRF3 predicts a heptahelical membrane protein with sequence similarity to CXCR1 and CXCR2. Expression of the recombinant protein in frog oocytes shows that it can bind and signal in response to IL-8, GRO-α, and NAP-2, making it a CXCR2-like molecule.174 Human cytomegalovirus has three open reading frames that predict heptahelical receptors. In particular, US28 is highly similar to CCR1, and when expressed in mammalian cells can bind a wide range of CC chemokines, but not CXC chemokines.156,175 These observations raise the interesting possibility that some viruses have selectively mimicked chemokine-related elements of host defense to inactivate them or to subvert them to virulent ends. Finally, the H saimiri–related virus isolated from Kaposi's sarcoma lesions also encodes a chemokine receptorlike molecule.176 Although it binds IL-8 with relatively high affinity, it is constitutively active when expressed in COS cells and enhances proliferation of transfected NRK cells. Thus, this viral product may be involved in transforming the cell of origin in Kaposi's sarcoma.
A recent insight into chemokine physiology comes from the demonstration that chemokine receptor expression can be regulated. For example, IL-2 strongly upregulates expression of CCR1 and CCR2 in circulating T cells.177 This provides another level of control over leukocyte migration because resting T lymphocytes may not be competent to migrate even in the presence of high concentrations of chemokines.
IN VIVO ACTIVITIES
Because there are so many examples of a single chemokine receptor binding several chemokines, it might reasonably be asked whether any individual chemokine could possibly play an essential role in an inflammatory response in vivo. If the answer is no, then a therapy that targets a single chemokine would be doomed to failure because other chemokines with related activities could easily compensate for its inactivation.
In fact, there are two arguments for the essential importance of individual chemokines. The first is theoretical. By examining Table 3, it is apparent that each CC chemokine binds to a unique subset of receptors. If every combination of activated receptors produces a distinct biological response in vivo, then the seven cloned CC receptors could provide 127 different combinations of receptor activation and 127 potentially different physiological actions. (This could be viewed as a single cell responding differently depending on which of its receptors were activated, or as leukocyte subpopulations being defined by their patterns of chemokine receptor expression.) Considering the known CC chemokines, and probably more at 17q11.1-12 or elsewhere, these numbers are quite reasonable.
The second argument for the specificity of individual chemokines has to do with expression patterns. It may be that many chemokines elicit similar responses, but only one is expressed in vivo in a particular setting. For example, several CC chemokines attract monocytes in vitro, but perhaps only MCP-1 is expressed by arterial smooth muscle cells during atherogenesis. This possibility is currently being tested directly in animal models using passive immunization or gene targeting.
However, the first question that must be asked is whether the in vitro properties of chemokines accurately predict their in vivo properties. For the ELR-containing CXC chemokines, the data are relatively straightforward. For example, intradermal injection of IL-8 in rabbits produces neutrophil accumulation.16,178,179 For CC chemokines, injection results are more controversial. Some investigators see monocytic infiltrates after intradermal injection of MCP-1, -2, or -3 in rat or mouse skin, while others do not.70,180 181
Regardless of the results, injection experiments are fraught with difficulty because of the possibilities of coinjecting contaminants and inducing unrelated tissue damage that could elicit leukocyte infiltration. Several groups have circumvented these problems by constructing transgenic mice that overexpress chemokines. Although these models have produced disparate results, in the aggregate they provide insight into how chemokines work in vivo.
Mice expressing IL-8 under the control of liver-specific promoter/enhancers did not develop neutrophil infiltrates in their livers.182 Instead, they had high serum levels of IL-8 that were associated with L-selectin shedding from circulating neutrophils and an inability to induce neutrophil extravasation in response to local stimuli. This is similar to the observation that intravenous administration of IL-8 in rabbits prevents local neutrophil accumulation (although L-selectin shedding was not observed).183,184 Along the same lines, mice overexpressing MCP-1 under the control of the MMTV-LTR had high serum levels of MCP-1 and no monocytic infiltrates in expressing organs.181 These mice were more susceptible than wild-type mice to intracellular pathogens such as Listeria monocytogenes and Mycobacterium tuberculosis, implicating MCP-1 in the host response to these organisms. This suggests that like IL-8 overexpression, MCP-1 overexpression rendered circulating monocytes incapable of responding to local physiological levels of MCP-1.
In contrast to these models, expression of chemokines at low levels in anatomically restricted areas can produce leukocytic infiltration consistent with their in vitro properties. For example, mice expressing murine GRO-α (KC) in the thymus or brain had neutrophil-rich infiltrates in these organs.185 There was no tissue damage associated with the acute infiltrate, although mice developed severe neurological disease long after GRO-α expression had declined and most of the neutrophils had departed.186 When murine MCP-1 was substituted for GRO-α, the infiltrates were monocytic, and, although quite mild, could be enhanced by systemic LPS treatment.187 In another model, mice expressing human MCP-1 under the control of a surfactant promoter had increased numbers of monocytes and lymphocytes in their bronchial lavage fluid.188 Similarly, mice expressing murine MCP-1 under the control of an insulin promoter had a substantial monocytic insulitis but no tissue damage or diabetes.188a This local effect of the insulin promoter–driven MCP-1 could be abrogated by mating these mice to the MMTV-LTR transgenic mice. Finally, MCP-1 expression controlled by a keratin promoter produced mice with increased numbers of Langerhans-like cells in their skin, and they responded to contact hypersensitivity challenges with exaggerated numbers of monocytes and T lymphocytes.189
These results provide two insights into how chemokines work in vivo. First, chemokines exert their attractant effects only when they are expressed locally at low levels; systemically administered chemokines actually antagonize the local effects. Second, chemokines appear to attract leukocytes without necessarily activating them. This suggests that chemokine function in leukocyte trafficking is restricted to attraction, and that other signals are necessary for activation.
CHEMOKINES IN NORMAL AND DISORDERED PHYSIOLOGY
Lymphocyte trafficking.A critical component of systemic immunity is lymphocyte trafficking.190 This includes macroenvironmental movement, eg, migration of T lymphocytes from the BM and thymus to the spleen and lymph nodes, and microenvironmental movement, eg, migration of B cells from mantle zones to germinal centers. Because of the effects of chemokines on the migration of specific leukocyte subsets, it has been suggested that they are involved in controlling this migration.191 In one model, leukocytes engage in selectin-mediated rolling along the vascular endothelium until they encounter a high local concentration of chemokines (or other attractants), presumably presented in the context of cell-surface macromolecules192,193 or bound to the membrane like the CX3C chemokine.3 3a The chemokines are thought to upregulate integrin affinities, leading to strong adhesive interactions and diapedesis. Extravasated leukocytes then follow a gradient of chemokine concentration and come to rest at a site of high concentration. Leukocyte infiltration in inflammatory sites would then be a special case of this general model of leukocyte movement.
The specificity of chemokine targets makes this an attractive model. For example, RANTES and MCP-1 target only CD45RO+ T cells, suggesting a role in trafficking memory cells.17 However, as appealing as this mechanism may be, there has been only one direct demonstration of its existence in vivo, and ironically, the chemokine involved has yet to be identified. This example involves BRL1, a 7-TMS protein expressed on mature B and a subpopulation of T cells.194 BLR1 has the DRYLAIV sequence in its second intracellular domain, strongly suggesting that it may be a chemokine receptor (see above), but its ligand has not been identified. In BLR1−/− mice created by targeted gene disruption, B lymphocytes are found in T-cell–rich zones of primary splenic follicles rather than in the usual B-cell regions, and no germinal centers develop in secondary follicles.195 Furthermore, the animals have few or no Peyer's patches. Thus, the ligand for BLR1, which is probably a chemokine, may be responsible for attracting B cells that normally populate these areas. These results suggest that chemokines may be involved in basal leukocyte trafficking, and are consistent with the observation that pertussis toxin prevents the normal migration of B and T lymphocytes to their proper microenvironmental locations in the spleen.196 Precise roles for specific chemokines and their receptors in this process will become clearer as more mice are engineered with genetic alterations in this system.
Inflammatory diseases.It is a small step to extend the model of normal leukocyte trafficking to leukocyte infiltration in inflammatory disease. In this case, elaboration of chemokines by sentinel cells at an inflammatory focus may be responsible for inducing strong adhesive interactions between rolling leukocytes and the endothelium. Diapedesing cells are then attracted to the inflammatory site by the chemokine concentration gradient.
Most of the evidence for this model is indirect, coming from experiments that merely document chemokine overexpression at inflammatory foci. For example, MCP-1 expression can be detected in human atheromatous plaques and in the aortic walls of primates fed high-cholesterol diets, consistent with a model of atherogenesis in which MCP-1 in the vessel wall attracts monocytes that eventually become foam cells.197,198 Similarly, the presence of inflammatory cells in the joints of patients with rheumatoid arthritis has been explained by IL-8 and MCP-1 in synovial fluid.199,200 Chemokine expression can be documented in glomerulonephritis,201 asthma,202,203 inflammatory bowel disease,204 and allogeneic transplant rejection.205-207
The critical question, of course, is whether chemokine expression is pathogenetically responsible for any of the manifestations of these diseases. This has been very difficult to address in humans because of the apparent absence of genetic abnormalities involving chemokines and their receptors (for an exception, see the section on HIV). In animals, chemokines can be shown to play important roles in inflammatory models that may or may not be relevant to disease. For example, passive immunization with anti–IL-8 or anti–MCP-1 antibodies can reduce the edema associated with delayed-type hypersensitivity reactions or the granulomas induced by Schistosoma mansoni eggs in sensitized mice.208,209 Anti–IL-8 can also significantly ameliorate tissue damage associated with reperfusion injury and chemical pneumonitis.210 211 These are striking results because the profoundly destructive inflammatory infiltrate associated with these models can be reversed by neutralizing IL-8 alone, without having to address any of numerous other proinflammatory byproducts of tissue damage.
Even if the singular role of IL-8 in these models is proved genetically, the relevance to human disease remains tenuous. In contrast, some animal models speak more directly to human illness. For example, experimental autoimmune encephalomyelitis closely mimics many of the manifestations of multiple sclerosis. Expression of MCP-1, IP-10, RANTES, MIP-1α, MIP-1β, MCP-3, and GRO-α occurs immediately before the appearance of infiltrating cells in the central nervous system (CNS).212-214 Interestingly, astrocytes appear to be the major source of MCP-1 and IP-10. A pathogenetic role for MIP-1α has been suggested by the observation that passive immunization with anti–MIP-1α antibodies ameliorates disease manifestations.215
Another model that closely mimics human disease is pulmonary inflammation induced by aerosol challenge in ovalbumin sensitized mice. This produces an eosinophilic infiltrate and bronchospasm that, although imperfect, resembles human asthma. Soon after challenge, monocytes and macrophages appear in the lung in association with MCP-1 expression.216 This is followed by T-lymphocyte and eosinophil accumulation that are associated with RANTES and eotaxin expression.99,101,216 Passive immunization with anti-eotaxin antibodies reduces eosinophil accumulation by 50%, pointing to a role for this chemokine in asthma-like inflammation.216
Finally, a genetic demonstration for the role of chemokines in viral disease has been shown. Mice engineered to be deficient in MIP-1α by targeted gene disruption show almost none of the presumably autoimmune myocarditis associated with Coxsackie virus injection.217 Furthermore, their pulmonary inflammatory responses to influenza virus are attenuated and clearance of virus is delayed. These results have two important implications. First, they show that MIP-1α plays a critical role in the inflammatory response to viruses. Second, they suggest that other chemokines with similar properties in vitro cannot substitute for MIP-1α in vivo.
HIV.Although it has been known for years that CD4 is an obligate receptor for HIV-1, expression of human CD4 in rodent cells is not sufficient to make them permissive for infection.218 This observation pointed to the existence of a coreceptor. Feng et al155 used an expression cloning strategy to identify the coreceptor for T-cell–tropic, syncytium-inducing HIV-1 strains, which they called “fusin.” We now know that fusin is CXCR4, and that one of its ligands, SDF-1, inhibits infection by these HIV-1 strains in cell expressing CD4 and CXCR4.50 54
The first hint about a connection between chemokines and HIV-1 came from Cocchi et al,219 who showed that MIP-1α, MIP-1β, or RANTES could prevent infection by macrophage-tropic, nonsyncytium-inducing strains of HIV-1. This was reported soon after the first descriptions of the ligand specificity of CCR5, and led several groups to begin testing HIV-1's interactions with CCR5. It is now clear that CCR5 is the major coreceptor for these HIV-1 strains, but CCR3 and, to a lesser extent CCR2B, are also active.220-223 CCR5 coreceptor function has been proven genetically by the discovery that a significant proportion of individuals who have been exposed to HIV-1 over extended periods of time, but are not infected, are homozygous for an inactive variant of CCR5.224-226
Evidence to date indicates that viral gp120 interacts directly with CD4 and with the chemokine receptor.227-229 Analyses of chimeric and mutated chemokine receptors point to the N-terminus and first extracellular loop as being critical specificity determinants for HIV-1 infection.230 231 Because some of these variants no longer signal in response to their cognate ligands, it has been suggested that receptor activation does not play a role in HIV-1 entry, but this remains to be proven rigorously.
These results identify chemokine receptors as new targets for anti-HIV therapy. Because individuals with inactive CCR5 seem to be otherwise well, it appears that full blockade of CCR5 is not detrimental. (However, because other CC chemokine receptors also act as coreceptors for some strains, the blockade might have to be more substantial and less specific.) These results also raise a series of interesting questions. First, why has the virus exploited chemokine receptors rather than other ubiquitous heptahelical receptors? If signaling is not part of HIV entry, this would suggest that the virus selected these receptors for structural reasons rather than for reasons of chemokine-like molecular mimicry. Second, the discovery of CCR5-negative individuals begs the question of what this receptor does. There are no obvious abnormalities in these individuals, and the inactive allele appears in certain populations with a frequency approaching 10% to 20%. The answers to these questions will provide as much insight into the physiology of chemokines as the pathophysiology of HIV.
Angiogenesis.CXC chemokines have been extensively investigated for their effects on angiogenesis in vivo and on endothelial cells in vitro. A variety of ELR-containing chemokines has been reported to be chemotactic for endothelial cells, including IL-8, ENA-78, GCP2, GRO-α, GRO-β, GRO-γ, and the processed products of PBP.232-234 All of these chemokines are also angiogenic in the rat cornea neovascularization assay.232,233 The non-ELR CXC chemokines, including PF4, IP-10, and MIG are not only inactive chemoattractants and nonangiogenic themselves, but they inhibit the angiogenic effects of ELR chemokines and basic FGF.40,232,235 Although these data appear to assign angiogenic properties to ELR chemokines and angiostatic properties to non-ELR CXC chemokines, there is a report that GRO-α and GRO-β inhibit endothelial cell proliferation in vitro and are potently anti-angiogenic in vivo.236 These disparate results may be related to differences in experimental systems.
PF4's angiostatic activity depends on the presence of its C-terminal heparin-binding domain and can be reversed by adding heparin. However, a PF4 variant that does not bind heparin (but does maintain the C-terminal α-helical structure) is an even more potent inhibitor.237 Another potent variant has a wild-type α-helix that binds heparin, but has undergone cleavage at Thr-16, suggesting that the determinants for angiostatic activity may be complex.41
Because the non-ELR CXC chemokines only inhibit chemokine– or growth factor–induced angiogenesis, one model for their angiostatic properties would be interference with activation of growth factor receptors on endothelial cells. In fact, PF4 binds to full-length VEGF (VEGF165 ) and prevents its heparin-dependent interaction with its receptor.238 However, PF4 also inhibits the mitogenic effects of a truncated VEGF (VEGF121 ) whose receptor binding is not heparin-dependent and is not prevented by PF4. This is a strong argument for angiostatic chemokines exerting their effects through their own cognate receptors, and effecting events at a post–growth factor receptor activation stage.
The angiostatic effects of PF4 can be produced in vivo (eg, in corneal neovascularization models) and this has led to testing PF4 for its ability to inhibit tumor angiogenesis. Systemic PF4 administered to mice after inoculation with B16F10 melanoma cells reduces the number and size of lung metastases without preventing their homing to the lung.239,240 The non–heparin-binding variant is also potent in vivo.237
In contrast, the observation that ELR chemokines are angiogenic suggests that they may play a role in promoting tumor angiogenesis. Several lung carcinomas have marginally increased levels of IL-8, and the angiogenic activity present in extracts from these tumors is almost entirely caused by IL-8.241 This has suggested a model in which the balance of ELR versus non-ELR chemokines produced by a tumor and its associated stroma dictate the degree of angiogenesis, and therefore aggressiveness, displayed by the tumor.233 Some evidence supporting this model comes from the beneficial effects of administering anti–IL-8 to SCID mice bearing human IL-8–expressing lung cancer xenografts.233
Hematopoiesis.Some chemokines have dual effects on hematopoiesis depending on the maturity of the progenitors being treated. For example, MIP-1α, MIP-1β, GRO-β, and GRO-γ enhance the formation of colony-forming unit granulocyte-macrophage (CFU-GM) from unfractionated BM, but only in the presence of M- or GM-colony-stimulating factor (CSF).242-245 In contrast, several chemokines suppress the proliferation of more immature progenitors, eg, CFU-S, CFU-A (macrophage-rich colonies derived from stem cells), and CFU-GM (colonies that require stem cell factor [SCF] and GM-CSF), as well as CFU-GEMM and burst-forming unit erythroid (BFU-E) (colonies that require SCF and erythropoietin).88,116,243,246-248 This effect is a direct one on the progenitor cells because the suppression is more complete on CD34+-selected cells.244,247 The same effect can be demonstrated by in vivo administration of MIP-1α, and pretreatment of animals with MIP-1α can enhance myeloid recovery after treatment with S-phase–active chemotherapeutic agents.246,249-251 Furthermore, analysis of these in vivo experiments has led to the suggestion that MIP-1α's target is not the earliest long-term reconstituting stem cell, but a somewhat more mature cell246 which may be Lin−Thy1+ in the mouse.242 Although the most direct explanation for these observations is that MIP-1α inhibits proliferation, it has also been suggested that MIP-1α may prevent factor-induced differentiation.248 252
Broxmeyer's group has systematically studied chemokines in the methylcellulose culture system and they have identified the suppressive chemokines as MIP-1α, GRO-β, PF4, IL-8, MCP-1, IP-10, and CCF18.116,243,247 Mixtures of these chemokines are synergistic in their suppression. Chemokines that have no suppressive effects on hematopoietic progenitors include: MIP-1β, GRO-α, GRO-γ, NAP-2, and RANTES. In fact, pretreatment of progenitors with MIP-1β blocks the suppressive effects of MIP-1α, and pretreatment with GRO-α or GRO-γ blocks the effects of IL-8 and PF4.243 In general, chemokines exert their suppressive effects with an ED50 of ≈5 nmol/L. However, in the case of MIP-1α, which oligomerizes at high concentrations, the active form is the monomer, which is 1,000-fold more potent than the usual mixture of multimers.253
Inspection of lists of suppressive and nonsuppressive chemokines suggests that this effect depends neither on CC versus CXC structure, nor on the presence or absence of the ELR motif. In fact, structure/activity analyses of IL-8 and PF4 show that neutrophil chemoattraction does not correlate with stem cell suppression.254 Some PF4/IL-8 chimeras were active at 10 pmol/L (similar to the MIP-1α monomers). Interestingly, mutations in IL-8 that involve the dimer interface destroy stem cell inhibitory activity, suggesting that dimerization may be important, although it remains possible that these amino acids are also required for interaction with the stem cell receptor for IL-8.
The fact that PF4 is as potent as IL-8 suggests that all suppressive effects do not occur via CXCR1 or CXCR2. Furthermore, the synergistic effects of some chemokines and the ability of chemokine pretreatment to block the effects of other chemokines implies the existence of multiple relevant receptors. At least one has been identified in mice. The gene encoding an IL-8 receptor homolog which binds murine MIP-2 (homologous to human GRO-β and GRO-γ) with high affinity has been disrupted in mice.255 Among other abnormalities, these mice have an expanded neutrophil compartment, especially when reared in a non–germ-free environment. BM progenitors from these mice are insensitive to the suppressive effects of IL-8 and murine MIP-2, but still sensitive to MIP-1α, PF4, IP-10, and MCP-1, arguing that multiple chemokine receptors are involved in hematopoiesis.256
These observations are clearly relevant to hematopoiesis in vivo. In the IL-8 receptor homolog-deficient mice reared in a germ-free environment, the number of BM progenitor cells was similar to that in wild-type mice.256 However, although the number of progenitors increased in both mice when reared in specific pathogen-free conditions, the increase in number of progenitors in the receptor-deficient mice was much greater than in the wild type. This is consistent with a model in which endogenous ligands for this receptor are involved in an inhibitory pathway for myeloid progenitors. Interestingly, although MIP-1α was the first stem cell inhibitor to be identified, mice deficient for MIP-1α do not have an expanded progenitor pool.217 Either MIP-1α's effects are not relevant in vivo, or other chemokines can compensate for its absence.
Although the focus of this discussion has been on hematopoietic stem cells, MIP-1α also inhibits the proliferation of keratinocyte stem cells.257 Thus, chemokines may have a more general effect on suppressing stem cell proliferation.
Antitumor effects.Angiostasis is only one means by which chemokines limit tumor growth. For example, because some chemokines inhibit hematopoietic and epithelial stem cell proliferation, it might be imagined that they could have direct inhibitory effects on malignant cells as well. So far, however, there is only a single report in which IL-8 directly inhibited the growth of non–small cell lung cancer cell lines in vitro.258
A more commonly investigated scenario exploits the leukocyte chemoattractant properties of chemokines to enhance a host antitumor response. Engineered expression of MCP-1, IP-10, TCA3, or lymphotactin in tumor cells has been shown to elicit antitumor effects when cells are injected in vivo.259-263 For example, MCP-1 expression in tumor cells prevents tumor formation after inoculation in athymic mice, suggesting that acute rejection is not T-cell–mediated.259 However, when syngeneic cells are irradiated and used as a tumor vaccine, engineered expression of MCP-1 in these cells prevents subsequent growth of wild-type tumor cells.264 This indicates that MCP-1 can enhance tumor specific immunity, presumably in a T-lymphocyte–dependent manner. Similar results have been reported for TCA3.262 Finally, lymphotactin itself has very little effect on tumor take or established tumors in syngeneic animals. However, when used in combination with IL-2, it has activity as a “therapeutic” tumor vaccine, reducing the size of established tumors.263 While the long-term immunity in immunocompetent animals in these models is T-lymphocyte–dependent, some effects may also reflect activation of NK cells by CC chemokines.66
IP-10's effects are more complicated. On one hand, engineered IP-10 expression in tumor cells prevents their growth in syngeneic animals, but not in athymic animals, suggesting that this effect is T-cell–dependent.260 However, injection of IP-10 into established Burkitt's tumors in nu/nu mice leads to tumor necrosis in association with microvascular thrombosis.265 This may be related to IP-10's antiangiogenic activity.
CONCLUSIONS
It is striking to recall that a family of proteins as large and ubiquitously expressed as the chemokines and their receptors was unknown less than 10 years ago. Their recent history may be one reason why we still understand so little about the true roles played by chemokines in normal and diseased physiology. In the absence of clear-cut underlying principles, a review about chemokines will necessarily seem like a series of unconnected observations.
Nonetheless, the picture that is emerging from genetically modified mouse models is that most of the in vitro properties of chemokines are recapitulated in vivo. This suggests that many of the early hypotheses about the proinflammatory function of chemokines in disease may turn out to be accurate. If so, that will provide an enormous number of new drug targets for diseases ranging from atherosclerosis to acquired immunodeficiency syndrome. Now the focus can start to shift away from proof-of-concept experiments that document chemokine-mediated pathology to mechanistic studies that investigate ways to interrupt chemokine/receptor interactions or chemokine receptor signal transduction.
NOTE ADDED IN PROOF
Two additional CC chemokine receptors have been characterized and their ligands identified. CCR6 is the receptor for MIP-3α/LARC and is expressed by CD4+ and CD8+ T lymphocytes and B lymphocytes, but not by NK cells, monocytes, or neutrophils.267 CCR7, formerly known as the orphan receptor EBI1, is the receptor for MIP-3β, also known as ELC (EBI1 ligand chemokine).268 CCR7 is expressed by activated T and B lymphocytes.
ACKNOWLEDGMENT
I apologize to any colleague whose work I may have unintentionally overlooked and not cited. I also want to thank Dr Craig Gerard for helpful advice and suggestions, and Laurie Geronimo for administrative assistance.
Supported by a grant from the National Institutes of Health. B.J.R. is a Scholar of The Leukemia Society.
Address reprint requests to Barrett J. Rollins, MD, PhD, Dana-Farber Cancer Institute, 44 Binney St, Boston, MA 02115.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal